Wastewater Treatment: Factors Affecting DBP Formation and Control
VerifiedAdded on 2022/11/26
|16
|2035
|327
Report
AI Summary
This report delves into the crucial topic of Disinfection Byproduct (DBP) formation in wastewater treatment. It highlights the necessity of wastewater treatment, driven by factors like water scarcity and environmental protection, and explores the use of disinfectants like chlorine. The report discusses the formation of DBPs, such as halonitromethanes, and their impact on aquatic ecosystems. It examines various factors affecting DBP formation, including chlorine contact time, pH, and temperature. The document contrasts DBP formation in wastewater and drinking water treatment, emphasizing the use of alternative disinfectants like ozone and ultrasound to minimize harmful byproducts. The report also outlines the processes involved in DBP formation, from the injection of wastewater to the final treatment stages. It concludes by emphasizing the importance of regulated DBP formation for safe drinking water and the need for alternatives to chlorine to mitigate adverse health effects. The paper makes it clear that regulated DBPs have fewer side effects when used in drinking water treatment, and the factors of temperature, turbidity, pH, the amount of organic and inorganic compounds found in wastewater, and the contact time affect the rate of formation of disinfection byproducts. The document cites multiple research papers and studies as references.

FORMATION OF DBP IN WASTEWATER 1
` FORMATION OF DBP IN WASTEWATER
By (Name)
Course
Tutor
Learning institution
Date
` FORMATION OF DBP IN WASTEWATER
By (Name)
Course
Tutor
Learning institution
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FORMATION OF DBP IN WASTEWATER 2
Abstract
In an attempt to protect the environment from being pullulated by the hazardous effluent. DBP
formation has significantly been employed in the wastewater treatment process to achieve the
standards required for the waste before their disposal. Factors such as limited drinking water
supply, massive irrigation, rapid industrialization, source protection, the increasing population
growth and the need for environmental protection sparked the need to treat the wastewater by the
use of BDP in water treatment to be able to recycle and reuse the same water for other processes.
The purpose of the DBP in wastewater treatment, however, is associated with specific adverse
outcomes. The DBPs formed significantly affects the aquatic life in the water. There is,
therefore, a need for an alternative disinfectant instead of the use of chlorine.
Abstract
In an attempt to protect the environment from being pullulated by the hazardous effluent. DBP
formation has significantly been employed in the wastewater treatment process to achieve the
standards required for the waste before their disposal. Factors such as limited drinking water
supply, massive irrigation, rapid industrialization, source protection, the increasing population
growth and the need for environmental protection sparked the need to treat the wastewater by the
use of BDP in water treatment to be able to recycle and reuse the same water for other processes.
The purpose of the DBP in wastewater treatment, however, is associated with specific adverse
outcomes. The DBPs formed significantly affects the aquatic life in the water. There is,
therefore, a need for an alternative disinfectant instead of the use of chlorine.

FORMATION OF DBP IN WASTEWATER 3
Introduction
The use of disinfection in the wastewater provides an alternative way to wastewater
reclamation. The application of environmental friendly wastewater DBP help in the attempt to
solve the water shortages affecting most countries in the world. Most countries in the world will
be soon reclaiming their barren land through irrigation as a result of the use and application of
the use and application disinfection technique, significantly improving the agricultural sector.
The main reason for the wastewater treatment through disinfection technique is to help
reduce the environmental pollution that may result from discharging untreated wastewater in the
primary water sources (Guyer, 2018). This research paper elucidates the application of
wastewater disinfectants such as DBP in the wastewater treatment process as well as various
factors affecting the operation of wastewater treatment using disinfection byproducts in the
wastewater and water treatment.
Introduction
The use of disinfection in the wastewater provides an alternative way to wastewater
reclamation. The application of environmental friendly wastewater DBP help in the attempt to
solve the water shortages affecting most countries in the world. Most countries in the world will
be soon reclaiming their barren land through irrigation as a result of the use and application of
the use and application disinfection technique, significantly improving the agricultural sector.
