Biology Report: Dornase Alfa Structure, Properties, and Purification
VerifiedAdded on 2022/07/27
|14
|2093
|51
Report
AI Summary
This report provides a comprehensive analysis of Dornase alfa, a recombinant human DNase I enzyme, focusing on its structure, physicochemical properties, and purification methods. The report begins by detailing the primary structure, including the amino acid sequence, signal peptide, glycosylation sites, and disulfide bonds. It then explores the physico-chemical properties, such as pI and molecular weight, and their significance in purification protocols. The report contrasts the initial purification methods with current industrial processes using recombinant DNA technology in CHO cells and affinity chromatography. The hydropathy plot analysis further illustrates the protein's hydrophobic and hydrophilic regions. The report explains the mechanism of action, application, and trade name of the enzyme, Pulmozyme, used for cystic fibrosis treatment.

Running head: BIOLOGY
DORNASE ALPHA
Name of the Student
Name of the University
Author Note
DORNASE ALPHA
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1BIOLOGY
Table of Contents
Introduction................................................................................................................................2
1. The primary structure (amino acid sequence):.......................................................................2
2. The physico-chemical properties (pI, MW value) of the protein that are important
considerations for a purification protocol:.................................................................................0
Predicted proteins..................................................................................................0
ProtScale..................................................................................................................................1
User-provided sequence:...................................................................................................1
3. How the protein was initially purified from its natural source when it was first discovered:3
4. How is the same protein now prepared for an industrial use, or in recombinant form:.........4
Bibliography...............................................................................................................................6
Table of Contents
Introduction................................................................................................................................2
1. The primary structure (amino acid sequence):.......................................................................2
2. The physico-chemical properties (pI, MW value) of the protein that are important
considerations for a purification protocol:.................................................................................0
Predicted proteins..................................................................................................0
ProtScale..................................................................................................................................1
User-provided sequence:...................................................................................................1
3. How the protein was initially purified from its natural source when it was first discovered:3
4. How is the same protein now prepared for an industrial use, or in recombinant form:.........4
Bibliography...............................................................................................................................6

2BIOLOGY
Introduction
Dornase alfa is a highly purified solution containing the recombinant DNase I protein of
human beings. This is an enzyme solution which is responsible for the selective cleavage of
DNA. This enzyme has been found to hydrolyse the DNA which has been found to be present
in the sputum/mucus of the patients suffering from cystic fibrosis and has been found to
reduce viscosity in lungs in order to facilitate airway clearance. The trade name of this
enzyme is Pulmozyme. This paper will discuss the primary structure, physicochemical
properties and purification processes of Dornase alfa.
1. The primary structure (amino acid sequence):
10 20 30 40 50
MRGMKLLGAL LALAALLQGA VSLKIAAFNI QTFGETKMSN ATLVSYIVQI
60 70 80 90 100
LSRYDIALVQ EVRDSHLTAV GKLLDNLNQD APDTYHYVVS EPLGRNSYKE
110 120 130 140 150
RYLFVYRPDQ VSAVDSYYYD DGCEPCGNDT FNREPAIVRF FSRFTEVREF
160 170 180 190 200
AIVPLHAAPG DAVAEIDALY DVYLDVQEKW GLEDVMLMGD FNAGCSYVRP
210 220 230 240 250
SQWSSIRLWT SPTFQWLIPD SADTTATPTH CAYDRIVVAG MLLRGAVVPD
260 270 280
SALPFNFQAA YGLSDQLAQA ISDHYPVEVM LK
Signal peptide sequence= Positions 1 to 22 [Yellow]
Mature chain sequence= Positions 23 to 282 [Green]
Glycosylation= N-linked glycosylation of 40th residue the protein (Asparagine) [Red] and N-
linked glycosylation of 128th residue the protein (Asparagine) [Red].
Disulphide bond= 123rd to 136th residue and 195th to 231st residue. The latter one is
responsible for enzyme activities
Introduction
Dornase alfa is a highly purified solution containing the recombinant DNase I protein of
human beings. This is an enzyme solution which is responsible for the selective cleavage of
DNA. This enzyme has been found to hydrolyse the DNA which has been found to be present
in the sputum/mucus of the patients suffering from cystic fibrosis and has been found to
reduce viscosity in lungs in order to facilitate airway clearance. The trade name of this
enzyme is Pulmozyme. This paper will discuss the primary structure, physicochemical
properties and purification processes of Dornase alfa.
