Density Determination for Regular and Irregular Objects Report
VerifiedAdded on 2023/06/14
|14
|3310
|88
Report
AI Summary
This report details an experiment focused on determining the density of regular and irregular objects. The experiment uses various methods, including direct measurement and water displacement, to find the volume and mass of different materials. Calculations and percentage errors are presented for both regular solids, metal pellets and liquids. The report also investigates the reaction between calcium carbonate and hydrochloric acid under varying conditions, measuring the rate of reaction by collecting evolved carbon dioxide. The procedures involve using graduated cylinders, pipettes, and density meters to accurately measure mass and volume, emphasizing the importance of nondestructive physical processes in material characterization. Experimental results are analyzed to compare measured and theoretical densities, highlighting the applications of density determination in various fields.

Surname 1
Name
Professor
Course
Date
Determination of Density for Regular and Irregular Objects
Abstract
The experiment in question would help in the understanding of the different ways in
which the density of the material can be determined. In the process the learner or anyone
interested would learn different methods applied in the determination of densities for different
shapes for instance regular and irregular objects. The objective is to help in the determination of
densities of various materials without any strain in the future (Watanabe and Tohma, et al 33).
To determine density of an object there is need for nondestructive physical process through
which various materials are distinguished from one another. It is sort of classification of
materials based on the understanding of their properties in relation to the primary aim which
Name
Professor
Course
Date
Determination of Density for Regular and Irregular Objects
Abstract
The experiment in question would help in the understanding of the different ways in
which the density of the material can be determined. In the process the learner or anyone
interested would learn different methods applied in the determination of densities for different
shapes for instance regular and irregular objects. The objective is to help in the determination of
densities of various materials without any strain in the future (Watanabe and Tohma, et al 33).
To determine density of an object there is need for nondestructive physical process through
which various materials are distinguished from one another. It is sort of classification of
materials based on the understanding of their properties in relation to the primary aim which
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Surname 2
in our case in density of regular and irregular objects. The laboratory experiment aims at
proving it right that the experimentation for density of materials applies in all cases and that no
material whether solid, liquid or gas lacks density.
Introduction
The aim of the experiment is to help in the understanding the meaning as well as the
importance of the significance that comes along with the density of substances. Density from the
theoretical understanding is a primary characteristic for homogeneous objects in their entirety. In
that regard we can say that density qualifies for intensive property, a property that that translates
to the meaning that density is dependent on the characteristics of the object in question in
terms of composition, size or amount in most cases. To determine density of an object there
should be first the nondestructive physical process through which various materials are
distinguished from one another (Watanabe and Tohma, et al 33). It is sort of classification of
materials based on the understanding of their properties in relation to the primary aim which
in our case in density of regular and irregular objects. The definition of density from the
formula point of view goes that density is the ratio of the objects mass (m) to its volume (V).
The SI unit for density in liquid or solid form is as follows g/mL or g/cm3 depending on
whether it is liquid or solid respectively. It should be noticed that cm3 is an equivalent to the mL
unit and no doubt about it should be made (Nikolić, Živković, Branković & Pavlović 559). The
units as put above work hand in hand and there should be no confusion about the use of the units.
In the experiment that is in question the main concern is the determination of the densities of
both regular and irregular objects putting more emphasis on the irregular object as the main
concern is how to accurately determine the density of the object that have undefined and
irregular size.
in our case in density of regular and irregular objects. The laboratory experiment aims at
proving it right that the experimentation for density of materials applies in all cases and that no
material whether solid, liquid or gas lacks density.
Introduction
The aim of the experiment is to help in the understanding the meaning as well as the
importance of the significance that comes along with the density of substances. Density from the
theoretical understanding is a primary characteristic for homogeneous objects in their entirety. In
that regard we can say that density qualifies for intensive property, a property that that translates
to the meaning that density is dependent on the characteristics of the object in question in
terms of composition, size or amount in most cases. To determine density of an object there
should be first the nondestructive physical process through which various materials are
distinguished from one another (Watanabe and Tohma, et al 33). It is sort of classification of
materials based on the understanding of their properties in relation to the primary aim which
in our case in density of regular and irregular objects. The definition of density from the
formula point of view goes that density is the ratio of the objects mass (m) to its volume (V).