The main reason for the wastewater treatment through disinfection technique is to help
reduce the environmental pollution that may result from discharging untreated wastewater in the
primary water sources (Guyer, 2018). This research paper elucidates the application of
wastewater disinfectants such as DBP in the wastewater treatment process as well as various
factors affecting the operation of wastewater treatment using disinfection byproducts in the
wastewater and water treatment.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

FORMATION OF DBP IN WASTEWATER 4
Discussion
Chlorine is one of the most commonly used disinfectants in the wastewater treatment
process. Its use in the effluent, however, results in the formation of carcinogenic disinfection
Byproduct. As a result of the reaction of the chlorine molecules in the effluent with both organic
and inorganic compounds in the effluent. The establishment of the disinfectant Byproducts in the
wastewater formed from the reaction of the chlorine with the various organic compounds to
interfere with the aquatic ecosystems. It is, therefore, necessary to save the life of the marine
creatures in the wastewater
Adequate wastewater treatment can be achieved by the use of Microtox bioassay with
Vibrio fischeri to intensively screen al the chemicals used in the wastewater treatment for the
removal of any disinfection byproduct formed. As the ratio of the chlorine molecule to
ammonium ions increases, the rate of toxicity of the wastewater also increases (Guyer, 2018).
Halonitromethanes (HNMs) is one of the examples of the disinfectant byproducts (DBPs) formed
from the reaction of the chlorine molecules with both organic and inorganic compounds in the
wastewater.
Total Halonitromethanes formed in the wastewater as a byproduct of chlorination is
directly proportional to the amount of bromine dissolved in the wastewater. As the bromine
concentration increases in the sewer, the amount of THMs also formed increases. Also, as the
bromide concentration increases, the rate of formation of the methyl bromide in the wastewater
also increases. In the differentiation between the freshwater and the sewage, the following
observations were made. At a higher concentration of the bromide, the distribution of the
monohalogenated, dihalogenated and trihalogeneted for the drinking water, differs from that of
Discussion
Chlorine is one of the most commonly used disinfectants in the wastewater treatment
process. Its use in the effluent, however, results in the formation of carcinogenic disinfection
Byproduct. As a result of the reaction of the chlorine molecules in the effluent with both organic
and inorganic compounds in the effluent. The establishment of the disinfectant Byproducts in the
wastewater formed from the reaction of the chlorine with the various organic compounds to
interfere with the aquatic ecosystems. It is, therefore, necessary to save the life of the marine
creatures in the wastewater
Adequate wastewater treatment can be achieved by the use of Microtox bioassay with
Vibrio fischeri to intensively screen al the chemicals used in the wastewater treatment for the
removal of any disinfection byproduct formed. As the ratio of the chlorine molecule to
ammonium ions increases, the rate of toxicity of the wastewater also increases (Guyer, 2018).
Halonitromethanes (HNMs) is one of the examples of the disinfectant byproducts (DBPs) formed
from the reaction of the chlorine molecules with both organic and inorganic compounds in the
wastewater.
Total Halonitromethanes formed in the wastewater as a byproduct of chlorination is
directly proportional to the amount of bromine dissolved in the wastewater. As the bromine
concentration increases in the sewer, the amount of THMs also formed increases. Also, as the
bromide concentration increases, the rate of formation of the methyl bromide in the wastewater
also increases. In the differentiation between the freshwater and the sewage, the following
observations were made. At a higher concentration of the bromide, the distribution of the
monohalogenated, dihalogenated and trihalogeneted for the drinking water, differs from that of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FORMATION OF DBP IN WASTEWATER 5
the wastewater. This is, therefore, the surest way to distinguish between the contaminated or the
pullulated water and the fresh drinking water.
Apart from the use of chlorine for the direct disinfection of the wastewater, several other
methods can also be used to disinfect the effluent water. Sodium hypochlorite can also be applied
in shallow doses at a retention time of about 30mins — different ways of disinfection such as
electrochemical disinfection, use of chlorine dioxide, and the disinfection using chloramines.
Several factors may affect the process of the formation of disinfection byproducts. They include
the chlorine contact time, the turbidity of the wastewater, the pH, Temperature as well as the
disinfectant dosage.
Generally, the formation of disinfection byproducts increases with the amount of time
the applied chlorine dosage will have with the wastewater under treatment. However, for the pH,
the rate of formation of the disinfection byproducts in the sewer is inversely proportional to the
pH value. As the pH of the wastewater increases, the rate of formation of disinfection byproducts
decreases monotonically (Guyer, 2018). The rise in temperature increases the kinetic energy of
both the chlorine molecules and the organic compounds in the wastewater. Therefore
temperature is directly proportional to the rate of formation of the disinfection byproducts. As
temperature increases, so do the rate of formation of the disinfection byproduct. The process in
figure 1 below shows the stepwise process involved in the application of disinfection byproducts
(DBPs) formation in the wastewater treatment process. Wastewater from the industries and other
sources is pumped to the injector. It is then disinfected with the supplied liquid sodium
hypochlorite. The resulting sewage then flows into the mixers where the effluent is thoroughly
mixed with the chlorine solution from the injector to form DBPs. The DBPs formed depends on
the contact time chlorine molecule have with the flowing wastewater within the mixer. The
the wastewater. This is, therefore, the surest way to distinguish between the contaminated or the
pullulated water and the fresh drinking water.