1. The primary structure (amino acid sequence):
10 20 30 40 50
MRGMKLLGAL LALAALLQGA VSLKIAAFNI QTFGETKMSN ATLVSYIVQI
60 70 80 90 100
LSRYDIALVQ EVRDSHLTAV GKLLDNLNQD APDTYHYVVS EPLGRNSYKE
110 120 130 140 150
RYLFVYRPDQ VSAVDSYYYD DGCEPCGNDT FNREPAIVRF FSRFTEVREF
160 170 180 190 200
AIVPLHAAPG DAVAEIDALY DVYLDVQEKW GLEDVMLMGD FNAGCSYVRP
210 220 230 240 250
SQWSSIRLWT SPTFQWLIPD SADTTATPTH CAYDRIVVAG MLLRGAVVPD
260 270 280
SALPFNFQAA YGLSDQLAQA ISDHYPVEVM LK
Signal peptide sequence= Positions 1 to 22 [Yellow]
Mature chain sequence= Positions 23 to 282 [Green]
Glycosylation= N-linked glycosylation of 40th residue the protein (Asparagine) [Red] and N-
linked glycosylation of 128th residue the protein (Asparagine) [Red].
Disulphide bond= 123rd to 136th residue and 195th to 231st residue. The latter one is
responsible for enzyme activities
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3BIOLOGY
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Running head: BIOLOGY
2. The physico-chemical properties (pI, MW value) of the protein that are important considerations for a
purification protocol:
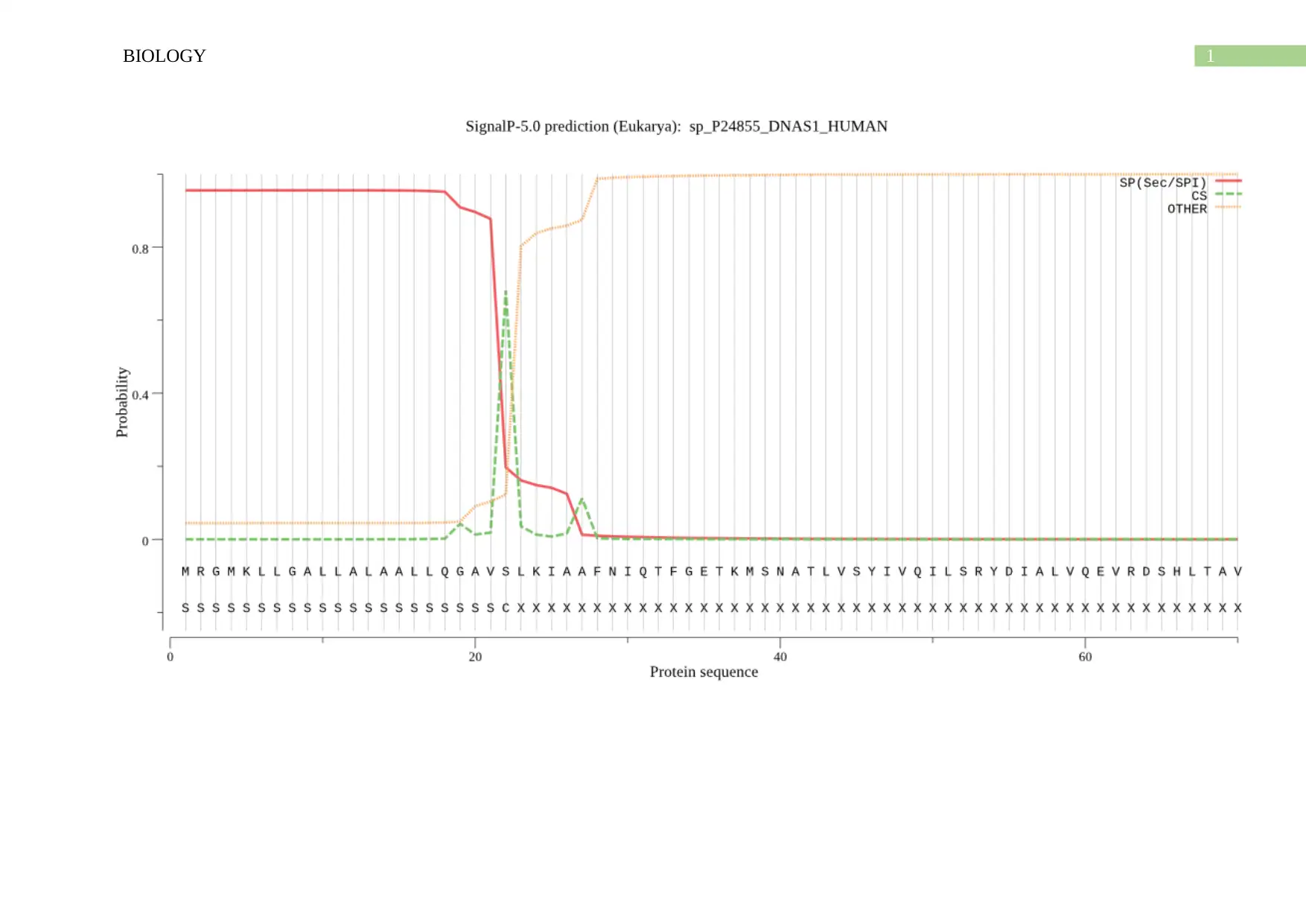
Prediction of signal peptide cleavage site by ExPASy-
Predicted proteins
