Chemical Synthesis and Diesel Hydrodesulfurization: A Detailed Report
VerifiedAdded on 2022/11/25
|16
|3181
|354
Report
AI Summary
This report delves into the process of Diesel Hydro Desulfurization (DHDS), a critical chemical synthesis process used to remove sulfur from diesel and other petroleum fuels. The report begins with an executive summary highlighting the environmental regulations mandating low sulfur content in fuels and the importance of DHDS in achieving this. It then introduces the role of petroleum, its composition, and the detrimental effects of sulfur, including environmental pollution and catalyst poisoning. The core of the report details the DHDS process, including hydrogenation and refining reactions, and catalytic hydrotreating. It explains the chemical reaction mechanisms, the role of catalysts, and the process flow, including reactor configurations. The report emphasizes the importance of DHDS in meeting environmental standards, particularly the reduction of sulfur content in fuels to minimize pollution and improve fuel efficiency. The study covers desulfurization, denitrification, and the application of hydrotreating in petroleum refineries, highlighting the use of hypothetical compounds and the significance of kinetic modeling. The report concludes by underlining the importance of catalytic hydrotreating in removing pollutants and producing high-quality fuels. Overall, the report provides a comprehensive overview of the DHDS process and its role in the petroleum industry.

Running head: DIESEL HYDRO DESULFURIZATION
Sulfur Elimination Through Hydrodesulfurization
Name of Student
Institution Affiliation
Sulfur Elimination Through Hydrodesulfurization
Name of Student
Institution Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

DIESEL HYDRO DESULFURIZATION 2
CHEMICAL SYNTHESIS PROCESS
Table of Contents
Executive summary.........................................................................................................................3
Introduction......................................................................................................................................4
CHEMICAL SYNTHESIS..............................................................................................................5
DESEL HYDRO DESULFURIZATION....................................................................................5
Hydro Desulfurization.............................................................................................................6
The main purpose of this process is to:..................................................................................7
Chemical reaction mechanism.................................................................................................7
Hydrogenation reactions..........................................................................................................7
Refining reactions....................................................................................................................8
Catalytic hydrotreating..............................................................................................................10
The process....................................................................................................................................11
Reactions and chemical equations.............................................................................................12
References......................................................................................................................................16
Executive summary
Growth of industries is the basis of trend in most countries in the world, industrialization
contributes to the development of the state in terms of infrastructure and raising the living
CHEMICAL SYNTHESIS PROCESS
Table of Contents
Executive summary.........................................................................................................................3
Introduction......................................................................................................................................4
CHEMICAL SYNTHESIS..............................................................................................................5
DESEL HYDRO DESULFURIZATION....................................................................................5
Hydro Desulfurization.............................................................................................................6
The main purpose of this process is to:..................................................................................7
Chemical reaction mechanism.................................................................................................7
Hydrogenation reactions..........................................................................................................7
Refining reactions....................................................................................................................8
Catalytic hydrotreating..............................................................................................................10
The process....................................................................................................................................11
Reactions and chemical equations.............................................................................................12
References......................................................................................................................................16
Executive summary
Growth of industries is the basis of trend in most countries in the world, industrialization
contributes to the development of the state in terms of infrastructure and raising the living

DIESEL HYDRO DESULFURIZATION 3
standards of the natives through employment opportunities that come with development of
industries.
Development of industries leads to an equivalent increase in power production to run heavy
machines in plant operations. This result in growing hydroprocessing, to achieve this combustion
of petroleum fuels to provide the driving energy for the industries. Combustion of petroleum
fuels if not properly regulated and monitored can lead to detrimental effects on the environment
[1]. In line with this aspect, Department of Petroleum and natural gas has maintained that a
maximum of 0.25 wt. % of sulfur from 1 wt % can be present in petroleum fuels [2]. This
regulation demands that all the refineries to establish desulfurization plant for diesel, to
encounter the objective of diesel production with revised specification of sulfur content. The
basic objective of a typical Diesel Hydro Desulfurization (DHDS) plant is to remove sulfur
content from diesel and other petroleum fuels to ensure that their use is environmentally friendly.