Apart from the use of chlorine for the direct disinfection of the wastewater, several other
methods can also be used to disinfect the effluent water. Sodium hypochlorite can also be applied
in shallow doses at a retention time of about 30mins — different ways of disinfection such as
electrochemical disinfection, use of chlorine dioxide, and the disinfection using chloramines.
Several factors may affect the process of the formation of disinfection byproducts. They include
the chlorine contact time, the turbidity of the wastewater, the pH, Temperature as well as the
disinfectant dosage.
Generally, the formation of disinfection byproducts increases with the amount of time
the applied chlorine dosage will have with the wastewater under treatment. However, for the pH,
the rate of formation of the disinfection byproducts in the sewer is inversely proportional to the
pH value. As the pH of the wastewater increases, the rate of formation of disinfection byproducts
decreases monotonically (Guyer, 2018). The rise in temperature increases the kinetic energy of
both the chlorine molecules and the organic compounds in the wastewater. Therefore
temperature is directly proportional to the rate of formation of the disinfection byproducts. As
temperature increases, so do the rate of formation of the disinfection byproduct. The process in
figure 1 below shows the stepwise process involved in the application of disinfection byproducts
(DBPs) formation in the wastewater treatment process. Wastewater from the industries and other
sources is pumped to the injector. It is then disinfected with the supplied liquid sodium
hypochlorite. The resulting sewage then flows into the mixers where the effluent is thoroughly
mixed with the chlorine solution from the injector to form DBPs. The DBPs formed depends on
the contact time chlorine molecule have with the flowing wastewater within the mixer. The
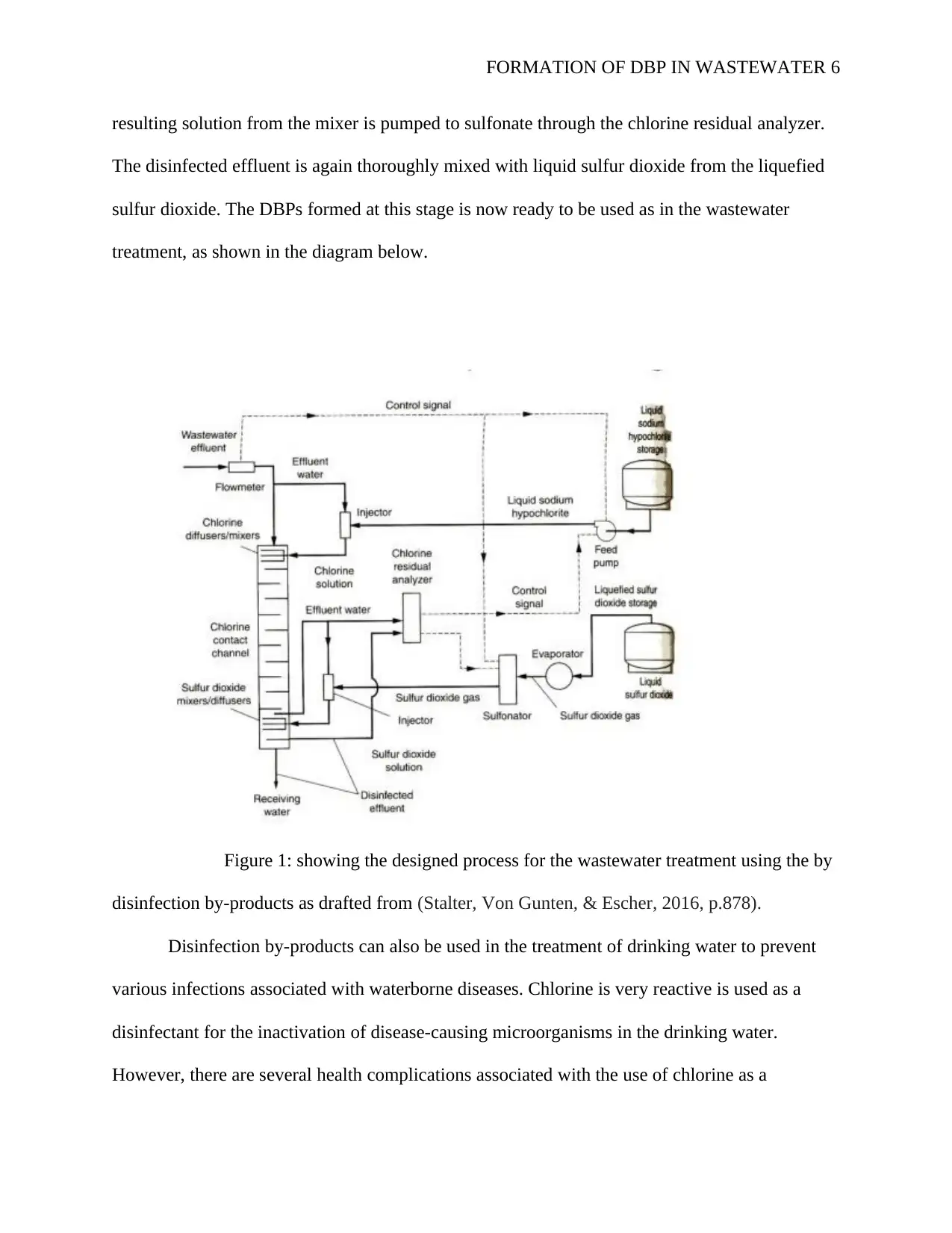
FORMATION OF DBP IN WASTEWATER 6
resulting solution from the mixer is pumped to sulfonate through the chlorine residual analyzer.
The disinfected effluent is again thoroughly mixed with liquid sulfur dioxide from the liquefied
sulfur dioxide. The DBPs formed at this stage is now ready to be used as in the wastewater
treatment, as shown in the diagram below.
Figure 1: showing the designed process for the wastewater treatment using the by
disinfection by-products as drafted from (Stalter, Von Gunten, & Escher, 2016, p.878).
Disinfection by-products can also be used in the treatment of drinking water to prevent
various infections associated with waterborne diseases. Chlorine is very reactive is used as a
disinfectant for the inactivation of disease-causing microorganisms in the drinking water.