sp_P24855_DNAS1_HUMAN
Prediction: Signal peptide (Sec/SPI)
Cleavage site between pos. 22 and 23: AVS-LK. Probability: 0.6807
Protein type Signal Peptide (Sec/SPI) Other
Likelihood 0.9547 0.0453
2. The physico-chemical properties (pI, MW value) of the protein that are important considerations for a
purification protocol:
Prediction of signal peptide cleavage site by ExPASy-
Predicted proteins
sp_P24855_DNAS1_HUMAN
Prediction: Signal peptide (Sec/SPI)
Cleavage site between pos. 22 and 23: AVS-LK. Probability: 0.6807
Protein type Signal Peptide (Sec/SPI) Other
Likelihood 0.9547 0.0453
1BIOLOGY
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Running head: BIOLOGY
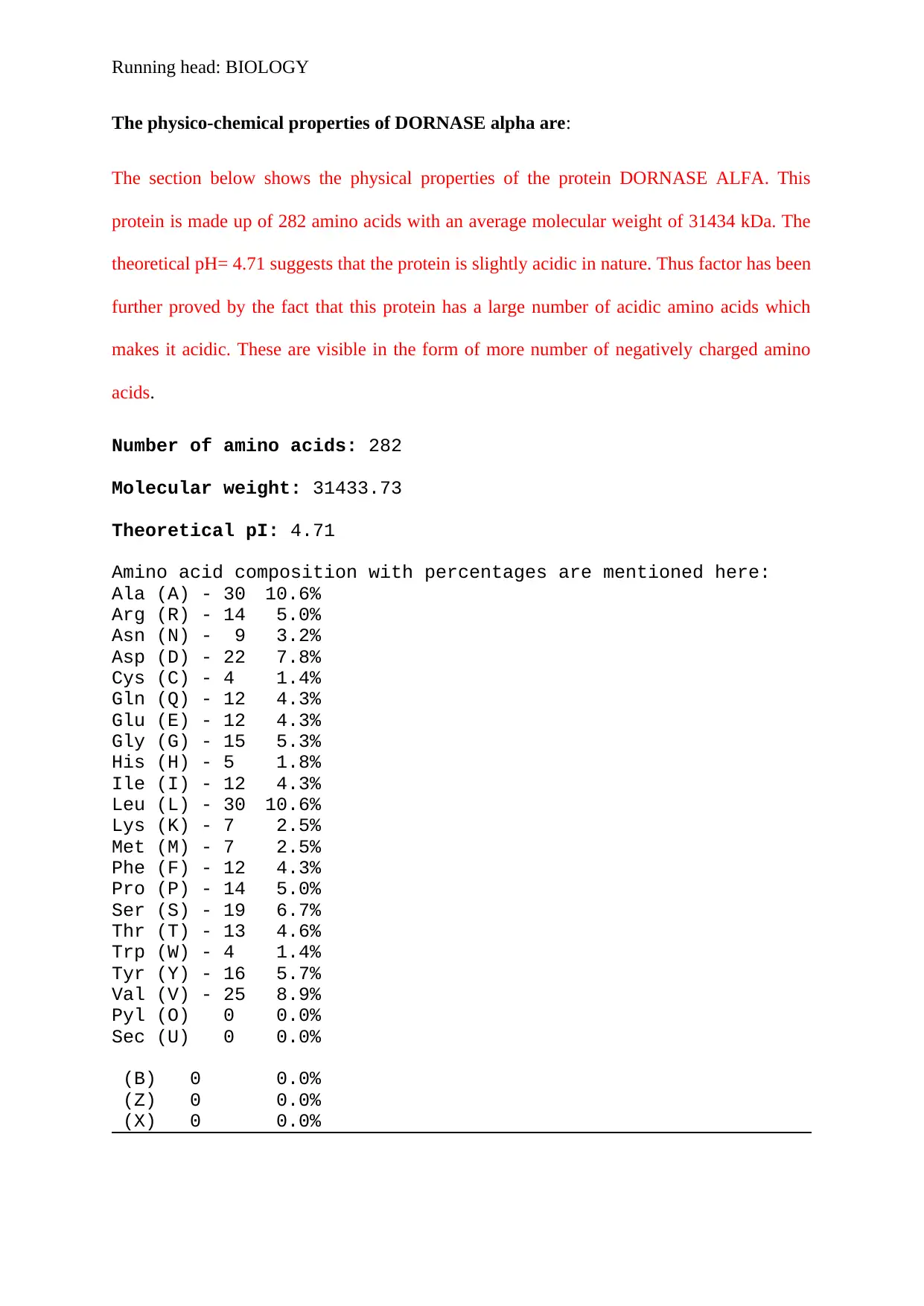
The physico-chemical properties of DORNASE alpha are:
The section below shows the physical properties of the protein DORNASE ALFA. This
protein is made up of 282 amino acids with an average molecular weight of 31434 kDa. The
theoretical pH= 4.71 suggests that the protein is slightly acidic in nature. Thus factor has been
further proved by the fact that this protein has a large number of acidic amino acids which
makes it acidic. These are visible in the form of more number of negatively charged amino
acids.