The SI unit for density in liquid or solid form is as follows g/mL or g/cm3 depending on
whether it is liquid or solid respectively. It should be noticed that cm3 is an equivalent to the mL
unit and no doubt about it should be made (Nikolić, Živković, Branković & Pavlović 559). The
units as put above work hand in hand and there should be no confusion about the use of the units.
In the experiment that is in question the main concern is the determination of the densities of
both regular and irregular objects putting more emphasis on the irregular object as the main
concern is how to accurately determine the density of the object that have undefined and
irregular size.

Surname 3
In this experiment focus would be on the determination of density of a liquid which is
defined in the procedure as water and use the same to compare the physical attributes or say
properties that the irregular object possess. Density is contained in the unit volume of any
object.
In as long as volume occupied by a gram water varies vary as temperature change, then
the density of the water for instance vary in accordance with the change in temperature. The
mass of the object in question would be attained in a different manner.
The mass is determined by the comparison of the mass of the object by the mass of said
known object. The mass in that regard is obtained through use of a weighing balance to be exact.
The volume for use in the experiment is obtained simply by use of a graduated measuring
cylinder, a graduated pipette or any other volumetric apparatus (Parihar, Anil Singh, et al). The
volume of a regular solid in most cases object like a spheres or cube is obtained by
measuring their dimensions then work out the volume through use of conventional
mathematical formulas.
There are some difficulties in the determination of the volume for irregular solids since
it is only obtained through use of the measure in the change in the water volume upon
immersing the object in water. The object in this regard displaces an equal volume of water in
accordance with the calibration (Wang, Yin, et al 379). The method however applies to
insoluble materials so in case the solid dissolves in water, then there should be in its place use a
liquid that the object in under study does not dissolve into for instance carbon tetrachloride if
the aim is to determine the volume of salt.
In this experiment focus would be on the determination of density of a liquid which is
defined in the procedure as water and use the same to compare the physical attributes or say
properties that the irregular object possess. Density is contained in the unit volume of any
object.
In as long as volume occupied by a gram water varies vary as temperature change, then
the density of the water for instance vary in accordance with the change in temperature. The
mass of the object in question would be attained in a different manner.
The mass is determined by the comparison of the mass of the object by the mass of said
known object. The mass in that regard is obtained through use of a weighing balance to be exact.
The volume for use in the experiment is obtained simply by use of a graduated measuring
cylinder, a graduated pipette or any other volumetric apparatus (Parihar, Anil Singh, et al). The
volume of a regular solid in most cases object like a spheres or cube is obtained by
measuring their dimensions then work out the volume through use of conventional
mathematical formulas.
There are some difficulties in the determination of the volume for irregular solids since
it is only obtained through use of the measure in the change in the water volume upon
immersing the object in water. The object in this regard displaces an equal volume of water in
accordance with the calibration (Wang, Yin, et al 379). The method however applies to
insoluble materials so in case the solid dissolves in water, then there should be in its place use a
liquid that the object in under study does not dissolve into for instance carbon tetrachloride if
the aim is to determine the volume of salt.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Surname 4
Theory and Objectives
In the event of determining density , mass and volume forms the most fundamental
part of the entire process. Mass and volume vary despite the similarity in the material used for
making the objects. Density comes in handy as it is the prevalent physical property for easier
characterization as well as classification of substances. It is important in that it applies in Quality
Monitoring and Process Control and for identity of heavier or lighter oil. The application of
density is vast as it cuts across various spheres of life be it environmental issues or
manufacturing life cycles in industries. The oil industry is however, the mostly affected with
expansive use of density and thus the emphasis on the determination of density of materials.