However, there are several health complications associated with the use of chlorine as a
resulting solution from the mixer is pumped to sulfonate through the chlorine residual analyzer.
The disinfected effluent is again thoroughly mixed with liquid sulfur dioxide from the liquefied
sulfur dioxide. The DBPs formed at this stage is now ready to be used as in the wastewater
treatment, as shown in the diagram below.
Figure 1: showing the designed process for the wastewater treatment using the by
disinfection by-products as drafted from (Stalter, Von Gunten, & Escher, 2016, p.878).
Disinfection by-products can also be used in the treatment of drinking water to prevent
various infections associated with waterborne diseases. Chlorine is very reactive is used as a
disinfectant for the inactivation of disease-causing microorganisms in the drinking water.
However, there are several health complications associated with the use of chlorine as a
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

FORMATION OF DBP IN WASTEWATER 7
disinfectant for the treatment of drinking water (Guyer, 2018). The formation of the various
byproducts of disinfection cases side effects on human health, DBPs are associated with liver
cancer, bladder cancers among many other side effects. However, the need for enough drinking
water calls for the application of disinfection byproducts formation for the treatment of drinking
water. The schematic diagram below shows the process involved in the formation of a regulated
DBPs that suits drinking water treatment.
As drafted from (Stalter, Von Gunten, & Escher, 2016, p.878).
The design process above shows how the recommended DBPs from the various series of
reactions involved. Natural organic matter, algae organic matter, and wastewater effluent
organic matter under controlled pH, contact time, and temperature reacts with inorganic DBP
such as bromide, iodide, and nitric (Guyer, 2018). The resulting products then react with the
various disinfectants such as chlorine, chloramines, chlorine dioxide, Ozone, and ultraviolet
under favorable pH, retention time, and temperature to form regulated emerging disinfection
disinfectant for the treatment of drinking water (Guyer, 2018). The formation of the various
byproducts of disinfection cases side effects on human health, DBPs are associated with liver
cancer, bladder cancers among many other side effects. However, the need for enough drinking
water calls for the application of disinfection byproducts formation for the treatment of drinking
water. The schematic diagram below shows the process involved in the formation of a regulated
DBPs that suits drinking water treatment.