Number of amino acids: 282
Molecular weight: 31433.73
Theoretical pI: 4.71
Amino acid composition with percentages are mentioned here:
Ala (A) - 30 10.6%
Arg (R) - 14 5.0%
Asn (N) - 9 3.2%
Asp (D) - 22 7.8%
Cys (C) - 4 1.4%
Gln (Q) - 12 4.3%
Glu (E) - 12 4.3%
Gly (G) - 15 5.3%
His (H) - 5 1.8%
Ile (I) - 12 4.3%
Leu (L) - 30 10.6%
Lys (K) - 7 2.5%
Met (M) - 7 2.5%
Phe (F) - 12 4.3%
Pro (P) - 14 5.0%
Ser (S) - 19 6.7%
Thr (T) - 13 4.6%
Trp (W) - 4 1.4%
Tyr (Y) - 16 5.7%
Val (V) - 25 8.9%
Pyl (O) 0 0.0%
Sec (U) 0 0.0%
(B) 0 0.0%
(Z) 0 0.0%
(X) 0 0.0%
The physico-chemical properties of DORNASE alpha are:
The section below shows the physical properties of the protein DORNASE ALFA. This
protein is made up of 282 amino acids with an average molecular weight of 31434 kDa. The
theoretical pH= 4.71 suggests that the protein is slightly acidic in nature. Thus factor has been
further proved by the fact that this protein has a large number of acidic amino acids which
makes it acidic. These are visible in the form of more number of negatively charged amino
acids.
Number of amino acids: 282
Molecular weight: 31433.73
Theoretical pI: 4.71
Amino acid composition with percentages are mentioned here:
Ala (A) - 30 10.6%
Arg (R) - 14 5.0%
Asn (N) - 9 3.2%
Asp (D) - 22 7.8%
Cys (C) - 4 1.4%
Gln (Q) - 12 4.3%
Glu (E) - 12 4.3%
Gly (G) - 15 5.3%
His (H) - 5 1.8%
Ile (I) - 12 4.3%
Leu (L) - 30 10.6%
Lys (K) - 7 2.5%
Met (M) - 7 2.5%
Phe (F) - 12 4.3%
Pro (P) - 14 5.0%
Ser (S) - 19 6.7%
Thr (T) - 13 4.6%
Trp (W) - 4 1.4%
Tyr (Y) - 16 5.7%
Val (V) - 25 8.9%
Pyl (O) 0 0.0%
Sec (U) 0 0.0%
(B) 0 0.0%
(Z) 0 0.0%
(X) 0 0.0%
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
1BIOLOGY
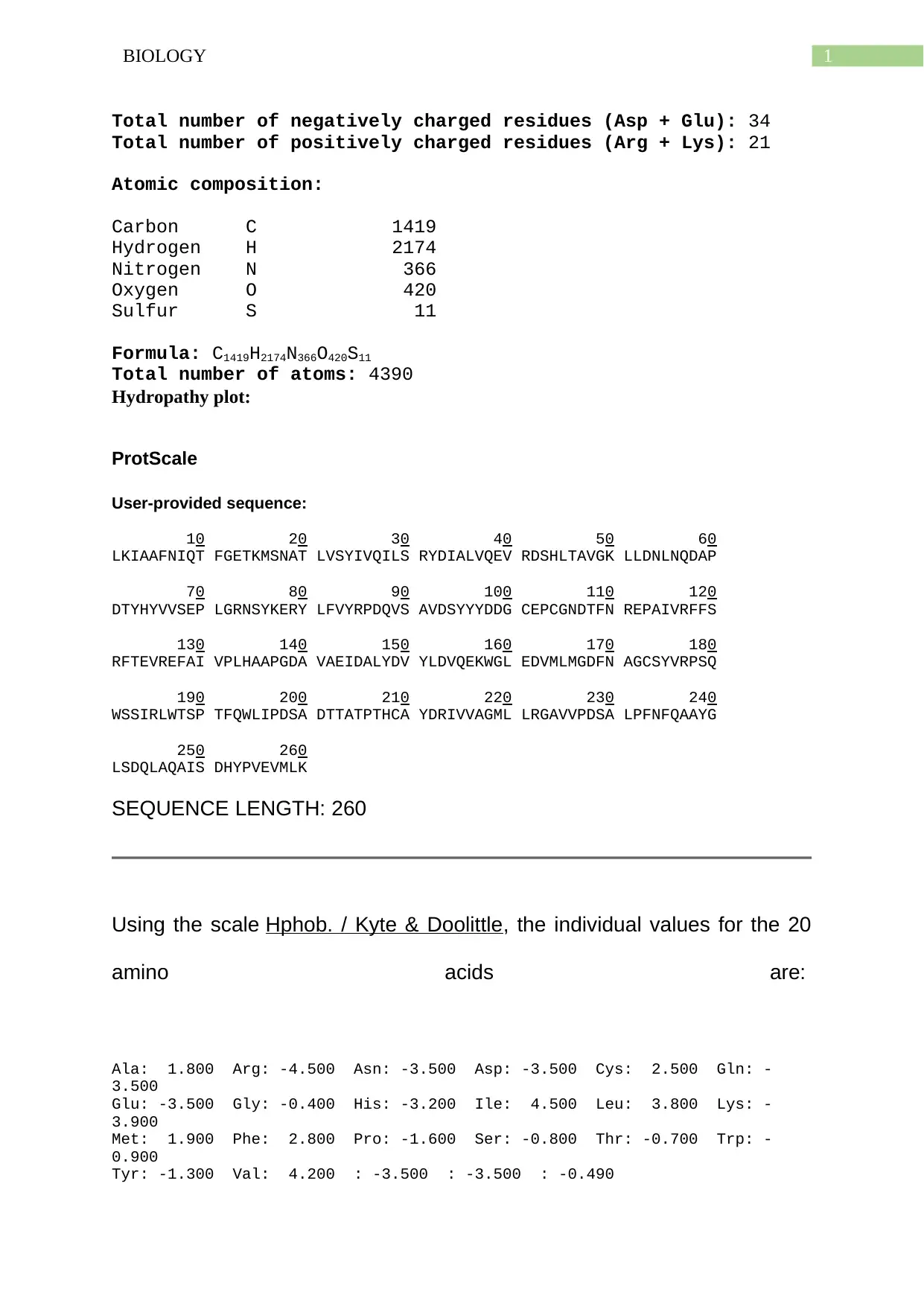
Total number of negatively charged residues (Asp + Glu): 34
Total number of positively charged residues (Arg + Lys): 21
Atomic composition:
Carbon C 1419
Hydrogen H 2174
Nitrogen N 366
Oxygen O 420
Sulfur S 11
Formula: C1419H2174N366O420S11
Total number of atoms: 4390
Hydropathy plot:
ProtScale
User-provided sequence:
10 20 30 40 50 60
LKIAAFNIQT FGETKMSNAT LVSYIVQILS RYDIALVQEV RDSHLTAVGK LLDNLNQDAP
70 80 90 100 110 120
DTYHYVVSEP LGRNSYKERY LFVYRPDQVS AVDSYYYDDG CEPCGNDTFN REPAIVRFFS
130 140 150 160 170 180
RFTEVREFAI VPLHAAPGDA VAEIDALYDV YLDVQEKWGL EDVMLMGDFN AGCSYVRPSQ
190 200 210 220 230 240
WSSIRLWTSP TFQWLIPDSA DTTATPTHCA YDRIVVAGML LRGAVVPDSA LPFNFQAAYG
250 260
LSDQLAQAIS DHYPVEVMLK
SEQUENCE LENGTH: 260
Using the scale Hphob. / Kyte & Doolittle, the individual values for the 20
amino acids are:
Ala: 1.800 Arg: -4.500 Asn: -3.500 Asp: -3.500 Cys: 2.500 Gln: -
3.500
Glu: -3.500 Gly: -0.400 His: -3.200 Ile: 4.500 Leu: 3.800 Lys: -
3.900
Met: 1.900 Phe: 2.800 Pro: -1.600 Ser: -0.800 Thr: -0.700 Trp: -
0.900
Tyr: -1.300 Val: 4.200 : -3.500 : -3.500 : -0.490
Total number of negatively charged residues (Asp + Glu): 34
Total number of positively charged residues (Arg + Lys): 21
Atomic composition:
Carbon C 1419
Hydrogen H 2174
Nitrogen N 366
Oxygen O 420
Sulfur S 11
Formula: C1419H2174N366O420S11
Total number of atoms: 4390
Hydropathy plot:
ProtScale
User-provided sequence:
10 20 30 40 50 60
LKIAAFNIQT FGETKMSNAT LVSYIVQILS RYDIALVQEV RDSHLTAVGK LLDNLNQDAP
70 80 90 100 110 120
DTYHYVVSEP LGRNSYKERY LFVYRPDQVS AVDSYYYDDG CEPCGNDTFN REPAIVRFFS
130 140 150 160 170 180
RFTEVREFAI VPLHAAPGDA VAEIDALYDV YLDVQEKWGL EDVMLMGDFN AGCSYVRPSQ
190 200 210 220 230 240
WSSIRLWTSP TFQWLIPDSA DTTATPTHCA YDRIVVAGML LRGAVVPDSA LPFNFQAAYG
250 260
LSDQLAQAIS DHYPVEVMLK
SEQUENCE LENGTH: 260
Using the scale Hphob. / Kyte & Doolittle, the individual values for the 20
amino acids are:
Ala: 1.800 Arg: -4.500 Asn: -3.500 Asp: -3.500 Cys: 2.500 Gln: -
3.500
Glu: -3.500 Gly: -0.400 His: -3.200 Ile: 4.500 Leu: 3.800 Lys: -
3.900
Met: 1.900 Phe: 2.800 Pro: -1.600 Ser: -0.800 Thr: -0.700 Trp: -
0.900
Tyr: -1.300 Val: 4.200 : -3.500 : -3.500 : -0.490
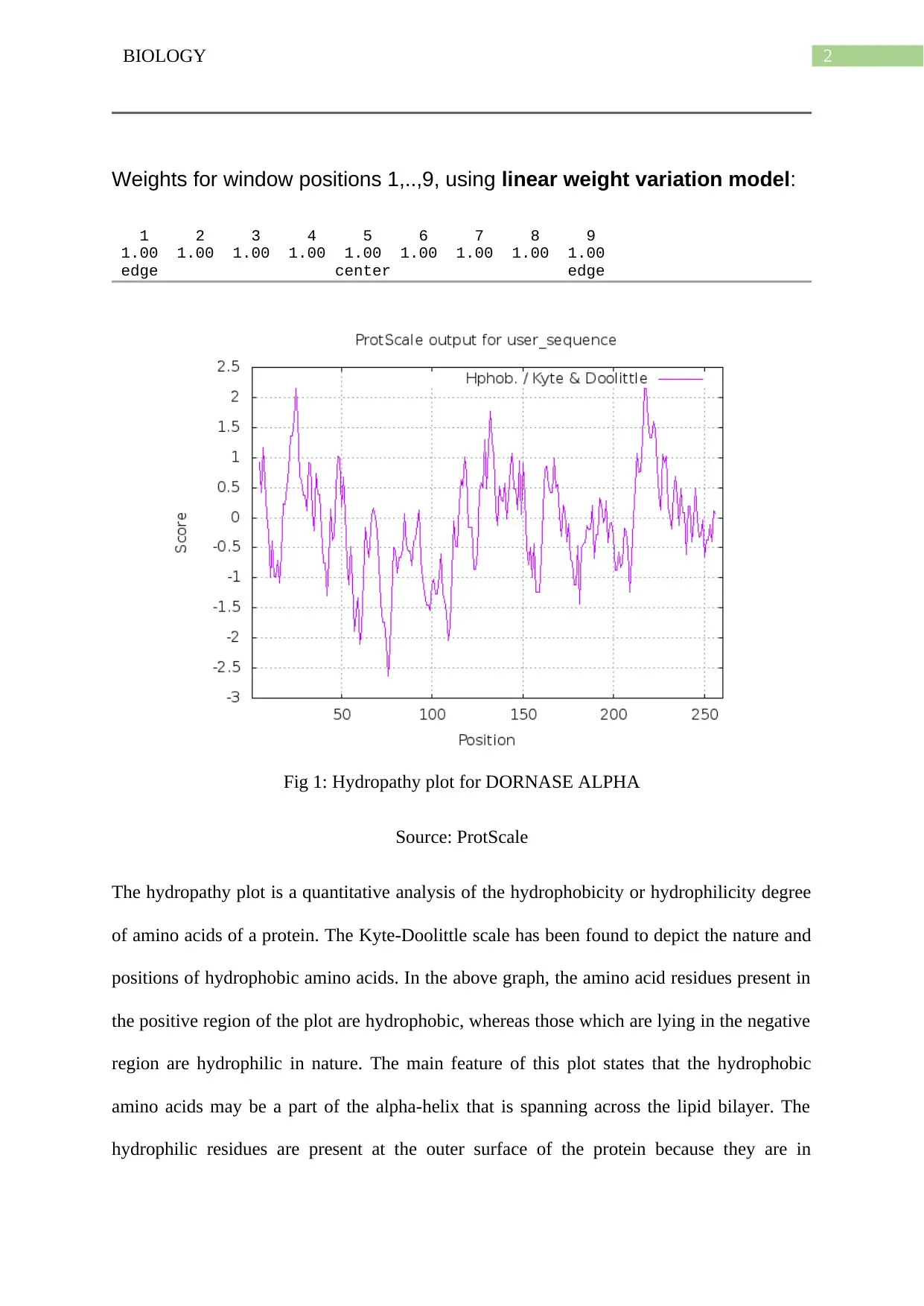
2BIOLOGY
Weights for window positions 1,..,9, using linear weight variation model:
1 2 3 4 5 6 7 8 9