This process involves hydro sulfurization, hydro nitrification and olefins, and aromatic
saturation, where these reactions are conducted in bi-functional catalysts. Reactor effluents are
divided into liquid and gas in chamber known as separator.
standards of the natives through employment opportunities that come with development of
industries.
Development of industries leads to an equivalent increase in power production to run heavy
machines in plant operations. This result in growing hydroprocessing, to achieve this combustion
of petroleum fuels to provide the driving energy for the industries. Combustion of petroleum
fuels if not properly regulated and monitored can lead to detrimental effects on the environment
[1]. In line with this aspect, Department of Petroleum and natural gas has maintained that a
maximum of 0.25 wt. % of sulfur from 1 wt % can be present in petroleum fuels [2]. This
regulation demands that all the refineries to establish desulfurization plant for diesel, to
encounter the objective of diesel production with revised specification of sulfur content. The
basic objective of a typical Diesel Hydro Desulfurization (DHDS) plant is to remove sulfur
content from diesel and other petroleum fuels to ensure that their use is environmentally friendly.
This process involves hydro sulfurization, hydro nitrification and olefins, and aromatic
saturation, where these reactions are conducted in bi-functional catalysts. Reactor effluents are
divided into liquid and gas in chamber known as separator.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

DIESEL HYDRO DESULFURIZATION 4
Introduction
Since time in memorials, petroleum has been essential to humans as it is used in one way or
another to aid daily operations. Petroleum contains hydrocarbons as the main component with
some considerable amount of nitrogen, sulfur, trace metals, and oxygen [5]. From petroleum,
fuels are derived such as diesel among many which are composed of about 75% saturated
hydrocarbons and 25% aromatic hydrocarbons [9]. In diesel sulfur makes the main contaminant
ranging between 0.1% and 0.5%, this is undesirable to the environment since it increases the
polarity of the fuel, poison catalysts, enhances emulsion stability and confers color [7]. During
combustion of diesel fuel, sulfur oxides may be released into environment either as gaseous or
particulate solid matter. Gaseous discharge of burning sulfur include Sulfur oxide (SO2) and
(SO3), these causes great damage to atmosphere and environment [11]
The target of international and domestic environment agencies has always been to control and
lessen the use of sulfur fuels to a level of environment-friendly. In situations where sulfur related
fuels are commonly used, the concentration of sulfur is also high to about 300ppm. The objective
of these agencies is to reduce this concentration level to about 100ppm. [6]
For effective and efficient removal of this sulfur from diesel fuels desulfurization process is most
ideal. With the current fast-growing of automobile industry and industrialization leads tom high
demand for crude oil and diesel fuel as the operating fuel [4]. These lead to a corresponding
increase in the amount of sulfur released to the environment. Thanks to the endless efforts geared
towards achieving the lowering of sulfur content in these fuels through various processes.
Refiners use desulfurization in context of eliminating production onboard transportation fuels
due to high compliance expenses to the set regulations [8]. Advanced technologies have been
Introduction
Since time in memorials, petroleum has been essential to humans as it is used in one way or
another to aid daily operations. Petroleum contains hydrocarbons as the main component with
some considerable amount of nitrogen, sulfur, trace metals, and oxygen [5]. From petroleum,
fuels are derived such as diesel among many which are composed of about 75% saturated
hydrocarbons and 25% aromatic hydrocarbons [9]. In diesel sulfur makes the main contaminant
ranging between 0.1% and 0.5%, this is undesirable to the environment since it increases the
polarity of the fuel, poison catalysts, enhances emulsion stability and confers color [7]. During
combustion of diesel fuel, sulfur oxides may be released into environment either as gaseous or
particulate solid matter. Gaseous discharge of burning sulfur include Sulfur oxide (SO2) and
(SO3), these causes great damage to atmosphere and environment [11]
The target of international and domestic environment agencies has always been to control and
lessen the use of sulfur fuels to a level of environment-friendly. In situations where sulfur related
fuels are commonly used, the concentration of sulfur is also high to about 300ppm. The objective
of these agencies is to reduce this concentration level to about 100ppm. [6]
For effective and efficient removal of this sulfur from diesel fuels desulfurization process is most
ideal. With the current fast-growing of automobile industry and industrialization leads tom high
demand for crude oil and diesel fuel as the operating fuel [4]. These lead to a corresponding
increase in the amount of sulfur released to the environment. Thanks to the endless efforts geared
towards achieving the lowering of sulfur content in these fuels through various processes.
