Solutions to Chemistry Homework Assignment on Coordination Chemistry
VerifiedAdded on 2022/09/15
|5
|834
|17
Homework Assignment
AI Summary
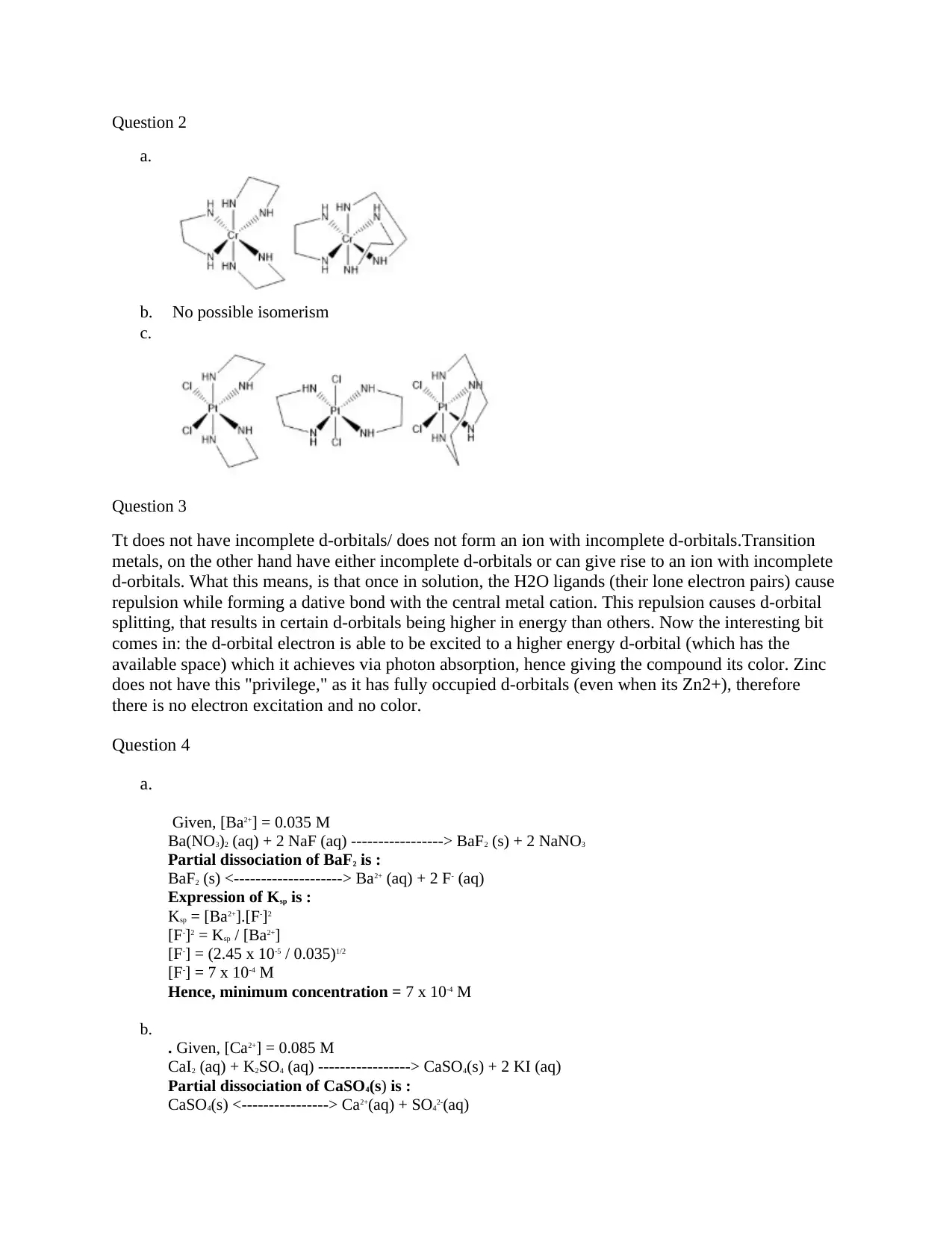
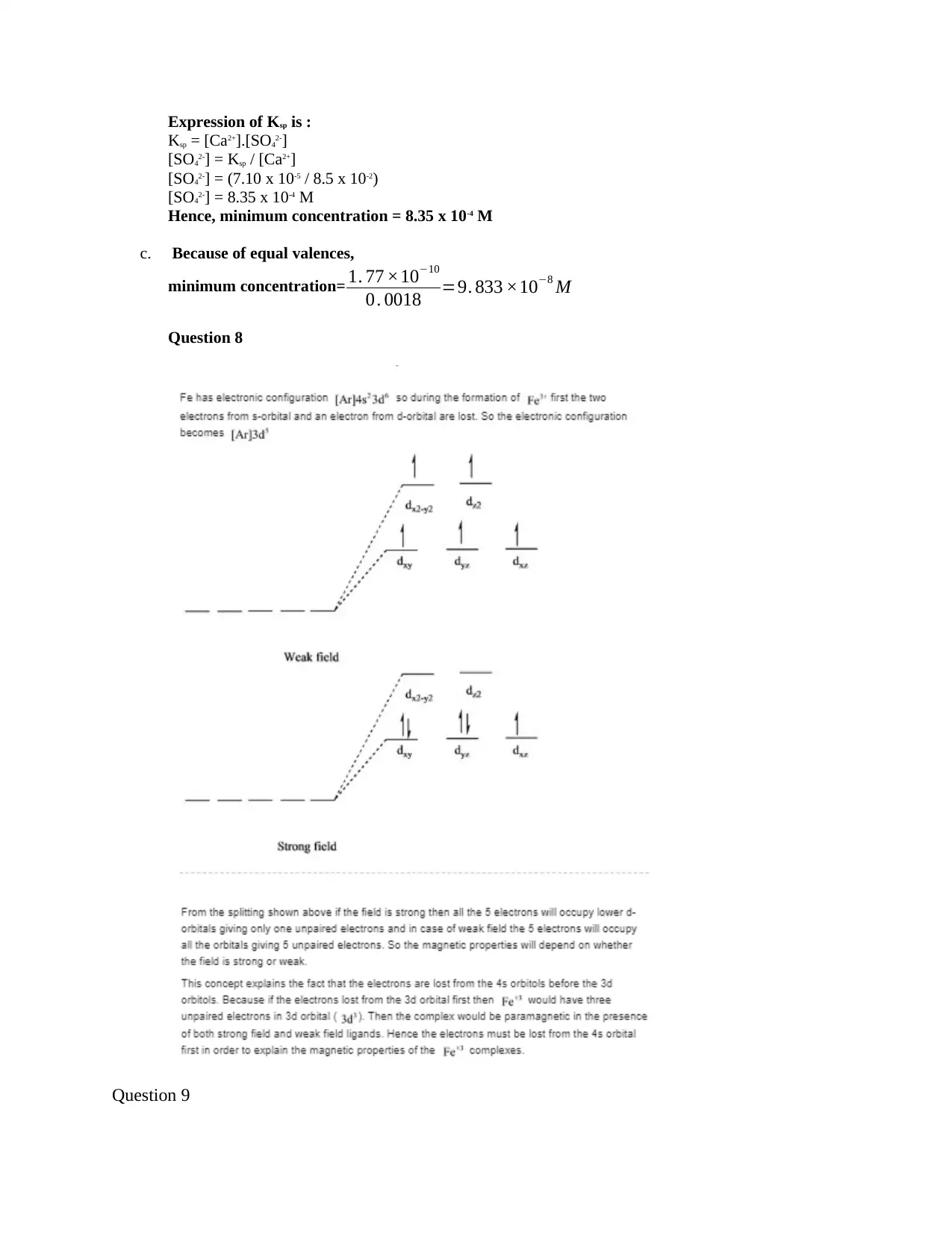
This document provides detailed solutions to a chemistry homework assignment. The solutions cover various topics including the absence of isomerism in a compound, the lack of color in zinc compounds explained by the absence of electron excitation due to fully occupied d-orbitals. The assignment includes calculations involving the solubility product (Ksp) to determine the minimum concentration of ions in solutions of barium fluoride and calcium sulfate. It explores the relationship between ligand strength, crystal field splitting, and the color of coordination complexes, explaining why blue light has higher energy than purple light. Furthermore, the document provides solutions to buffer calculations, determining the pH and molar ratios required for a dimethylamine buffer, and calculating the crystal field splitting energy from the absorbance spectrum of a complex. The solutions are comprehensive and provide step-by-step explanations.
1 out of 5




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)