Materials
1. Measuring Cylinder
2. Pipette
3. Graduated cylinder
4. Water
5. Density meter
6. Materials Used:
7. Graduated Cylinder
8. 2. Regular Shaped Solid
9. 3. Metal Sample
10. 4. Distilled Water
11. 5. Electronic Balance
Theory and Objectives
In the event of determining density , mass and volume forms the most fundamental
part of the entire process. Mass and volume vary despite the similarity in the material used for
making the objects. Density comes in handy as it is the prevalent physical property for easier
characterization as well as classification of substances. It is important in that it applies in Quality
Monitoring and Process Control and for identity of heavier or lighter oil. The application of
density is vast as it cuts across various spheres of life be it environmental issues or
manufacturing life cycles in industries. The oil industry is however, the mostly affected with
expansive use of density and thus the emphasis on the determination of density of materials.
Materials
1. Measuring Cylinder
2. Pipette
3. Graduated cylinder
4. Water
5. Density meter
6. Materials Used:
7. Graduated Cylinder
8. 2. Regular Shaped Solid
9. 3. Metal Sample
10. 4. Distilled Water
11. 5. Electronic Balance
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Surname 5
Procedure (Method)
The experiment started off by first carrying out determination of volume of regular objects then
that of irregular objects.
1. The mass is determined weighing balance ( Applicable to regular and irregular objects)
2. The volume for use in the experiment is obtained simply by use of a graduated measuring
cylinder, a graduated pipette or any other volumetric apparatus.
3. The volume of a regular solid obtained by measuring their dimensions use mathematical
formulas to calculate the figures from the measurement.
4. Irregular objects volume is then obtained through use of water via immersion
5. Density meter used in the determination of densities for liquids
The reaction between a carbonate and an acid forms a salt, carbon (IV) oxide, and water.
In This experiment therefore, calcium carbonate reacted with hydrochloric acid and resulted in
the formation of calcium chloride, water, and carbon (IV) oxide just as indicted in the following
chemical reaction:
CaCO3 + 2HCl CaCl3 + H2O + CO2
For the rate of the reaction to be measured, one of the products was supposed to be
measured, and the rate at which it was being produced was used to determine the rate of the
reaction. The experiment progressed and was observed by collecting the carbon dioxide evolved
in a gas syringe and recording the volume at regular intervals based on the below presented
diagram:
The variables and/or factors that were changed included the acid concentration,
temperature of the acid, and particle size of calcium carbonate (Zhao and Dong et al 239)
Procedure (Method)
The experiment started off by first carrying out determination of volume of regular objects then
that of irregular objects.
1. The mass is determined weighing balance ( Applicable to regular and irregular objects)
2. The volume for use in the experiment is obtained simply by use of a graduated measuring
cylinder, a graduated pipette or any other volumetric apparatus.
3. The volume of a regular solid obtained by measuring their dimensions use mathematical
formulas to calculate the figures from the measurement.
4. Irregular objects volume is then obtained through use of water via immersion
5. Density meter used in the determination of densities for liquids
The reaction between a carbonate and an acid forms a salt, carbon (IV) oxide, and water.
In This experiment therefore, calcium carbonate reacted with hydrochloric acid and resulted in
the formation of calcium chloride, water, and carbon (IV) oxide just as indicted in the following
chemical reaction:
CaCO3 + 2HCl CaCl3 + H2O + CO2
For the rate of the reaction to be measured, one of the products was supposed to be
measured, and the rate at which it was being produced was used to determine the rate of the
reaction. The experiment progressed and was observed by collecting the carbon dioxide evolved
in a gas syringe and recording the volume at regular intervals based on the below presented
diagram:
The variables and/or factors that were changed included the acid concentration,
temperature of the acid, and particle size of calcium carbonate (Zhao and Dong et al 239)

Surname 6
Concentrated and dilute acid had different impact when they were reacted with marble chips and
the time taken to turn the mixture clear also varied depending on the acid concentration. High
and low temperatures were also investigated on their impact to the reaction between marble chips
and hydrochloric acid. The experiment also required to use different sizes of marble chips to
determine which particle size could react faster as compared to others.
