Detailed Lab Report: Measuring Enthalpy Change of Copper (II) Sulfate
VerifiedAdded on 2023/06/15
|16
|2501
|71
Report
AI Summary
This laboratory report details an experiment conducted to determine the enthalpy change for the reaction between anhydrous copper (II) sulfate and water, resulting in hydrated copper (II) sulfate. The experiment employs calorimetry and Hess's Law to calculate the enthalpy change, addressing the challenges of direct measurement due to reaction speed and potential by-products. The report includes two experiments: one involving the addition of anhydrous copper (II) sulfate to water and another using hydrated copper (II) sulfate. Temperature changes are meticulously recorded, and enthalpy changes are calculated using the formula ΔH = m * cp * ΔT. The results are then compared with theoretical values, and percentage errors are analyzed, with sources of error identified as potential inaccuracies in temperature and mass measurements. The conclusion affirms that the experimental aim was achieved, and the calculated enthalpy change closely aligns with the theoretical value, validating the experiment's accuracy.

Laboratory Report 1
LABORATORY REPORT
By Name
Course
Instructor
Institution
Location
Date
LABORATORY REPORT
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Laboratory Report 2
ABSTRACT
In a thermochemical equation, it is important to note state symbols (solid, liquid or gas)
because the enthalpy change, ∆H depends on the phase of the substances. In some cases, it is
very difficult to determine the enthalpy changes for reactions through experiment like in
situations where the reaction is too slow or too fast or by-products are formed. In such situation,
Hess's law is applied. When anhydrous copper (II) sulfate is added to water then there will be a
reaction which involves the physical change as the anhydrous copper (II) sulfate (white in
colour) changes to hydrated copper (II) sulfate (blue in colour). And during that reaction there
will be change in temperature. There will increase in temperature as this reaction takes place
therefore the reaction is an exothermic reaction. When water is added to the anhydrous copper
(II) sulfate, the water molecule will hence chemically combine with the anhydrous copper (II)
sulfate to form a hydrated copper (II) sulfate having water molecules chemically combined as
seen below;
CuSO4 (s) + 5H2O (l) CuSO4.5H2O (s)
ABSTRACT
In a thermochemical equation, it is important to note state symbols (solid, liquid or gas)
because the enthalpy change, ∆H depends on the phase of the substances. In some cases, it is
very difficult to determine the enthalpy changes for reactions through experiment like in
situations where the reaction is too slow or too fast or by-products are formed. In such situation,
Hess's law is applied. When anhydrous copper (II) sulfate is added to water then there will be a
reaction which involves the physical change as the anhydrous copper (II) sulfate (white in
colour) changes to hydrated copper (II) sulfate (blue in colour). And during that reaction there
will be change in temperature. There will increase in temperature as this reaction takes place
therefore the reaction is an exothermic reaction. When water is added to the anhydrous copper
(II) sulfate, the water molecule will hence chemically combine with the anhydrous copper (II)
sulfate to form a hydrated copper (II) sulfate having water molecules chemically combined as
seen below;
CuSO4 (s) + 5H2O (l) CuSO4.5H2O (s)

Laboratory Report 3
AIM OF THE PRACTICAL
To obtain the enthalpy change for the reaction when anhydrous copper (ii) sulfate is
reacted with water to get hydrated copper (ii) sulfate, this enthalpy cannot be measured directly.
INTRODUCTION
Enthalpy change is the amount of energy which is absorbed or evolved during chemical reaction
at a constant pressure. An easier way to obtain the change in enthalpy for a chemical reaction is
to take change in the temperature as a result of the same reaction.
ΔH = m cp ΔT…………………………………………………………………………………… 1
ΔH = the enthalpy of the reaction
ΔT = change in temperature
Cp = the heat capacity of the substance which changes temperature
m = the mass of the sample which changes the temperature
In a thermochemical equation, it is important to note state symbols (solid, liquid or gas)
because the enthalpy change, ∆H, depends on the phase of the substances. In some cases, it is
very difficult to determine the enthalpy changes for reactions through experiment like in
situations where the reaction is too slow or too fast or by-products are formed (Cemic, 2015). In
such situation, Hess's law is applied. The Law states that the enthalpy change for a chemical
reaction is independent of the route taken. This implies that the change in enthalpy for the overall
reaction will be identical irrespective of how many steps are covered (Dahm, 2014). This law can
be illustrated as below.