As drafted from (Stalter, Von Gunten, & Escher, 2016, p.878).
The design process above shows how the recommended DBPs from the various series of
reactions involved. Natural organic matter, algae organic matter, and wastewater effluent
organic matter under controlled pH, contact time, and temperature reacts with inorganic DBP
such as bromide, iodide, and nitric (Guyer, 2018). The resulting products then react with the
various disinfectants such as chlorine, chloramines, chlorine dioxide, Ozone, and ultraviolet
under favorable pH, retention time, and temperature to form regulated emerging disinfection
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FORMATION OF DBP IN WASTEWATER 8
byproducts. The examples of the byproducts include; trihalomethane (THMs), haloacetic acids
(HAAs), haloacetaldehydes, Halonitromethanes as well as the nitrosamines that has negligible
side effects on human health when used in the water treatment for human consumption.
In comparing the DBPs formation in case of wastewater and water, there is an extensive
use of chlorine as disinfectant in the case of wastewater leading to the formation of excess of
trihalomethane (THMs), haloacetic acids (HAAs), haloacetaldehydes, Halonitromethanes as well
as the nitrosamines byproducts that causes side effects on human health. These DBPs are
associated with liver cancer, bladder cancers among many other side effects hence making the
resulting water from the treated wastewater not recommended for human consumption.
However, in the case of water treatment alternatives of chlorine such as ozone, ultraviolet, and
ultrasound technology are used as disinfectants as they produce a regulated amount of the DBPs
formation that has a negligible side effect on the human health. Furthermore, the use of
ultrasound in water treatment eliminates the production of DBPs, such as trihalomethane (THMs
harmful to human health.
Conclusion
In summary, the formation of various disinfectants products such as trihalomethane
(THMs), haloacetic acids (HAAs), haloacetaldehydes, Halonitromethanes as well as the
nitrosamines formed from the reaction of the disinfectants with both organic and inorganic
compounds in the wastewater can be used for both wastewater treatment as well as in the
treatment of drinking water (Guyer, 2018). Factors such as temperature, turbidity, pH, the
byproducts. The examples of the byproducts include; trihalomethane (THMs), haloacetic acids
(HAAs), haloacetaldehydes, Halonitromethanes as well as the nitrosamines that has negligible
side effects on human health when used in the water treatment for human consumption.
In comparing the DBPs formation in case of wastewater and water, there is an extensive
use of chlorine as disinfectant in the case of wastewater leading to the formation of excess of
trihalomethane (THMs), haloacetic acids (HAAs), haloacetaldehydes, Halonitromethanes as well
as the nitrosamines byproducts that causes side effects on human health. These DBPs are
associated with liver cancer, bladder cancers among many other side effects hence making the
resulting water from the treated wastewater not recommended for human consumption.
However, in the case of water treatment alternatives of chlorine such as ozone, ultraviolet, and
ultrasound technology are used as disinfectants as they produce a regulated amount of the DBPs
formation that has a negligible side effect on the human health. Furthermore, the use of
ultrasound in water treatment eliminates the production of DBPs, such as trihalomethane (THMs
harmful to human health.
Conclusion
In summary, the formation of various disinfectants products such as trihalomethane
(THMs), haloacetic acids (HAAs), haloacetaldehydes, Halonitromethanes as well as the
nitrosamines formed from the reaction of the disinfectants with both organic and inorganic
compounds in the wastewater can be used for both wastewater treatment as well as in the
treatment of drinking water (Guyer, 2018). Factors such as temperature, turbidity, pH, the

FORMATION OF DBP IN WASTEWATER 9
amount of both organic and inorganic compounds found in the wastewater as well as the contact
time affects the rate of formation of disinfection byproducts. Finally, the regulated form of the
DBPs has little adverse side effects on human health hence suitable for use in drinking water
treatment as opposed to the use of hazardous chlorine as the disinfectant.
amount of both organic and inorganic compounds found in the wastewater as well as the contact
time affects the rate of formation of disinfection byproducts. Finally, the regulated form of the
DBPs has little adverse side effects on human health hence suitable for use in drinking water
treatment as opposed to the use of hazardous chlorine as the disinfectant.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

FORMATION OF DBP IN WASTEWATER 10
Bibliography
Beauchamp, N., Dorea, C., Bouchard, C., and Rodriguez, M., 2018. Use of differential
absorbance to estimate concentrations of chlorinated disinfection by-product in drinking
water: Critical review and research needs. Critical reviews in environmental science and
technology, 48(2), pp.210-241.