Previous experiments indicated that calcium carbonate when reacted with hydrochloric
acid, can turn the acid luminous and that it itself will bubble and turn cloudy. These experiments
also had it that time consumed for a particular size of marble chips to disappear completely is
used to determine the rate of the reaction. The rate of reaction according to research can be
defined as the speed at which a reaction is expected to occur. Chemically, the rates of reaction is
defined as the rate of a chemical reaction that increases when reactants and products
concentration is increased and is measured by unit time. Increase in the concentration, gas
pressure, temperature, the surface area, reactants, and use of a catalyst are some of the factors
that affects the rate of a reaction.
The experiment commenced by ensuring that the apparatus was gas tight and then
clamped up as shown in the diagram in the introduction part above, and the graduated syringe as
well as the boiling tube were held in position by the aid of appropriate clamps and six marble
chips placed in the boiling tube. A 10 cm3 measuring cylinder was then used to measure exactly
4 cm3 of bench hydrochloric acid. To get the accurate volume of the bench acid, a teat pipette
was utilized. The stop watch was then switched on when hydrochloric acid was added in the
boiling tube, and the stopper replaced almost immediately (Zhao and Dong et al 246). The
volume of the gas (carbon dioxide) was recorded every 15 seconds for a total of four minutes just
Concentrated and dilute acid had different impact when they were reacted with marble chips and
the time taken to turn the mixture clear also varied depending on the acid concentration. High
and low temperatures were also investigated on their impact to the reaction between marble chips
and hydrochloric acid. The experiment also required to use different sizes of marble chips to
determine which particle size could react faster as compared to others.
Previous experiments indicated that calcium carbonate when reacted with hydrochloric
acid, can turn the acid luminous and that it itself will bubble and turn cloudy. These experiments
also had it that time consumed for a particular size of marble chips to disappear completely is
used to determine the rate of the reaction. The rate of reaction according to research can be
defined as the speed at which a reaction is expected to occur. Chemically, the rates of reaction is
defined as the rate of a chemical reaction that increases when reactants and products
concentration is increased and is measured by unit time. Increase in the concentration, gas
pressure, temperature, the surface area, reactants, and use of a catalyst are some of the factors
that affects the rate of a reaction.
The experiment commenced by ensuring that the apparatus was gas tight and then
clamped up as shown in the diagram in the introduction part above, and the graduated syringe as
well as the boiling tube were held in position by the aid of appropriate clamps and six marble
chips placed in the boiling tube. A 10 cm3 measuring cylinder was then used to measure exactly
4 cm3 of bench hydrochloric acid. To get the accurate volume of the bench acid, a teat pipette
was utilized. The stop watch was then switched on when hydrochloric acid was added in the
boiling tube, and the stopper replaced almost immediately (Zhao and Dong et al 246). The
volume of the gas (carbon dioxide) was recorded every 15 seconds for a total of four minutes just
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Surname 7
as it is shown in the result table. When the reaction came to completion, the used hydrochloric
acid was poured off, taking care not to lose the calcium carbonate.
The gas syringe was rest to zero and using a 10 cm3 measuring again, 4 cm3 of bench
hydrochloric acid was measured with the teat pipette being used to acquire exact volume of the
acid. The 4 cm3 was then added to distilled water and the teat pipette used to obtain the exact
final volume of 8cm3. The clock was then set on and the step 4 above repeated.
The measuring cylinder was then set to zero and using a 10 cm3 cylinder, 4 cm3 of bench
acid was measured and just as above a teat pipette was used to obtain the exact volume. 4 cm3 of
distilled water was measured using a teat pipette to get the exact volume and the two volumes
were placed into the second boiling tube which was warmed until the temperature was about
350C. The apparatus were then emptied and the marble chips tried using a paper towel
(Khatmullina, Liliya and Isachenko 871).The same calcium carbonate were crushed by the help
of a mortar and pestle and then returned to the boiling tube. The syringe was set to zero, and
using a 10 cm3 measuring cylinder, 4 cm3 of bench acid was measured, with the help of a teat
pipette so the accurate volume was obtained. The volume of the acid was then added to the
crushed marble chips in the boiling tube and the stopper replaced immediately.
Density Determinations
Calculations:
Density = M/V
Regular solid - 173.084g/318.226cm^3 = 5.43903x10^-1
Metal Pellets- Mass = 50.046g
as it is shown in the result table. When the reaction came to completion, the used hydrochloric
acid was poured off, taking care not to lose the calcium carbonate.