AIM OF THE PRACTICAL
To obtain the enthalpy change for the reaction when anhydrous copper (ii) sulfate is
reacted with water to get hydrated copper (ii) sulfate, this enthalpy cannot be measured directly.
INTRODUCTION
Enthalpy change is the amount of energy which is absorbed or evolved during chemical reaction
at a constant pressure. An easier way to obtain the change in enthalpy for a chemical reaction is
to take change in the temperature as a result of the same reaction.
ΔH = m cp ΔT…………………………………………………………………………………… 1
ΔH = the enthalpy of the reaction
ΔT = change in temperature
Cp = the heat capacity of the substance which changes temperature
m = the mass of the sample which changes the temperature
In a thermochemical equation, it is important to note state symbols (solid, liquid or gas)
because the enthalpy change, ∆H, depends on the phase of the substances. In some cases, it is
very difficult to determine the enthalpy changes for reactions through experiment like in
situations where the reaction is too slow or too fast or by-products are formed (Cemic, 2015). In
such situation, Hess's law is applied. The Law states that the enthalpy change for a chemical
reaction is independent of the route taken. This implies that the change in enthalpy for the overall
reaction will be identical irrespective of how many steps are covered (Dahm, 2014). This law can
be illustrated as below.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Laboratory Report 4
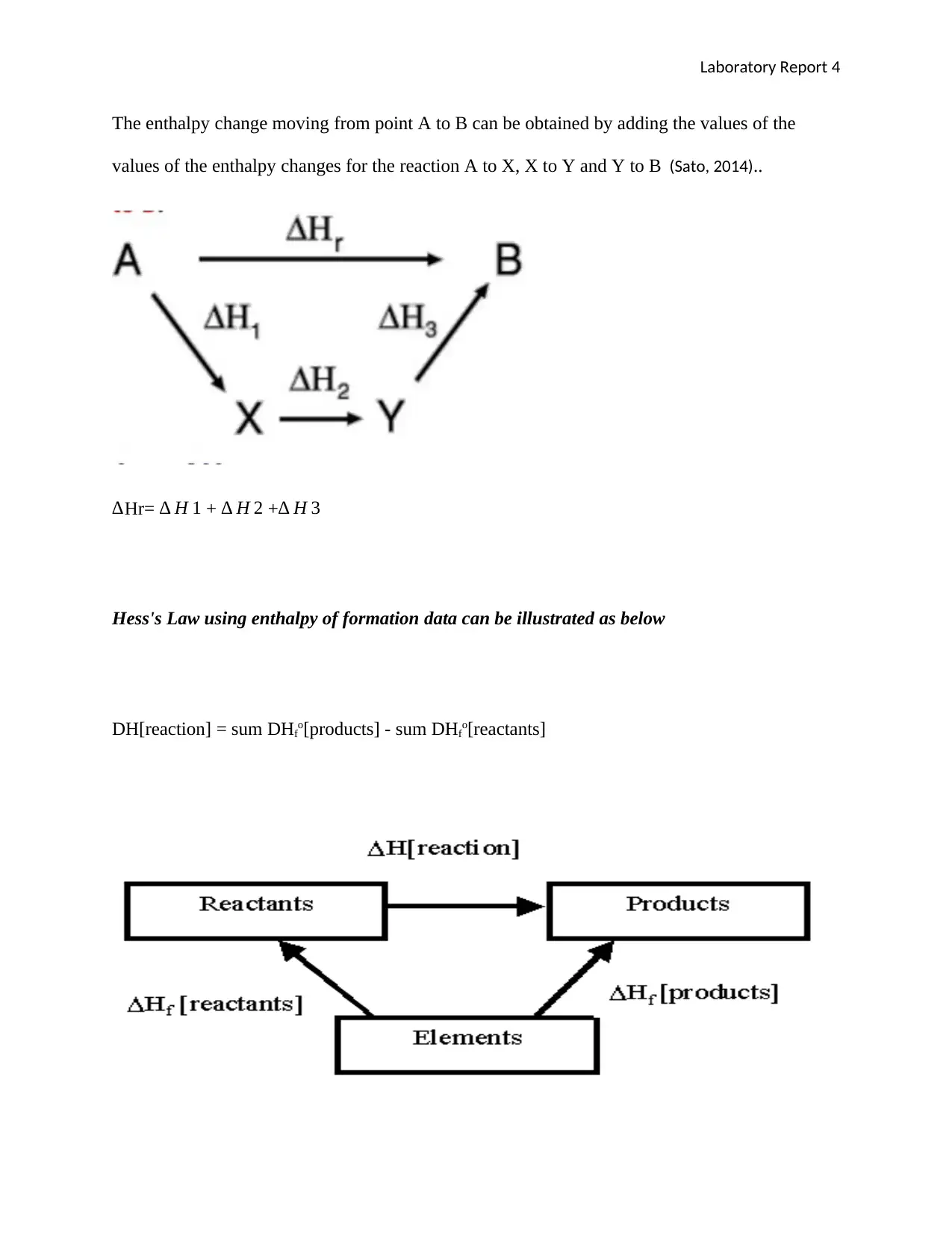
The enthalpy change moving from point A to B can be obtained by adding the values of the
values of the enthalpy changes for the reaction A to X, X to Y and Y to B (Sato, 2014)..