Refiners use desulfurization in context of eliminating production onboard transportation fuels
due to high compliance expenses to the set regulations [8]. Advanced technologies have been
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

DIESEL HYDRO DESULFURIZATION 5
established to obtain the most viable and economical methods of sulfur removal from petroleum
fuels. Through the major source of sulfur pollution, petroleum refining industries, both direct and
indirect motor fumes, are in front line for the struggle to achieve environment-friendly and
sustainable operation.
CHEMICAL SYNTHESIS.
Chemical synthesis is the process that involves the disorientation of the already existing
chemical bonds that both hold molecules or compounds together and form new bonds in
molecule or compounds that are more valuable than the existing ones. Synthesis of more
complex molecules at times involve quite a several individual reactions that eventually undergo
sequential reaction from available initial materials to the desired final product with each step
involving reaction at only single chemical bond in the molecule [2]. This process requires
planning and analysis of the route of chemical reactions, and this demands that the chemists have
to visualize the end product and develop a backward work formula towards increasingly simpler
compounds. These are often used depending on many other factors such as availability of
starting material together with their costs [5], amount of energy required to start and sustain these
reactions and separating costs and purification of end product.
DIESEL HYDRO DESULFURIZATION
The issue of desulfurization is gaining a lot of focus since crude oil is getting higher in sulfur
content. To control the level of sulfur in hydrocarbon fuels for fuel use and protection of
environment[11], diesel hydrodesulfurization of model duel and real fuel are studied by Selective
absorption for removing Sulfur (SARS) process using appropriate adsorbent [6], examining the
temperature during the desulfurization process. With technology availability, the current
established to obtain the most viable and economical methods of sulfur removal from petroleum
fuels. Through the major source of sulfur pollution, petroleum refining industries, both direct and
indirect motor fumes, are in front line for the struggle to achieve environment-friendly and
sustainable operation.
CHEMICAL SYNTHESIS.
Chemical synthesis is the process that involves the disorientation of the already existing
chemical bonds that both hold molecules or compounds together and form new bonds in
molecule or compounds that are more valuable than the existing ones. Synthesis of more
complex molecules at times involve quite a several individual reactions that eventually undergo
sequential reaction from available initial materials to the desired final product with each step
involving reaction at only single chemical bond in the molecule [2]. This process requires
planning and analysis of the route of chemical reactions, and this demands that the chemists have
to visualize the end product and develop a backward work formula towards increasingly simpler
compounds. These are often used depending on many other factors such as availability of
starting material together with their costs [5], amount of energy required to start and sustain these
reactions and separating costs and purification of end product.
DIESEL HYDRO DESULFURIZATION
The issue of desulfurization is gaining a lot of focus since crude oil is getting higher in sulfur
content. To control the level of sulfur in hydrocarbon fuels for fuel use and protection of
environment[11], diesel hydrodesulfurization of model duel and real fuel are studied by Selective
absorption for removing Sulfur (SARS) process using appropriate adsorbent [6], examining the
temperature during the desulfurization process. With technology availability, the current

DIESEL HYDRO DESULFURIZATION 6
hydrodesulfurization is hard to control the sulfur concentration in diesel fuels below 49 parts per
million since the outstanding sulfur concentration in profitable diesel fuels are 4-
methylbenzothiophene, 4, 6-dimethyl dibenzothiophene and other alkyl dibenzothiophenes
which are quite difficult to eliminate[8]. In recent research the process of sulfur removal from
transportation fuels has become of great importance to every organization and nations worldwide
due to heightened interest to have clean air in the atmosphere hence increasingly stringent laws
and regulations for sulfur fuels to the environment [8]. The high demand for ultra-low sulfur
fuels for fuel cells with modern automobiles that are much more efficient and friendly to the
environment. [11]
Liquid hydrocarbon fuels are on the rise because they are promising ingredient because of their
high energy density, storage, and transportation safe [7]. Automotive fuel cells, for example, the
developing PEFC and SOFC auxiliary power units in vehicles diesel and gasoline are the most
preferred fuels since they are easily available and ease in their production, storage, and delivery.