Dong, H., Qiang, Z., and Richardson, S.D., 2019. Formation of Iodinated Disinfection
Byproducts (I-DBPs) in Drinking Water: Emerging Concerns and Current Issues.
Accounts of chemical research.
Guyer, J.P. ed., 2018. An Introduction to Domestic Water Treatment. Guyer Partners.
Li, X.F., and Mitch, W.A., 2018. Drinking water disinfection byproducts (DBPs) and human
health effects: multidisciplinary challenges and opportunities.
Ma, C., Xu, H., Zhang, L., Pei, H., and Jin, Y., 2018. Use of fluorescence excitation-emission
matrices coupled with parallel factor analysis to monitor C-and N-DBPs formation in
drinking water recovered from cyanobacteria-laden sludge dewatering. Science of The
Total Environment, 640, pp.609-618.
Sawade, E., Fabris, R., Humpage, A., and Drikas, M., 2016. Effect of increasing bromide
concentration on toxicity in treated drinking water. Journal of water and health, 14(2),
pp.183-191.
Stalter, D., O'Malley, E., Von Gunten, U. and Escher, B.I., 2016. Point-of-use water filters can
effectively remove disinfection by-products and toxicity from chlorinated and
chloraminated tap water. Environmental Science: Water Research & Technology, 2(5),
pp.875-883.
Bibliography
Beauchamp, N., Dorea, C., Bouchard, C., and Rodriguez, M., 2018. Use of differential
absorbance to estimate concentrations of chlorinated disinfection by-product in drinking
water: Critical review and research needs. Critical reviews in environmental science and
technology, 48(2), pp.210-241.
Dong, H., Qiang, Z., and Richardson, S.D., 2019. Formation of Iodinated Disinfection
Byproducts (I-DBPs) in Drinking Water: Emerging Concerns and Current Issues.
Accounts of chemical research.
Guyer, J.P. ed., 2018. An Introduction to Domestic Water Treatment. Guyer Partners.
Li, X.F., and Mitch, W.A., 2018. Drinking water disinfection byproducts (DBPs) and human
health effects: multidisciplinary challenges and opportunities.
Ma, C., Xu, H., Zhang, L., Pei, H., and Jin, Y., 2018. Use of fluorescence excitation-emission
matrices coupled with parallel factor analysis to monitor C-and N-DBPs formation in
drinking water recovered from cyanobacteria-laden sludge dewatering. Science of The
Total Environment, 640, pp.609-618.
Sawade, E., Fabris, R., Humpage, A., and Drikas, M., 2016. Effect of increasing bromide
concentration on toxicity in treated drinking water. Journal of water and health, 14(2),
pp.183-191.
Stalter, D., O'Malley, E., Von Gunten, U. and Escher, B.I., 2016. Point-of-use water filters can
effectively remove disinfection by-products and toxicity from chlorinated and
chloraminated tap water. Environmental Science: Water Research & Technology, 2(5),
pp.875-883.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FORMATION OF DBP IN WASTEWATER 11
Tan, X., Chen, C., Hu, Y., Wen, J., Qin, Y., Cheng, J., and Chen, Y., 2018. Novel AgNWs-
PAN/TPU membrane for point-of-use drinking water electrochemical disinfection.
Science of The Total Environment, 637, pp.408-417.
Zwiener, C., 2017. K. Clive Thompson, Simon Gillespie, and Emma H. Goslan (Eds.):
Disinfection by-products in drinking water. Analytical and bioanalytical chemistry,
409(7), pp.1727-1728.
Tan, X., Chen, C., Hu, Y., Wen, J., Qin, Y., Cheng, J., and Chen, Y., 2018. Novel AgNWs-
PAN/TPU membrane for point-of-use drinking water electrochemical disinfection.
Science of The Total Environment, 637, pp.408-417.
Zwiener, C., 2017. K. Clive Thompson, Simon Gillespie, and Emma H. Goslan (Eds.):
Disinfection by-products in drinking water. Analytical and bioanalytical chemistry,
409(7), pp.1727-1728.

FORMATION OF DBP IN WASTEWATER 12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.