∆Hr= ∆ H 1 + ∆ H 2 +∆ H 3
Hess's Law using enthalpy of formation data can be illustrated as below
DH[reaction] = sum DHfo[products] - sum DHfo[reactants]
The enthalpy change moving from point A to B can be obtained by adding the values of the
values of the enthalpy changes for the reaction A to X, X to Y and Y to B (Sato, 2014)..
∆Hr= ∆ H 1 + ∆ H 2 +∆ H 3
Hess's Law using enthalpy of formation data can be illustrated as below
DH[reaction] = sum DHfo[products] - sum DHfo[reactants]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Laboratory Report 5
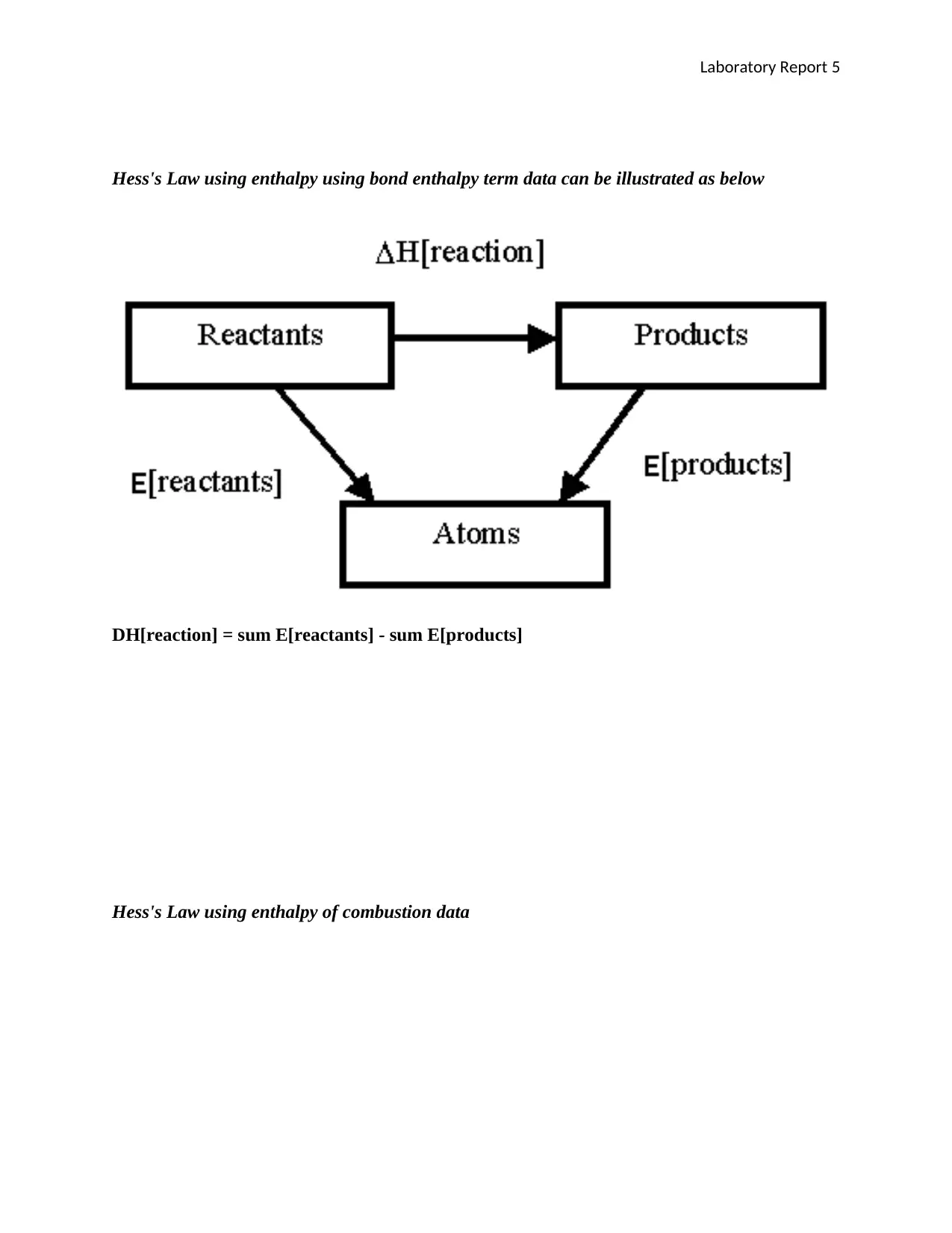
Hess's Law using enthalpy using bond enthalpy term data can be illustrated as below