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00
edge center edge
Fig 1: Hydropathy plot for DORNASE ALPHA
Source: ProtScale
The hydropathy plot is a quantitative analysis of the hydrophobicity or hydrophilicity degree
of amino acids of a protein. The Kyte-Doolittle scale has been found to depict the nature and
positions of hydrophobic amino acids. In the above graph, the amino acid residues present in
the positive region of the plot are hydrophobic, whereas those which are lying in the negative
region are hydrophilic in nature. The main feature of this plot states that the hydrophobic
amino acids may be a part of the alpha-helix that is spanning across the lipid bilayer. The
hydrophilic residues are present at the outer surface of the protein because they are in
Weights for window positions 1,..,9, using linear weight variation model:
1 2 3 4 5 6 7 8 9
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00
edge center edge
Fig 1: Hydropathy plot for DORNASE ALPHA
Source: ProtScale
The hydropathy plot is a quantitative analysis of the hydrophobicity or hydrophilicity degree
of amino acids of a protein. The Kyte-Doolittle scale has been found to depict the nature and
positions of hydrophobic amino acids. In the above graph, the amino acid residues present in
the positive region of the plot are hydrophobic, whereas those which are lying in the negative
region are hydrophilic in nature. The main feature of this plot states that the hydrophobic
amino acids may be a part of the alpha-helix that is spanning across the lipid bilayer. The
hydrophilic residues are present at the outer surface of the protein because they are in
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3BIOLOGY
constant contact with the solvent. The highest hydrophobic peak at the region 250 shows that
the residue is completely embedded inside the protein core. The highest negative peak at 75
shows that the region is completely outside the protein core, in contact with the surrounding
solvent (hydrophilic).