The gas syringe was rest to zero and using a 10 cm3 measuring again, 4 cm3 of bench
hydrochloric acid was measured with the teat pipette being used to acquire exact volume of the
acid. The 4 cm3 was then added to distilled water and the teat pipette used to obtain the exact
final volume of 8cm3. The clock was then set on and the step 4 above repeated.
The measuring cylinder was then set to zero and using a 10 cm3 cylinder, 4 cm3 of bench
acid was measured and just as above a teat pipette was used to obtain the exact volume. 4 cm3 of
distilled water was measured using a teat pipette to get the exact volume and the two volumes
were placed into the second boiling tube which was warmed until the temperature was about
350C. The apparatus were then emptied and the marble chips tried using a paper towel
(Khatmullina, Liliya and Isachenko 871).The same calcium carbonate were crushed by the help
of a mortar and pestle and then returned to the boiling tube. The syringe was set to zero, and
using a 10 cm3 measuring cylinder, 4 cm3 of bench acid was measured, with the help of a teat
pipette so the accurate volume was obtained. The volume of the acid was then added to the
crushed marble chips in the boiling tube and the stopper replaced immediately.
Density Determinations
Calculations:
Density = M/V
Regular solid - 173.084g/318.226cm^3 = 5.43903x10^-1
Metal Pellets- Mass = 50.046g
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Surname 8
Volume - 27.2mL – 20.0mL = 7.20mL
50.046g/7.20mL= 6.95mL
Percent error= (6.95g/mL - 6.80)/6.80 X 100 = 2.21%
Calculations
Density = M/V
Mass – Empty graduated cylinder = 51.395
Cylinder plus water = 73.688g
73.688g-51.395g = 22.293g
Volume- 23mL
22.293/23 = .96926g/mL
Percentage error = (.96926g/mL - .99714g/mL)/.99714g/mL X 100 = -2.80%
Mass – Unknown liquid + Cylinder = 77.923g
Empty graduated cylinder- 51.395g
77.923-51.395= 26.528g
Volume – 24mL
26.528g/24mL = 1.1053
Percent error = (1.1053g/mL – 1.132g/mL) / 1.132g/mL X 100 = -2.36%
The Density of Measured Solutions
Volume - 27.2mL – 20.0mL = 7.20mL
50.046g/7.20mL= 6.95mL
Percent error= (6.95g/mL - 6.80)/6.80 X 100 = 2.21%
Calculations
Density = M/V
Mass – Empty graduated cylinder = 51.395
Cylinder plus water = 73.688g
73.688g-51.395g = 22.293g
Volume- 23mL
22.293/23 = .96926g/mL
Percentage error = (.96926g/mL - .99714g/mL)/.99714g/mL X 100 = -2.80%