However gasoline and diesel fuels contain significant amount of sulfur ranging between 300 to
450 ppm [9]. The sulfur content in fuel and hydrogen sulfide H2S produced by these fuels are
very poisonous to catalysts both electrode catalysts and in hydrocarbon fuel processor catalysts.
Thus for fuel cell application in industries and in automobiles, sulfur content in the fuels needs to
be maintained at the lowest level possible [5]
Hydro Desulfurization
This is referred to a catalytic chemical reaction process where sulfur is eliminated or removed
from petroleum fuels such as petrol, diesel fuels, jet fuels, kerosene and natural gas [2]. The
process is usually used to elevate octane rating in naphtha rivulets [10]. Fuels obtained through
hydrodesulfurization is hard to control the sulfur concentration in diesel fuels below 49 parts per
million since the outstanding sulfur concentration in profitable diesel fuels are 4-
methylbenzothiophene, 4, 6-dimethyl dibenzothiophene and other alkyl dibenzothiophenes
which are quite difficult to eliminate[8]. In recent research the process of sulfur removal from
transportation fuels has become of great importance to every organization and nations worldwide
due to heightened interest to have clean air in the atmosphere hence increasingly stringent laws
and regulations for sulfur fuels to the environment [8]. The high demand for ultra-low sulfur
fuels for fuel cells with modern automobiles that are much more efficient and friendly to the
environment. [11]
Liquid hydrocarbon fuels are on the rise because they are promising ingredient because of their
high energy density, storage, and transportation safe [7]. Automotive fuel cells, for example, the
developing PEFC and SOFC auxiliary power units in vehicles diesel and gasoline are the most
preferred fuels since they are easily available and ease in their production, storage, and delivery.
However gasoline and diesel fuels contain significant amount of sulfur ranging between 300 to
450 ppm [9]. The sulfur content in fuel and hydrogen sulfide H2S produced by these fuels are
very poisonous to catalysts both electrode catalysts and in hydrocarbon fuel processor catalysts.
Thus for fuel cell application in industries and in automobiles, sulfur content in the fuels needs to
be maintained at the lowest level possible [5]
Hydro Desulfurization
This is referred to a catalytic chemical reaction process where sulfur is eliminated or removed
from petroleum fuels such as petrol, diesel fuels, jet fuels, kerosene and natural gas [2]. The
process is usually used to elevate octane rating in naphtha rivulets [10]. Fuels obtained through
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

DIESEL HYDRO DESULFURIZATION 7
this process reduce sulfur and its oxide emission in vehicles, train, power plant, aircraft,
residential furnace, and industrial furnaces and other fuel combustion forms. [8]
The main purpose of this process is to:
1. To perform desulfurization of the diesel fuels in the reactor feed with the aim of
obtaining the desired specification
2. To use a catalyst to determine hydrogenation of eventual olefin structure.
Chemical reaction mechanism
1. Hydrogenation reactions
2. Refining reactions
Process of hydrogenation.
Hydrogenation is process reactions that affects aromatics, olefins directly and are associated with
high temperatures where olefins and diolefins are transformed to saturated mixtures [1].
Reaction rate involving aromatics is always limited.
The intake to diesel hydrodesulfurization chamber is merger of pure and fractured gas oils.
These intake fuels are then sieved through a sieve package to lower drum where pressure is
maintained at a constant level through gas blanketing [1].
The liquid stage intake pumps that are pumped to underflow control by inlet pumps, combined
with hydrogen gas from recycle compressor outlet stream then allows heat exchanger to regulate
the temperatures. The injection inhibitor that prevents polymerization at the inlet of the reactor
facilitates the fraternization of the recycle hydrogen together with inlets to ensure tolerable
hydrogen fractional pressure. [1]
this process reduce sulfur and its oxide emission in vehicles, train, power plant, aircraft,
residential furnace, and industrial furnaces and other fuel combustion forms. [8]
The main purpose of this process is to:
1. To perform desulfurization of the diesel fuels in the reactor feed with the aim of
obtaining the desired specification
2. To use a catalyst to determine hydrogenation of eventual olefin structure.
Chemical reaction mechanism
1. Hydrogenation reactions
2. Refining reactions
Process of hydrogenation.