3. How the protein was initially purified from its natural source when it
was first discovered:
There is no initial purification processes or historical purification processes of the protein
since this enzyme is novel and only has recombinant methods of preparation and purification
that will be discussed in the next part. The same process which has been used in the next
section was used in 1973, during the purification of Dornase alpha. Initial drug preparation
was done by using recombinant DNA technology in CHO, followed by selection of the
recombinant cells. After the cells are selected, protein is extracted from the cells. However,
this protein is a crude extract and contains contaminations of trypsin and chymotrypsin. Thus,
gel filtration chromatography was used to separate the recombinant protein at an initial stage
which has been found to be modified today on being substituted by affinity chromatography.
constant contact with the solvent. The highest hydrophobic peak at the region 250 shows that
the residue is completely embedded inside the protein core. The highest negative peak at 75
shows that the region is completely outside the protein core, in contact with the surrounding
solvent (hydrophilic).
3. How the protein was initially purified from its natural source when it
was first discovered:
There is no initial purification processes or historical purification processes of the protein
since this enzyme is novel and only has recombinant methods of preparation and purification
that will be discussed in the next part. The same process which has been used in the next
section was used in 1973, during the purification of Dornase alpha. Initial drug preparation
was done by using recombinant DNA technology in CHO, followed by selection of the
recombinant cells. After the cells are selected, protein is extracted from the cells. However,
this protein is a crude extract and contains contaminations of trypsin and chymotrypsin. Thus,
gel filtration chromatography was used to separate the recombinant protein at an initial stage
which has been found to be modified today on being substituted by affinity chromatography.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4BIOLOGY
4. How is the same protein now prepared for an industrial use, or in
recombinant form:
Dornase alfa is a recombinant DNase I enzyme of human beings which selectively cleaves
DNA. Dornase alfa is a highly purified solution containing the recombinant DNase I protein
of human beings. This is an enzyme solution which is responsible for the selective cleavage
of DNA. This enzyme has been found to hydrolyse the DNA which has been found to be
present in the sputum/mucus of the patients suffering from cystic fibrosis and has been found
to reduce viscosity in lungs in order to facilitate airway clearance. The trade name of this
enzyme is Pulmozyme. Thus, it can be stated that the definition itself justifies the purification
process. Only recombinant DNA technology can be used to prepare and purify it. This protein
has been first produced inside Chinese Hamster Ovary or the CHO cells which contained the
DNA for encoding human DNase I.
Flow chart:
Chinese hamster
ovary cells were
isolated.
The cells were grown
on selective culture
media.
In the media,
human cDNA
was added.
The process
is known as
transfection.
4. How is the same protein now prepared for an industrial use, or in
recombinant form:
Dornase alfa is a recombinant DNase I enzyme of human beings which selectively cleaves
DNA. Dornase alfa is a highly purified solution containing the recombinant DNase I protein
of human beings. This is an enzyme solution which is responsible for the selective cleavage
of DNA. This enzyme has been found to hydrolyse the DNA which has been found to be
present in the sputum/mucus of the patients suffering from cystic fibrosis and has been found
to reduce viscosity in lungs in order to facilitate airway clearance. The trade name of this
enzyme is Pulmozyme. Thus, it can be stated that the definition itself justifies the purification
process. Only recombinant DNA technology can be used to prepare and purify it. This protein
has been first produced inside Chinese Hamster Ovary or the CHO cells which contained the
DNA for encoding human DNase I.
Flow chart:
Chinese hamster
ovary cells were
isolated.
The cells were grown
on selective culture
media.
In the media,
human cDNA
was added.
The process
is known as
transfection.