Mass – Unknown liquid + Cylinder = 77.923g
Empty graduated cylinder- 51.395g
77.923-51.395= 26.528g
Volume – 24mL
26.528g/24mL = 1.1053
Percent error = (1.1053g/mL – 1.132g/mL) / 1.132g/mL X 100 = -2.36%
The Density of Measured Solutions
Surname 9
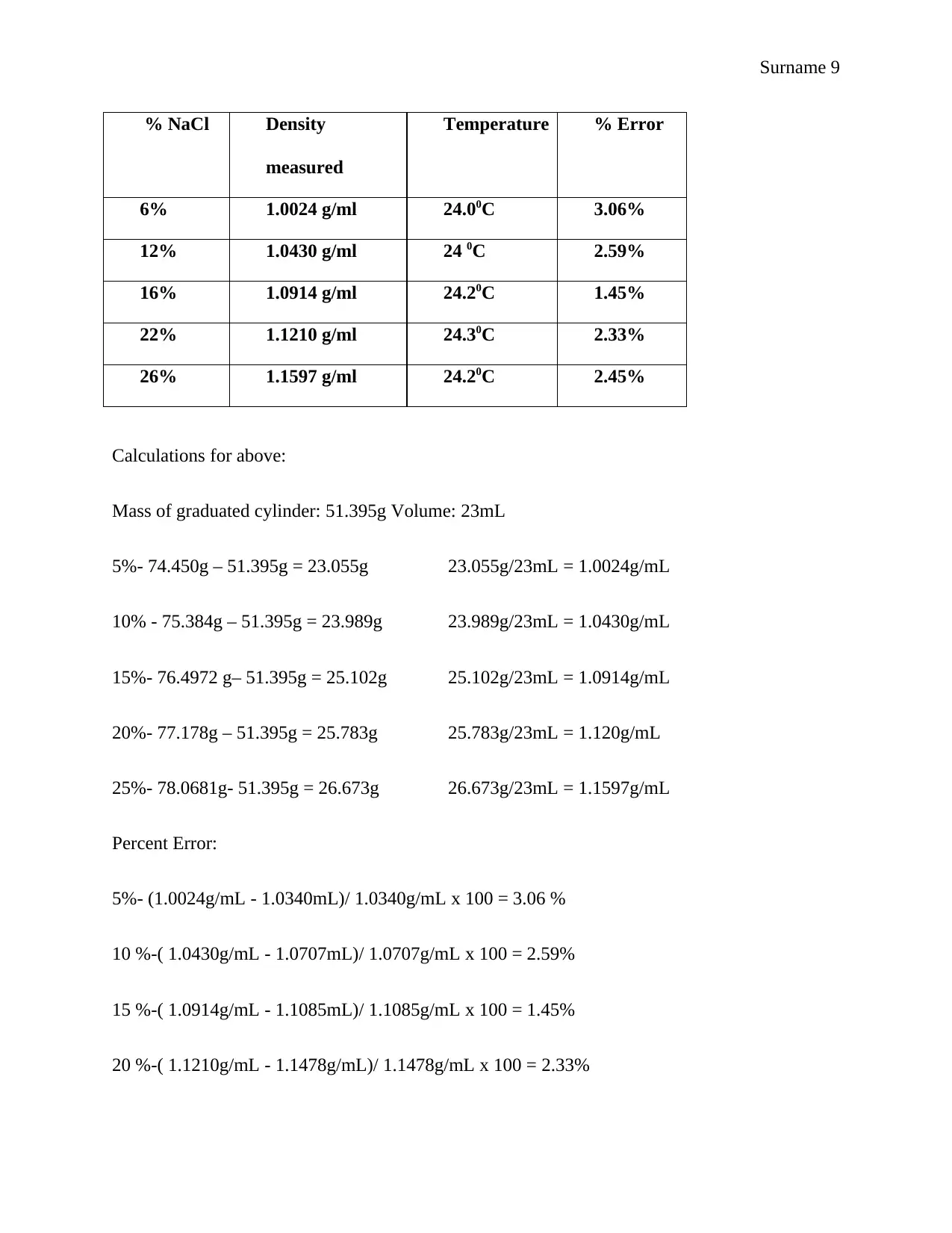
Calculations for above:
Mass of graduated cylinder: 51.395g Volume: 23mL
5%- 74.450g – 51.395g = 23.055g 23.055g/23mL = 1.0024g/mL
10% - 75.384g – 51.395g = 23.989g 23.989g/23mL = 1.0430g/mL
15%- 76.4972 g– 51.395g = 25.102g 25.102g/23mL = 1.0914g/mL
20%- 77.178g – 51.395g = 25.783g 25.783g/23mL = 1.120g/mL
25%- 78.0681g- 51.395g = 26.673g 26.673g/23mL = 1.1597g/mL
Percent Error:
5%- (1.0024g/mL - 1.0340mL)/ 1.0340g/mL x 100 = 3.06 %
10 %-( 1.0430g/mL - 1.0707mL)/ 1.0707g/mL x 100 = 2.59%
15 %-( 1.0914g/mL - 1.1085mL)/ 1.1085g/mL x 100 = 1.45%
20 %-( 1.1210g/mL - 1.1478g/mL)/ 1.1478g/mL x 100 = 2.33%
% NaCl Density
measured
Temperature % Error
6% 1.0024 g/ml 24.00C 3.06%
12% 1.0430 g/ml 24 0C 2.59%
16% 1.0914 g/ml 24.20C 1.45%
22% 1.1210 g/ml 24.30C 2.33%
26% 1.1597 g/ml 24.20C 2.45%
Calculations for above:
Mass of graduated cylinder: 51.395g Volume: 23mL
5%- 74.450g – 51.395g = 23.055g 23.055g/23mL = 1.0024g/mL
10% - 75.384g – 51.395g = 23.989g 23.989g/23mL = 1.0430g/mL
15%- 76.4972 g– 51.395g = 25.102g 25.102g/23mL = 1.0914g/mL
20%- 77.178g – 51.395g = 25.783g 25.783g/23mL = 1.120g/mL
25%- 78.0681g- 51.395g = 26.673g 26.673g/23mL = 1.1597g/mL
Percent Error:
5%- (1.0024g/mL - 1.0340mL)/ 1.0340g/mL x 100 = 3.06 %
10 %-( 1.0430g/mL - 1.0707mL)/ 1.0707g/mL x 100 = 2.59%
15 %-( 1.0914g/mL - 1.1085mL)/ 1.1085g/mL x 100 = 1.45%
20 %-( 1.1210g/mL - 1.1478g/mL)/ 1.1478g/mL x 100 = 2.33%
% NaCl Density
measured
Temperature % Error
6% 1.0024 g/ml 24.00C 3.06%
12% 1.0430 g/ml 24 0C 2.59%
16% 1.0914 g/ml 24.20C 1.45%
22% 1.1210 g/ml 24.30C 2.33%
26% 1.1597 g/ml 24.20C 2.45%
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Surname 10
25 %-( 1.1597g/mL – 1.1888g/mL)/1.1888g/mL x 100 = 2.45%
There would be more precision in weighing due to having mass significant figures.
Was not used due to it being harder to determine the differences and a lot harder to be
that precise.
Analysis of Data:
Part A – The data acquired from Part A shows that the dimensions of the solid will equal
the volume of it. Once the volume has been solved for, the mass of the object is able to be found,