DH[reaction] = sum E[reactants] - sum E[products]
Hess's Law using enthalpy of combustion data
Hess's Law using enthalpy using bond enthalpy term data can be illustrated as below
DH[reaction] = sum E[reactants] - sum E[products]
Hess's Law using enthalpy of combustion data
Laboratory Report 6
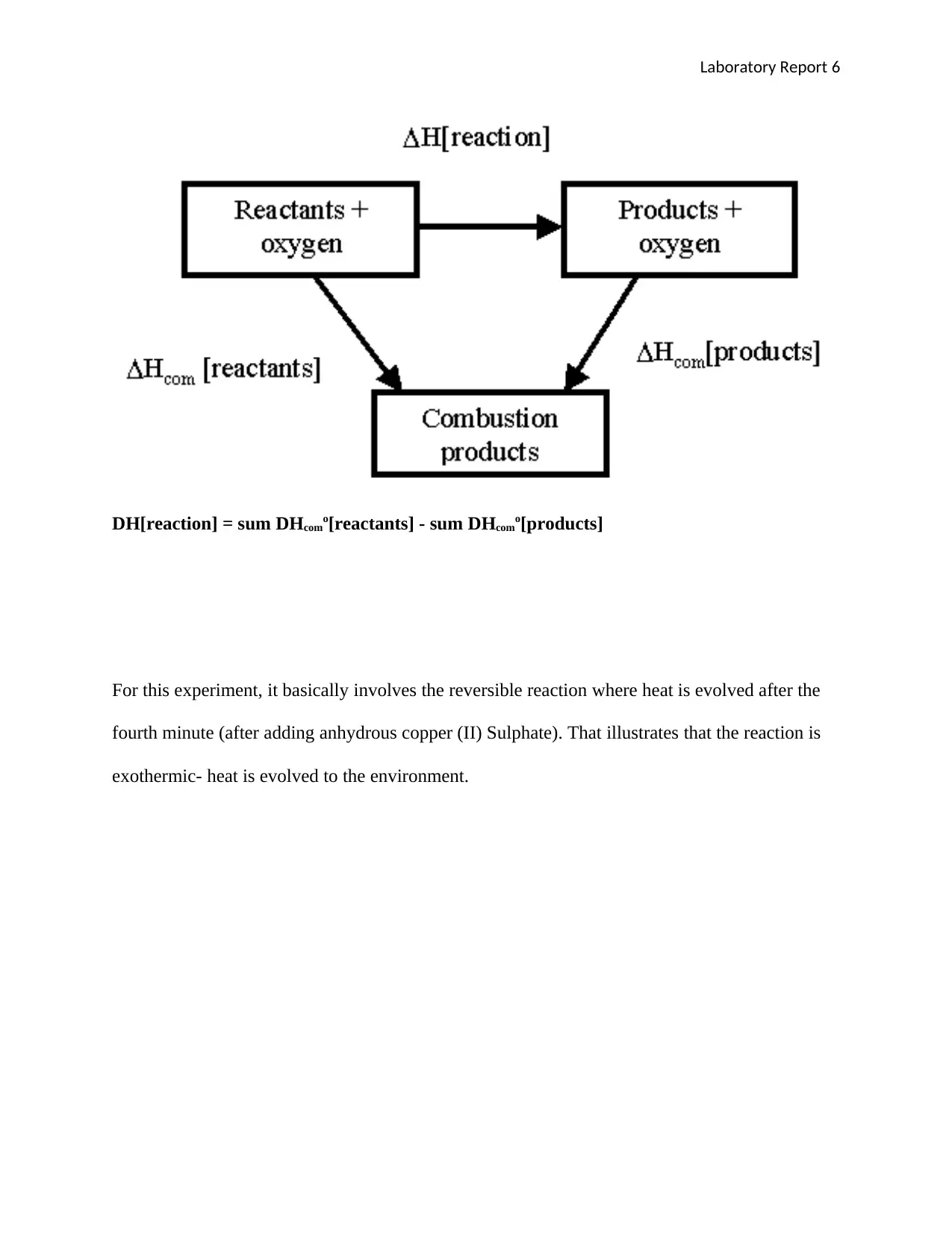
DH[reaction] = sum DHcomo[reactants] - sum DHcomo[products]
For this experiment, it basically involves the reversible reaction where heat is evolved after the
fourth minute (after adding anhydrous copper (II) Sulphate). That illustrates that the reaction is
exothermic- heat is evolved to the environment.