Hydrogenation is process reactions that affects aromatics, olefins directly and are associated with
high temperatures where olefins and diolefins are transformed to saturated mixtures [1].
Reaction rate involving aromatics is always limited.
The intake to diesel hydrodesulfurization chamber is merger of pure and fractured gas oils.
These intake fuels are then sieved through a sieve package to lower drum where pressure is
maintained at a constant level through gas blanketing [1].
The liquid stage intake pumps that are pumped to underflow control by inlet pumps, combined
with hydrogen gas from recycle compressor outlet stream then allows heat exchanger to regulate
the temperatures. The injection inhibitor that prevents polymerization at the inlet of the reactor
facilitates the fraternization of the recycle hydrogen together with inlets to ensure tolerable
hydrogen fractional pressure. [1]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

DIESEL HYDRO DESULFURIZATION 8
Hydrogenation of olefin compounds
RCH=CH2 + H2 RCH2 –CH3
Hydrogenation of aromatic compounds
C6H6 + 3H2 C6H12
The incoming hydrogen makes up gas is directed through chlorine adsorbent before the
compressor compresses it. Some part of this gas is channeled into the reactor as quench gas. In
heat exchanger the mixed stream is heated before passed to the second exchanger and lastly to
the reactor heater where it’s heated to the desired initial temperature [1]. Reactor temperature are
regulated by scheming fuel oil to gas into a heater, and then the stream is then introduced to the
reactor with catalysts. This is done in three beds, and the temperature of each bed is controlled
by introducing cold quench hydrogen from the recycling compressor [11].
Water is introduced at the inlet of overflow to prevent deposition of ammonium salts that might
cause corrosion of the reactor. The sour water is partly recycled since it contains ammonium salt
and can be used in washing.
Refining reactions
Denitrification- this reaction only occurs in cases of heterocyclic compounds with an aromatic
structure such as pyridine and the rate of reaction of denitrification is lower compared to the rate
of desulfurization. Denitrification leads to the formation of ammonia gas and the consumption of
hydrogen gas. [7]
Denitrification
C2H2N + 5H2 C2H2 + NH 3
Hydrogenation of olefin compounds
RCH=CH2 + H2 RCH2 –CH3
Hydrogenation of aromatic compounds
C6H6 + 3H2 C6H12
The incoming hydrogen makes up gas is directed through chlorine adsorbent before the
compressor compresses it. Some part of this gas is channeled into the reactor as quench gas. In
heat exchanger the mixed stream is heated before passed to the second exchanger and lastly to
the reactor heater where it’s heated to the desired initial temperature [1]. Reactor temperature are
regulated by scheming fuel oil to gas into a heater, and then the stream is then introduced to the
reactor with catalysts. This is done in three beds, and the temperature of each bed is controlled
by introducing cold quench hydrogen from the recycling compressor [11].
Water is introduced at the inlet of overflow to prevent deposition of ammonium salts that might
cause corrosion of the reactor. The sour water is partly recycled since it contains ammonium salt
and can be used in washing.
Refining reactions
Denitrification- this reaction only occurs in cases of heterocyclic compounds with an aromatic
structure such as pyridine and the rate of reaction of denitrification is lower compared to the rate
of desulfurization. Denitrification leads to the formation of ammonia gas and the consumption of
hydrogen gas. [7]
Denitrification
C2H2N + 5H2 C2H2 + NH 3

DIESEL HYDRO DESULFURIZATION 9
Desulfurization is the reaction that involves saturated sulfides, disulfide, and aromatic
compounds. Sulfur combine in the cycle of aromatic structure is not easy to eliminate like in case
of thiophene. This reaction leads to formation of hydrogen sulfide and hydrogen consumption.
[5]
Desulfurization
RSH + H2 R-H + H2S
Diesel hydrodesulfurization is also referred as hydrotreating as it is widely used in petroleum
refineries and mining industries to produce high quality fuels as well as upgrade crude oil by
eliminating or lowering concentration of metal, sulfur or nitrogen. For the design and
installation of new plants [2], detailed research is of essence to operate at optimal conditions.