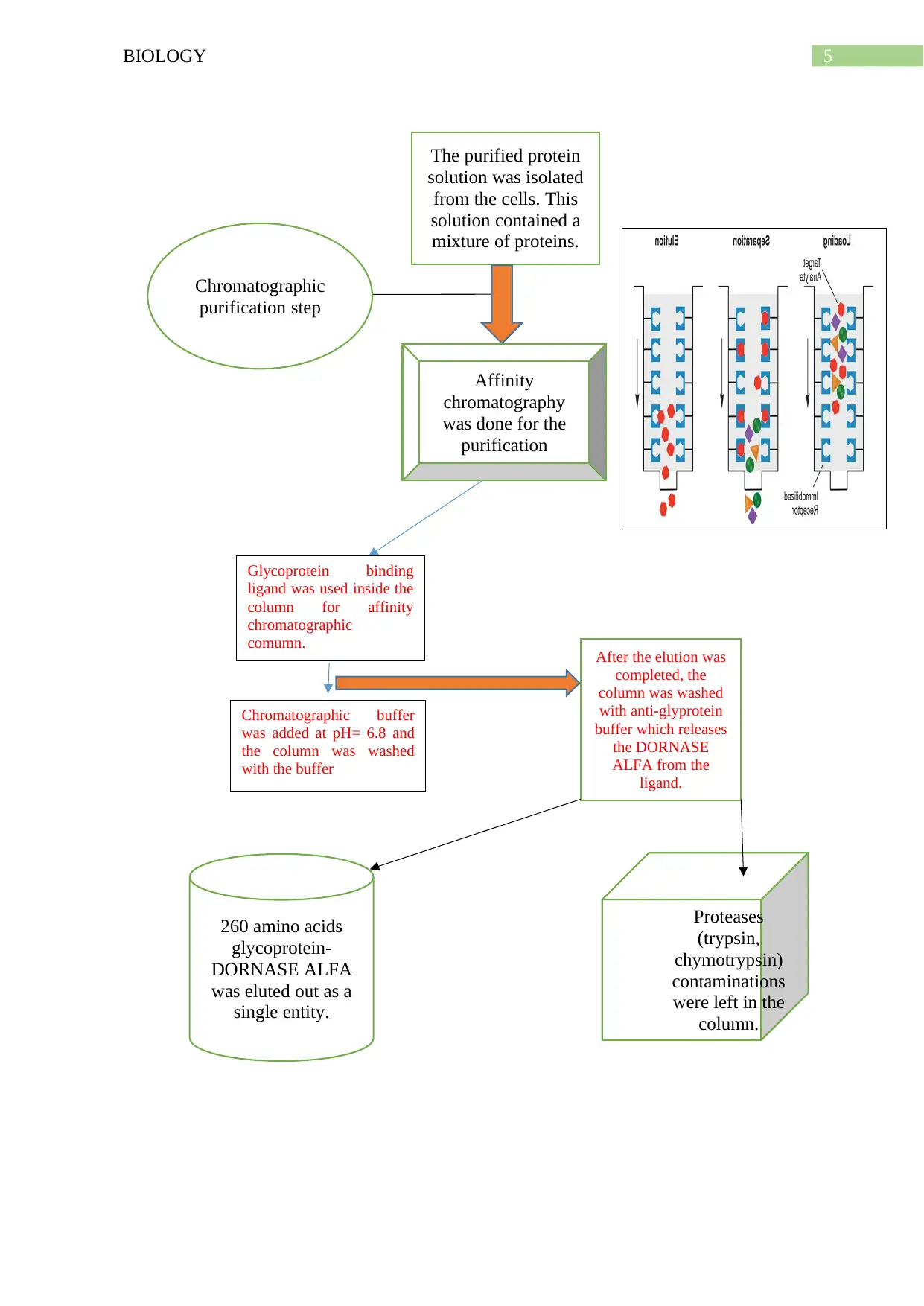
5BIOLOGY
The purified protein
solution was isolated
from the cells. This
solution contained a
mixture of proteins.
Affinity
chromatography
was done for the
purification
260 amino acids
glycoprotein-
DORNASE ALFA
was eluted out as a
single entity.
Proteases
(trypsin,
chymotrypsin)
contaminations
were left in the
column.
Chromatographic
purification step
Glycoprotein binding
ligand was used inside the
column for affinity
chromatographic
comumn.
Chromatographic buffer
was added at pH= 6.8 and
the column was washed
with the buffer
After the elution was
completed, the
column was washed
with anti-glyprotein
buffer which releases
the DORNASE
ALFA from the
ligand.
The purified protein
solution was isolated
from the cells. This
solution contained a
mixture of proteins.
Affinity
chromatography
was done for the
purification
260 amino acids
glycoprotein-
DORNASE ALFA
was eluted out as a
single entity.
Proteases
(trypsin,
chymotrypsin)
contaminations
were left in the
column.
Chromatographic
purification step
Glycoprotein binding
ligand was used inside the
column for affinity
chromatographic
comumn.
Chromatographic buffer
was added at pH= 6.8 and
the column was washed
with the buffer
After the elution was
completed, the
column was washed
with anti-glyprotein
buffer which releases
the DORNASE
ALFA from the
ligand.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