DH[reaction] = sum DHcomo[reactants] - sum DHcomo[products]
For this experiment, it basically involves the reversible reaction where heat is evolved after the
fourth minute (after adding anhydrous copper (II) Sulphate). That illustrates that the reaction is
exothermic- heat is evolved to the environment.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Laboratory Report 7
METHOD
Experiment 1
Collecting data for the determination of ∆H1
A suitable table of results is constructed to allow recording of the temperatures at an
interval of 15 minutes. Anhydrous copper (ii) sulfate was weighed between 3.9 g and 4.10 g in a
dry stoppered weighing bottle. The stock of the solid is kept in a closed container during
weighing. The skin contact with chemical was avoided and precious mass was recorded. A
measuring cylinder was used to place 25cm3of deionized water into a polystyrene cup and its
temperature was recorded every minute as the liquid was stirred continuously (Letcher, 2017).
At the fourth minute powdered anhydrous copper (ii) sulfate was added rapidly to the
water in the polystyrene cup as stirring continuous but at this time the temperature was not
recorded. The solution was stirred and its temperature was recorded in the polystyrene cup. At
the fifth minutes and every minute up to the fifteenth minute, the solution was stirred and its
temperature was recorded in the polystyrene cup (Lvov, 2012). A graph of temperature (on the y-
axis) against time was plotted. Two separate best fit was drawn one which joins the points before
addition while the other which joins the points after addition, both lines were extrapolated to the
fourth minute and the temperature change in the experiment was determined.
Experiment 2
Collecting data for the determination of ∆H2
METHOD
Experiment 1
Collecting data for the determination of ∆H1
A suitable table of results is constructed to allow recording of the temperatures at an
interval of 15 minutes. Anhydrous copper (ii) sulfate was weighed between 3.9 g and 4.10 g in a
dry stoppered weighing bottle. The stock of the solid is kept in a closed container during
weighing. The skin contact with chemical was avoided and precious mass was recorded. A
measuring cylinder was used to place 25cm3of deionized water into a polystyrene cup and its
temperature was recorded every minute as the liquid was stirred continuously (Letcher, 2017).
At the fourth minute powdered anhydrous copper (ii) sulfate was added rapidly to the
water in the polystyrene cup as stirring continuous but at this time the temperature was not
recorded. The solution was stirred and its temperature was recorded in the polystyrene cup. At
the fifth minutes and every minute up to the fifteenth minute, the solution was stirred and its
temperature was recorded in the polystyrene cup (Lvov, 2012). A graph of temperature (on the y-
axis) against time was plotted. Two separate best fit was drawn one which joins the points before
addition while the other which joins the points after addition, both lines were extrapolated to the
fourth minute and the temperature change in the experiment was determined.
Experiment 2
Collecting data for the determination of ∆H2
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Laboratory Report 8
A suitable table of results was constructed to allow recording of temperatures at an
interval of 15 minutes. Anhydrous copper (ii) sulfate was weighed between 6.20 g and 6.30 g in
a dry stoppered weighing bottle. A measuring cylinder was used to place 24cm3of deionized
water into a polystyrene cup and its temperature was recorded every minute as the liquid was
stirred continuously. The total amount of water would be approximately the same as in
experiment 1 since hydrated crystal contains water.
At the fourth minute powdered copper (ii) sulfate was added rapidly to the water in the
polystyrene cup as stirring continuous but at this time the temperature was not recorded. At the
fifth minutes and every minute up to the fifteenth minute, the solution was stirred and its
temperature was recorded in the polystyrene cup (Tsao, 2012). A graph of temperature (on the y-
axis) against time was plotted. Two separate best fit was drawn one which joins the points before
addition while the other which joins the points after addition, both lines were extrapolated to the
fourth minute and the temperature change in the experiment was determined.