This involves rigorous steady-state reactor that becomes an imperative tool to face the actual
task of refining [3]. When considering light-medium reactors and because it involves
probably mixture hundreds of compounds, which not all of these compounds can be
explicitly considered. In such case, hypothetical compounds are clearly defined to take the
basis of feed. This is followed by characterization step of feed to obtain feed composition
about the hypothetical components [8]. Some previous approach to tackle this process was by
defining pseudo compounds generated as a result of true boiling point distillation curve [3].
Another approach is to employ lumping of species where their chemical families group
different species with similarities in their structural and chemical behavior. [3]
Desulfurization is the reaction that involves saturated sulfides, disulfide, and aromatic
compounds. Sulfur combine in the cycle of aromatic structure is not easy to eliminate like in case
of thiophene. This reaction leads to formation of hydrogen sulfide and hydrogen consumption.
[5]
Desulfurization
RSH + H2 R-H + H2S
Diesel hydrodesulfurization is also referred as hydrotreating as it is widely used in petroleum
refineries and mining industries to produce high quality fuels as well as upgrade crude oil by
eliminating or lowering concentration of metal, sulfur or nitrogen. For the design and
installation of new plants [2], detailed research is of essence to operate at optimal conditions.
This involves rigorous steady-state reactor that becomes an imperative tool to face the actual
task of refining [3]. When considering light-medium reactors and because it involves
probably mixture hundreds of compounds, which not all of these compounds can be
explicitly considered. In such case, hypothetical compounds are clearly defined to take the
basis of feed. This is followed by characterization step of feed to obtain feed composition
about the hypothetical components [8]. Some previous approach to tackle this process was by
defining pseudo compounds generated as a result of true boiling point distillation curve [3].
Another approach is to employ lumping of species where their chemical families group
different species with similarities in their structural and chemical behavior. [3]
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

DIESEL HYDRO DESULFURIZATION 10
Hydrotreating of diesel fuels is based in three phases that are heterogeneous catalysis: where
the model must have proper consideration on how to formulate the starting points stating all
kinetics that defines the reactions, then estimate this kinetic essentials with data obtained at
pilot plant scale eventually scaling up for instances of commercial units performance [3].
Even though this aspect of hypothetical considerations, it is associated by some setbacks that
might arise in the refinery reactor modeling and as new feeds which prevent formulation of a
fixed reaction set. The best way to overcome this, there should be a hydrocarbon mixture
starting from the feedstock properties where molecular configuration techniques have given
adequate results. [3]
Catalytic hydrotreating
Catalytic hydrotreating refers to the hydrogenation process that is used to remove
environmental pollutants such as nitrogen, metals, and sulfur from petroleum fractions such
as diesel, petrol kerosene and gasoline [4]. Elimination of these pollutants is very crucial
since when they are carelessly released to atmosphere, they have detrimental effects on the
equipment’s, end products and even the catalysts. Hydrotreating is also efficient since it
removes about 90% of these contaminants from crude petroleum [9]. This process of
hydrotreating is done before catalytic reforming to avoid contamination of catalysts by
untreated feedstock. It also comes before catalytic cracking to boost product yield and
upgrade middle distillate petroleum portions into finished end products such as heating fuel
oils, kerosene, and diesel fuels [3]. It also enhances the conversion of olefins and aromatics
to saturated compounds [4]
Hydrotreating of diesel fuels is based in three phases that are heterogeneous catalysis: where
the model must have proper consideration on how to formulate the starting points stating all
kinetics that defines the reactions, then estimate this kinetic essentials with data obtained at
pilot plant scale eventually scaling up for instances of commercial units performance [3].