by using the equation (Cuban and Mark 14). Having the graduated cylinder reach 20mL the
volume then increased when adding the substance then subtracting the final from the initial
would give the volume of the object. Divide the mass of the pellets to once again get the density.
Part B – The data from part B shows how the mass of the empty graduated cylinder
subtracted by the cylinder mass plus water is equal to the exact mass. Next, the volume of the
water is stated, so this will be the equation for density. To solver for the unknown liquid the mass
of the liquid minus the mass of the empty graduated cylinder to get the mass, then use the
volume recorded to obtain the density.
Part C – The results from part C how the weight variation of each NaCl percent mixture
can help solver for the density using the methods that have been used in part B of pure liquids,
which if graphed will show a steady line.
Reporting Values
1. Fill the table with the results
25 %-( 1.1597g/mL – 1.1888g/mL)/1.1888g/mL x 100 = 2.45%
There would be more precision in weighing due to having mass significant figures.
Was not used due to it being harder to determine the differences and a lot harder to be
that precise.
Analysis of Data:
Part A – The data acquired from Part A shows that the dimensions of the solid will equal
the volume of it. Once the volume has been solved for, the mass of the object is able to be found,
by using the equation (Cuban and Mark 14). Having the graduated cylinder reach 20mL the
volume then increased when adding the substance then subtracting the final from the initial
would give the volume of the object. Divide the mass of the pellets to once again get the density.
Part B – The data from part B shows how the mass of the empty graduated cylinder
subtracted by the cylinder mass plus water is equal to the exact mass. Next, the volume of the
water is stated, so this will be the equation for density. To solver for the unknown liquid the mass
of the liquid minus the mass of the empty graduated cylinder to get the mass, then use the
volume recorded to obtain the density.
Part C – The results from part C how the weight variation of each NaCl percent mixture
can help solver for the density using the methods that have been used in part B of pure liquids,
which if graphed will show a steady line.