Equipment and materials used
Balance
Stand and clamp
Beaker (250 or 400 cm3)
Measuring cylinder × 2 (25 cm3)
Weighing bottle × 2
Thermometer (0.10 C divisions)
Polystyrene cup (calorimeter)
A suitable table of results was constructed to allow recording of temperatures at an
interval of 15 minutes. Anhydrous copper (ii) sulfate was weighed between 6.20 g and 6.30 g in
a dry stoppered weighing bottle. A measuring cylinder was used to place 24cm3of deionized
water into a polystyrene cup and its temperature was recorded every minute as the liquid was
stirred continuously. The total amount of water would be approximately the same as in
experiment 1 since hydrated crystal contains water.
At the fourth minute powdered copper (ii) sulfate was added rapidly to the water in the
polystyrene cup as stirring continuous but at this time the temperature was not recorded. At the
fifth minutes and every minute up to the fifteenth minute, the solution was stirred and its
temperature was recorded in the polystyrene cup (Tsao, 2012). A graph of temperature (on the y-
axis) against time was plotted. Two separate best fit was drawn one which joins the points before
addition while the other which joins the points after addition, both lines were extrapolated to the
fourth minute and the temperature change in the experiment was determined.
Equipment and materials used
Balance
Stand and clamp
Beaker (250 or 400 cm3)
Measuring cylinder × 2 (25 cm3)
Weighing bottle × 2
Thermometer (0.10 C divisions)
Polystyrene cup (calorimeter)
Laboratory Report 9
Stopwatch
Stirrer
Hydrated copper (ii) sulfate small crystals – harmful, dangerous to the environment
Anhydrous copper (ii) sulfate powder – harmful, dangerous to the environment
Lab coat and eye protection.
RESULT
Experiment 1
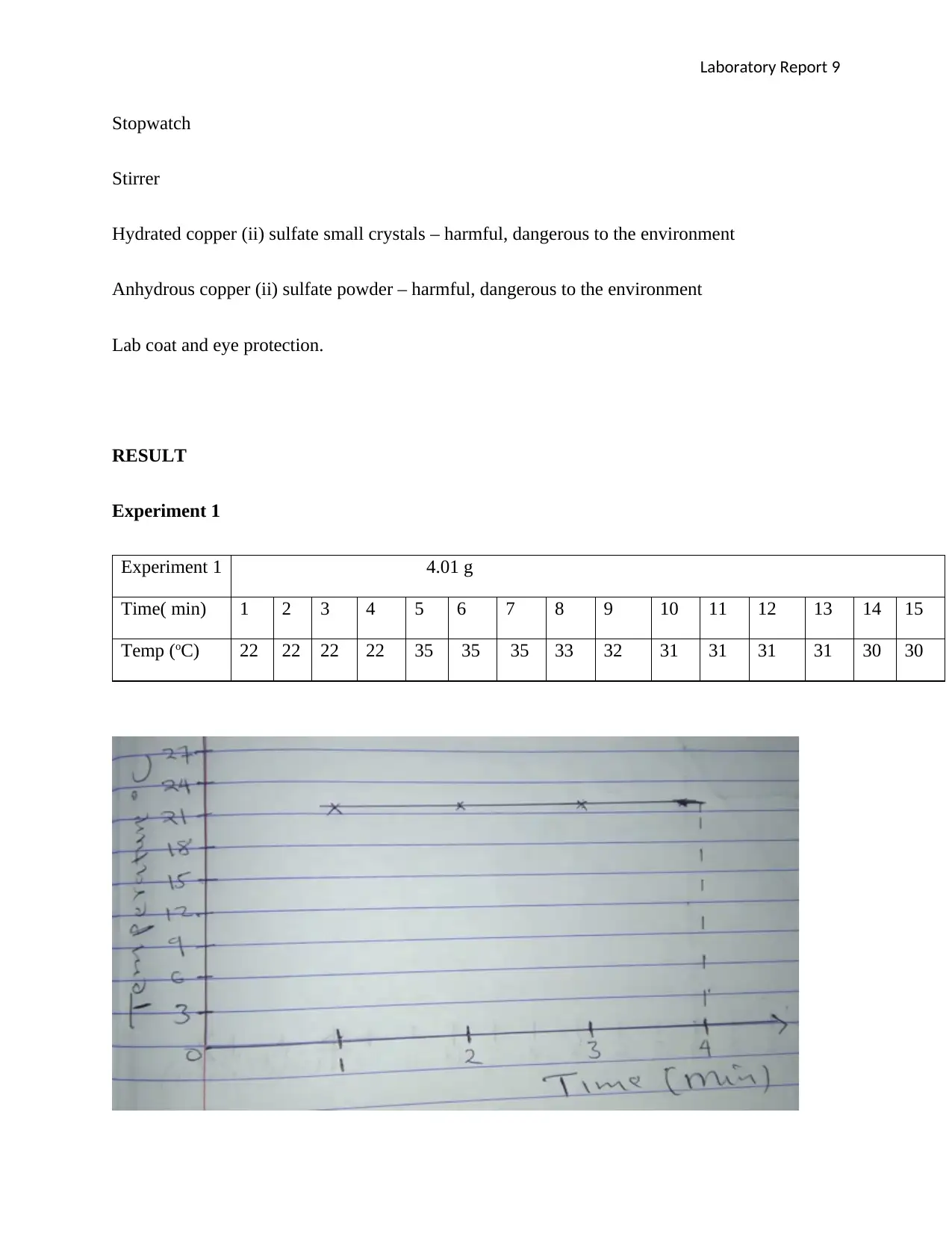
Experiment 1 4.01 g
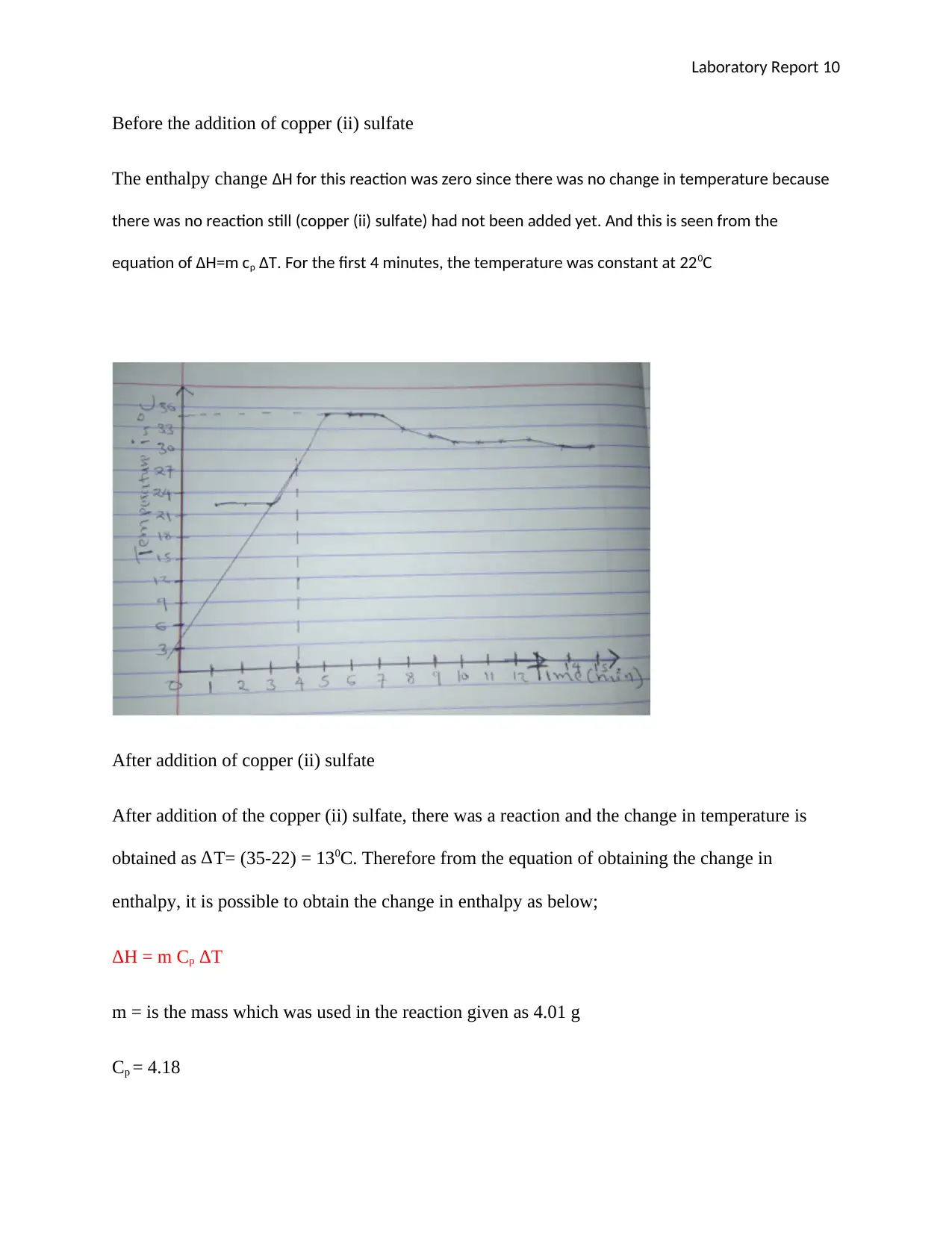
Time( min) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Temp (oC) 22 22 22 22 35 35 35 33 32 31 31 31 31 30 30
Stopwatch
Stirrer
Hydrated copper (ii) sulfate small crystals – harmful, dangerous to the environment
Anhydrous copper (ii) sulfate powder – harmful, dangerous to the environment
Lab coat and eye protection.