Even though this aspect of hypothetical considerations, it is associated by some setbacks that
might arise in the refinery reactor modeling and as new feeds which prevent formulation of a
fixed reaction set. The best way to overcome this, there should be a hydrocarbon mixture
starting from the feedstock properties where molecular configuration techniques have given
adequate results. [3]
Catalytic hydrotreating
Catalytic hydrotreating refers to the hydrogenation process that is used to remove
environmental pollutants such as nitrogen, metals, and sulfur from petroleum fractions such
as diesel, petrol kerosene and gasoline [4]. Elimination of these pollutants is very crucial
since when they are carelessly released to atmosphere, they have detrimental effects on the
equipment’s, end products and even the catalysts. Hydrotreating is also efficient since it
removes about 90% of these contaminants from crude petroleum [9]. This process of
hydrotreating is done before catalytic reforming to avoid contamination of catalysts by
untreated feedstock. It also comes before catalytic cracking to boost product yield and
upgrade middle distillate petroleum portions into finished end products such as heating fuel
oils, kerosene, and diesel fuels [3]. It also enhances the conversion of olefins and aromatics
to saturated compounds [4]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
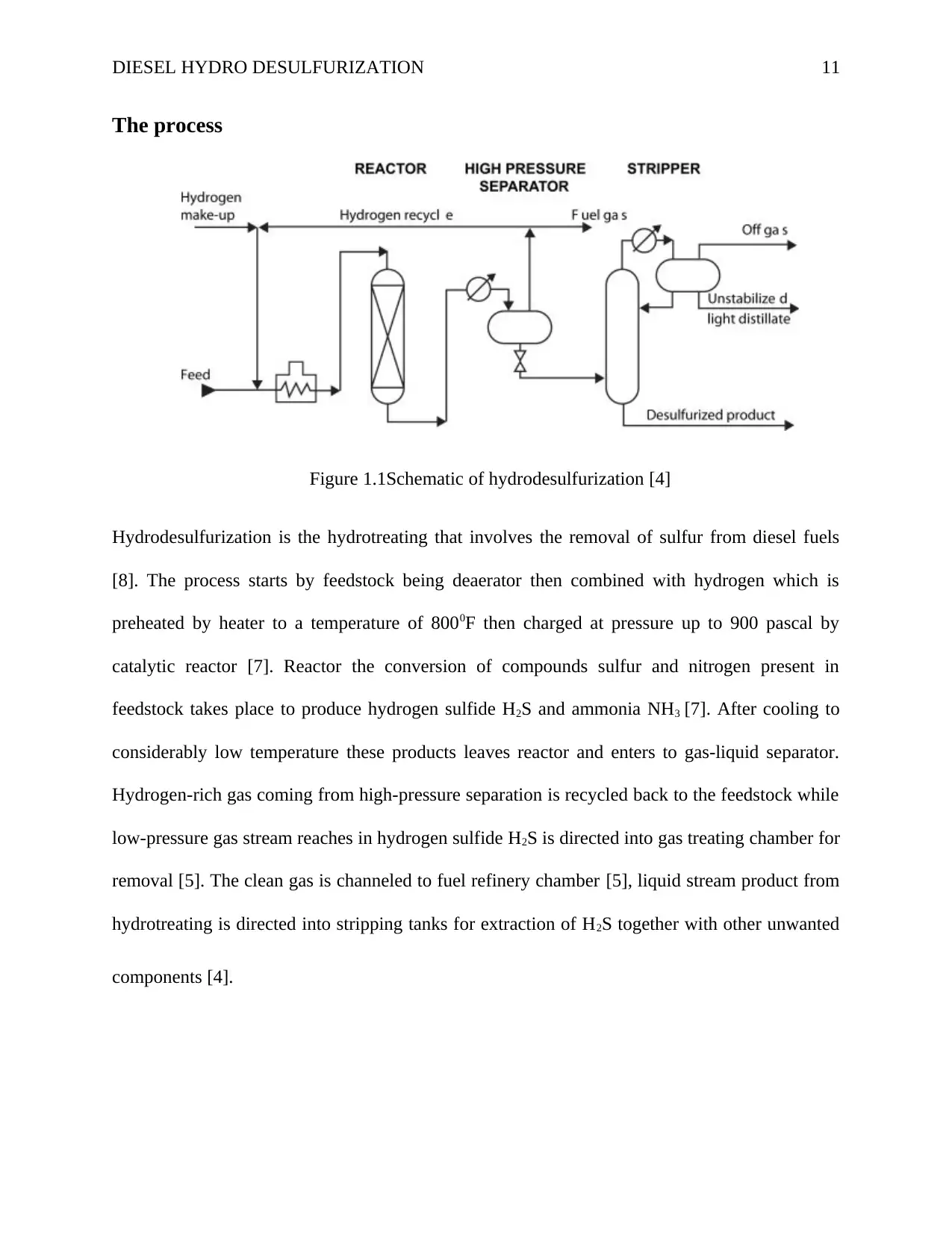
DIESEL HYDRO DESULFURIZATION 11
The process
Figure 1.1Schematic of hydrodesulfurization [4]
Hydrodesulfurization is the hydrotreating that involves the removal of sulfur from diesel fuels
[8]. The process starts by feedstock being deaerator then combined with hydrogen which is
preheated by heater to a temperature of 8000F then charged at pressure up to 900 pascal by
catalytic reactor [7]. Reactor the conversion of compounds sulfur and nitrogen present in
feedstock takes place to produce hydrogen sulfide H2S and ammonia NH3 [7]. After cooling to
considerably low temperature these products leaves reactor and enters to gas-liquid separator.