Reporting Values
1. Fill the table with the results
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Surname 11
Discussion /Conclusion
Density as a unit is one important physical property for easier characterization as well as
classification of substances. It is to the best knowledge of the person carrying out the experiment
to realize the substances required for determination of the parameters used for density
calculations are carried out in the above processes. The emphasis on use of density emanates
from its importance in the oil industry and manufacturing of various products in many industries
on the globe. Density as a unit of measurement applies in many areas and is useful for
identifying finer physical properties of substances.
Works Cited
Discussion /Conclusion
Density as a unit is one important physical property for easier characterization as well as
classification of substances. It is to the best knowledge of the person carrying out the experiment
to realize the substances required for determination of the parameters used for density
calculations are carried out in the above processes. The emphasis on use of density emanates
from its importance in the oil industry and manufacturing of various products in many industries
on the globe. Density as a unit of measurement applies in many areas and is useful for
identifying finer physical properties of substances.
Works Cited

Surname 12
Apinyavisit, Krit, et al. "Heat and mass transfer properties of longan shrinking from a spherical
to an irregular shape during drying." Biosystems Engineering 169 (2018): 11-21.
Avramović, Ljiljana, et al. "Comparative Morphological and Crystallographic Analysis of
Electrochemically-and Chemically-Produced Silver Powder Particles." Metals 7.5 (2017):
160.
Cava, A., Biviano, A., Mamon, G. A., Varela, J., Bettoni, D., D’Onofrio, M., & Poggianti, B.
(2017). Structural and dynamical modeling of WINGS clusters-I. The distribution of
cluster galaxies of different morphological classes within regular and irregular
clusters. Astronomy & Astrophysics, 606, A108.
Chen, Youming, Raj Das, and Mark Battley. "Experimental study on in-plane compressive
response of irregular honeycombs." Journal of Composite Materials (2017):
0021998317749964.
Cuban, Mark, Joyce Reitman, and Jeffrey Orion Pritchard. "Object detection and analysis via
unmanned aerial vehicle." U.S. Patent Application No. 14/831,739.
Dando, Kerrick R., et al. "Production and characterization of epoxy syntactic foams highly
loaded with thermoplastic microballoons." Journal of Cellular Plastics (2017):
0021955X17700093.
Hu, Yile, et al. "Thermomechanical peridynamic analysis with irregular non-uniform domain
discretization." Engineering Fracture Mechanics (2018).
Khatmullina, Liliya, and Igor Isachenko. "Settling velocity of microplastic particles of regular
shapes." Marine pollution bulletin 114.2 (2017): 871-880.
Apinyavisit, Krit, et al. "Heat and mass transfer properties of longan shrinking from a spherical
to an irregular shape during drying." Biosystems Engineering 169 (2018): 11-21.
Avramović, Ljiljana, et al. "Comparative Morphological and Crystallographic Analysis of
Electrochemically-and Chemically-Produced Silver Powder Particles." Metals 7.5 (2017):
160.
Cava, A., Biviano, A., Mamon, G. A., Varela, J., Bettoni, D., D’Onofrio, M., & Poggianti, B.
(2017). Structural and dynamical modeling of WINGS clusters-I. The distribution of
cluster galaxies of different morphological classes within regular and irregular
clusters. Astronomy & Astrophysics, 606, A108.
Chen, Youming, Raj Das, and Mark Battley. "Experimental study on in-plane compressive
response of irregular honeycombs." Journal of Composite Materials (2017):
0021998317749964.
Cuban, Mark, Joyce Reitman, and Jeffrey Orion Pritchard. "Object detection and analysis via
unmanned aerial vehicle." U.S. Patent Application No. 14/831,739.
Dando, Kerrick R., et al. "Production and characterization of epoxy syntactic foams highly
loaded with thermoplastic microballoons." Journal of Cellular Plastics (2017):
0021955X17700093.
Hu, Yile, et al. "Thermomechanical peridynamic analysis with irregular non-uniform domain
discretization." Engineering Fracture Mechanics (2018).
Khatmullina, Liliya, and Igor Isachenko. "Settling velocity of microplastic particles of regular
shapes." Marine pollution bulletin 114.2 (2017): 871-880.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.