RESULT
Experiment 1
Experiment 1 4.01 g
Time( min) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Temp (oC) 22 22 22 22 35 35 35 33 32 31 31 31 31 30 30
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Laboratory Report 10
Before the addition of copper (ii) sulfate
The enthalpy change ΔH for this reaction was zero since there was no change in temperature because
there was no reaction still (copper (ii) sulfate) had not been added yet. And this is seen from the
equation of ΔH=m cp ΔT. For the first 4 minutes, the temperature was constant at 220C
After addition of copper (ii) sulfate
After addition of the copper (ii) sulfate, there was a reaction and the change in temperature is
obtained as ∆T= (35-22) = 130C. Therefore from the equation of obtaining the change in
enthalpy, it is possible to obtain the change in enthalpy as below;
ΔH = m Cp ΔT
m = is the mass which was used in the reaction given as 4.01 g
Cp = 4.18
Before the addition of copper (ii) sulfate
The enthalpy change ΔH for this reaction was zero since there was no change in temperature because
there was no reaction still (copper (ii) sulfate) had not been added yet. And this is seen from the
equation of ΔH=m cp ΔT. For the first 4 minutes, the temperature was constant at 220C
After addition of copper (ii) sulfate
After addition of the copper (ii) sulfate, there was a reaction and the change in temperature is
obtained as ∆T= (35-22) = 130C. Therefore from the equation of obtaining the change in
enthalpy, it is possible to obtain the change in enthalpy as below;
ΔH = m Cp ΔT
m = is the mass which was used in the reaction given as 4.01 g
Cp = 4.18
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Laboratory Report 11
Therefore
ΔH = 4.01×4.18 × 13
ΔH= 217.903 kJ
The theoretical value of the change in enthalpy for the temperature change of 130C is 219.2,
therefore, it is possible to obtain the error.
Percentage error = 219.2−217.903
219.2 × 100
Percentage error = 1.297
219.2 × 100
Percentage error = 0.592 %
Experiment 2
Experiment
2
6.29 g
Time( min) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Temp (oC) 21 21 21 19 19 19 19 19 19 19 19 18 18 18
Therefore
ΔH = 4.01×4.18 × 13
ΔH= 217.903 kJ
The theoretical value of the change in enthalpy for the temperature change of 130C is 219.2,
therefore, it is possible to obtain the error.
Percentage error = 219.2−217.903
219.2 × 100
Percentage error = 1.297
219.2 × 100
Percentage error = 0.592 %
Experiment 2
Experiment
2
6.29 g
Time( min) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Temp (oC) 21 21 21 19 19 19 19 19 19 19 19 18 18 18

Laboratory Report 12
Before the addition of copper (ii) sulfate
The enthalpy change ΔH for this reaction was zero since there was no change in temperature because
there was no reaction still (copper (ii) sulfate) had not been added yet. And this is seen from the
equation of ΔH=m cp ΔT. For the first 4 minutes, the temperature was constant at 210C
Before the addition of copper (ii) sulfate
The enthalpy change ΔH for this reaction was zero since there was no change in temperature because
there was no reaction still (copper (ii) sulfate) had not been added yet. And this is seen from the
equation of ΔH=m cp ΔT. For the first 4 minutes, the temperature was constant at 210C
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

