CHEM 101 Lab: Experiment 7 - Determining the Mole Volume of a Gas
VerifiedAdded on 2021/12/15
|5
|1225
|73
Report
AI Summary
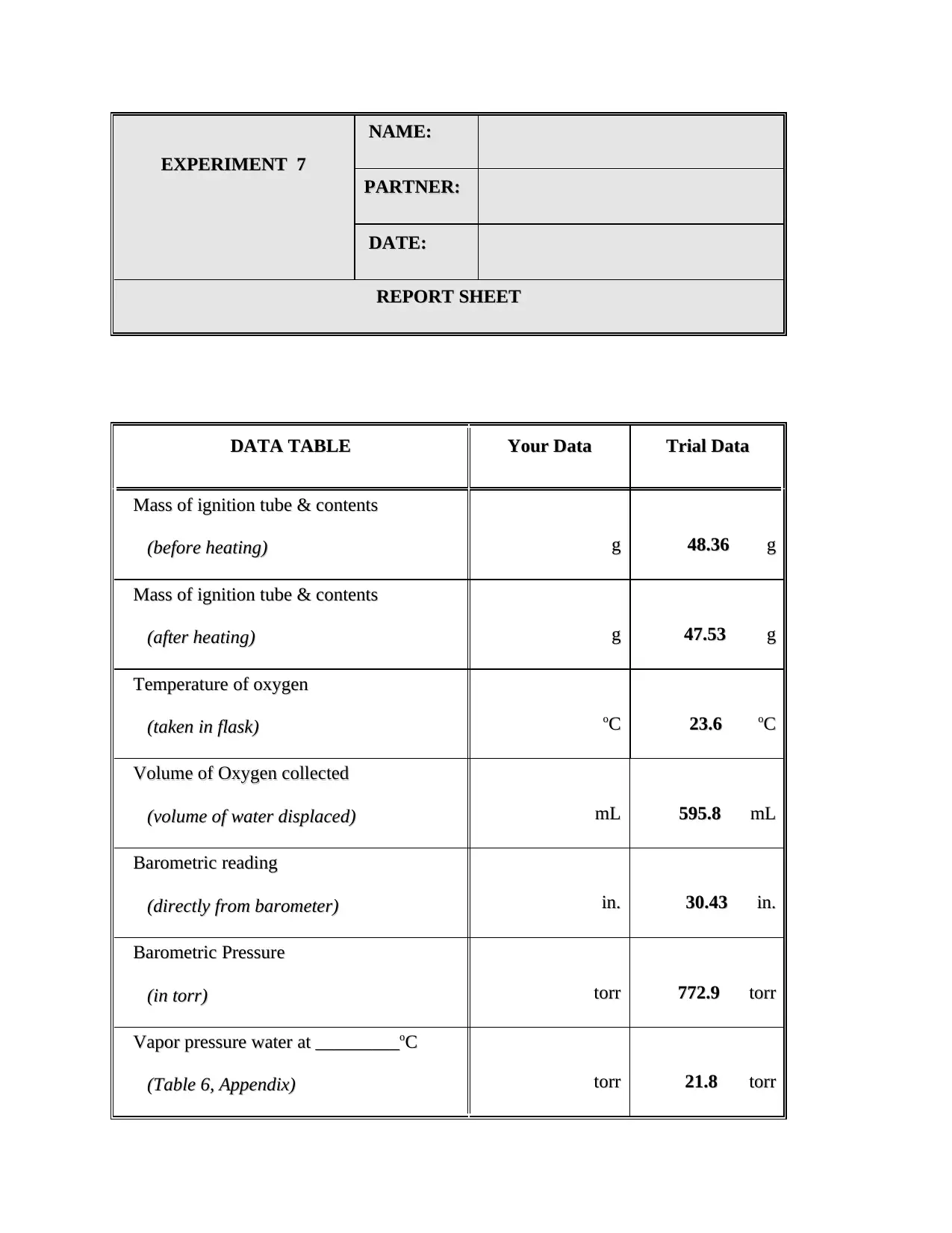
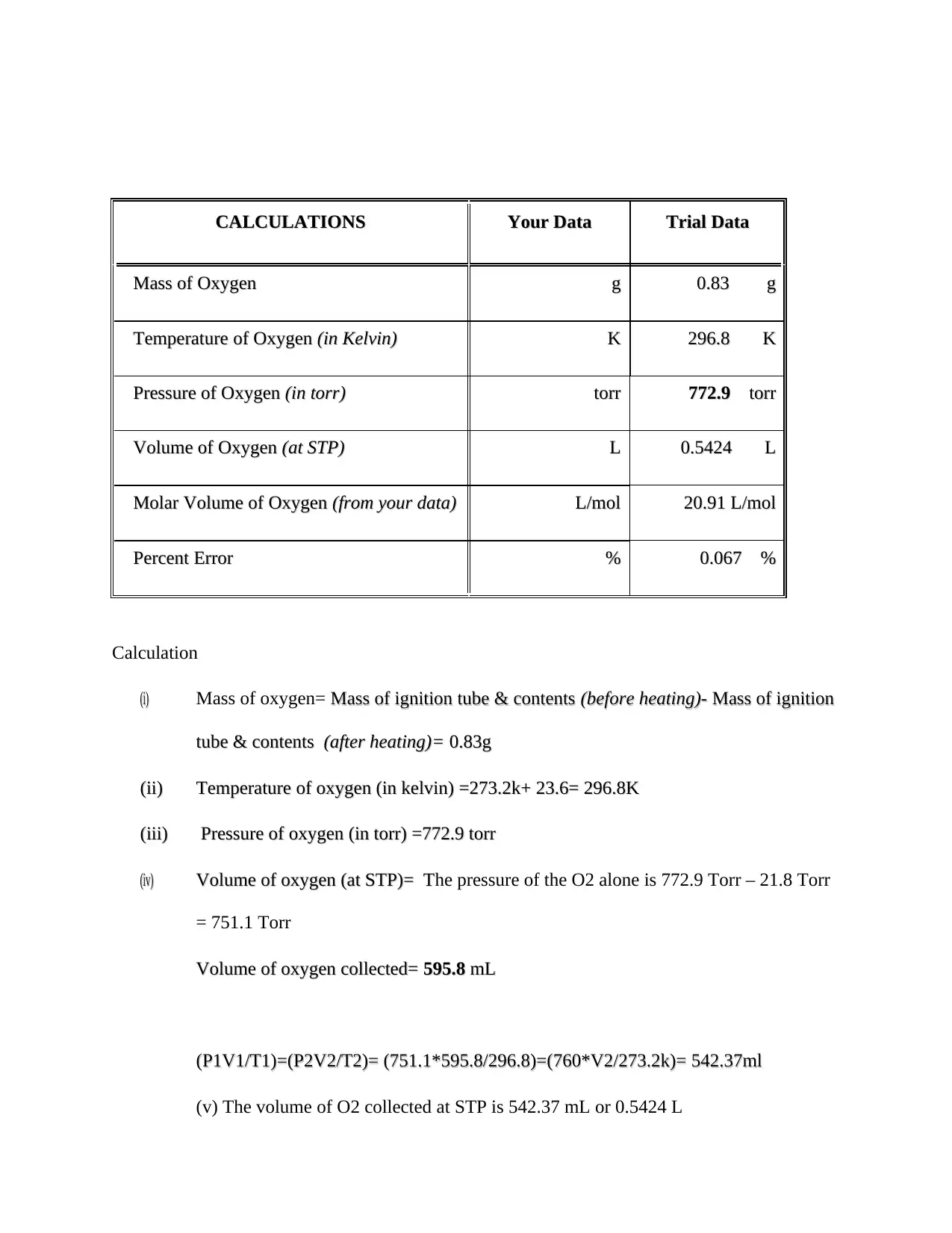
This chemistry lab report details Experiment 7, which focuses on determining the mole volume of a gas, specifically oxygen. The experiment involves the thermal decomposition of potassium chlorate (KClO3) in the presence of manganese dioxide (MnO2) as a catalyst to produce oxygen gas. The report includes a data table with the student's data and trial data, detailing the masses before and after heating, temperature, volume, and barometric readings. Calculations are provided to determine the mass of oxygen, temperature in Kelvin, pressure, volume at STP, molar volume, and percent error. The report also answers questions regarding the experimental procedure, such as the importance of a dry test tube, the function of the catalyst, and the reasons for specific steps like equalizing water levels. A balanced chemical equation is provided, and the report explains the relationship between the water volume in the beaker and the oxygen produced. The report concludes with references to support the findings and explanations.
1 out of 5




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)