Hydrogen-rich gas coming from high-pressure separation is recycled back to the feedstock while
low-pressure gas stream reaches in hydrogen sulfide H2S is directed into gas treating chamber for
removal [5]. The clean gas is channeled to fuel refinery chamber [5], liquid stream product from
hydrotreating is directed into stripping tanks for extraction of H2S together with other unwanted
components [4].
The process
Figure 1.1Schematic of hydrodesulfurization [4]
Hydrodesulfurization is the hydrotreating that involves the removal of sulfur from diesel fuels
[8]. The process starts by feedstock being deaerator then combined with hydrogen which is
preheated by heater to a temperature of 8000F then charged at pressure up to 900 pascal by
catalytic reactor [7]. Reactor the conversion of compounds sulfur and nitrogen present in
feedstock takes place to produce hydrogen sulfide H2S and ammonia NH3 [7]. After cooling to
considerably low temperature these products leaves reactor and enters to gas-liquid separator.
Hydrogen-rich gas coming from high-pressure separation is recycled back to the feedstock while
low-pressure gas stream reaches in hydrogen sulfide H2S is directed into gas treating chamber for
removal [5]. The clean gas is channeled to fuel refinery chamber [5], liquid stream product from
hydrotreating is directed into stripping tanks for extraction of H2S together with other unwanted
components [4].

DIESEL HYDRO DESULFURIZATION 12
Reactions and chemical equations
The removal of sulfur demands for special conditions to efficiently eliminate sulfur from diesel
fuels [12]. In case of organosulfur compounds involving reaction with hydrogen gas producing
hydrogen sulfide, this reaction requires high hydrogen pressure greater than 200 atm and
temperature of 4500C [3]
Different types of catalysts escalate various reactions depending on the requirements for the
reaction to occur [2]. The selection is also pegged on the type of oil being purified. The most
commonly used catalysts are mixture of Mo and Co sulfides [2] with alumina, mixture of Ni and
Mo. There numerous models giving detailed explanation on how thiophene moietie is oriented
on the catalysts, generally the Co-Mo-S model developed by Topsoe. Under this catalysts are
formed in single layer of Mos2 with Co or Ni at the edges [2].
The coordination of thiophenes Moieties occurs in a way that all thiophenes molecules, available
the sides with high electronic density forming the lone pair over sulfur atoms and the double
bond within carbon atoms [2]. Some of the coordination is involved in hydrogenolysis process.
The common coordination mode of the thiophenes is shown in the figure 1.2 below.
Reactions and chemical equations
The removal of sulfur demands for special conditions to efficiently eliminate sulfur from diesel
fuels [12]. In case of organosulfur compounds involving reaction with hydrogen gas producing
hydrogen sulfide, this reaction requires high hydrogen pressure greater than 200 atm and
temperature of 4500C [3]
Different types of catalysts escalate various reactions depending on the requirements for the
reaction to occur [2]. The selection is also pegged on the type of oil being purified. The most
commonly used catalysts are mixture of Mo and Co sulfides [2] with alumina, mixture of Ni and
Mo. There numerous models giving detailed explanation on how thiophene moietie is oriented
on the catalysts, generally the Co-Mo-S model developed by Topsoe. Under this catalysts are
formed in single layer of Mos2 with Co or Ni at the edges [2].
The coordination of thiophenes Moieties occurs in a way that all thiophenes molecules, available
the sides with high electronic density forming the lone pair over sulfur atoms and the double
bond within carbon atoms [2]. Some of the coordination is involved in hydrogenolysis process.
The common coordination mode of the thiophenes is shown in the figure 1.2 below.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
