University Healthcare: NURS5069 DVT Treatment Literature Search
VerifiedAdded on 2022/08/28
|33
|3717
|15
Homework Assignment
AI Summary
This assignment presents a literature search strategy conducted to compare the effectiveness of heparin and clexane injections in preventing thrombus reformation among adult patients with deep venous thrombosis (DVT). The research question was developed using the PICOT framework. The literature review involved searching the CINAHL database using keywords related to DVT, heparin, clexane, and thrombus. Boolean operators were used to refine the search. Inclusion criteria included peer-reviewed articles published in English between 2005 and 2020, focusing on primary research designs. Exclusion criteria removed animal studies, case studies, and secondary research. The PRISMA flowchart was used to filter articles, resulting in the inclusion of 6 articles. The CINAHL database was chosen for its comprehensive coverage of nursing and allied health literature. Randomised control trials and cohort studies were selected to establish effectiveness and associations. The PICOT framework was used to frame the research question and ensure the relevance of retrieved articles. Justification is provided for the selection of search terms, inclusion/exclusion criteria, and the systematic approach employed to minimize bias and ensure replicability. The assignment includes a detailed methodology, justification, and references.

Running head: HEALTHCARE
NURS5069 Research in Nursing and Health Care: Literature Search Strategy
Name of the Student
Name of the University
Author Note
NURS5069 Research in Nursing and Health Care: Literature Search Strategy
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1HEALTHCARE
Research question
Does the use of heparin injection decrease the re-formation of thrombus, in comparison
to clexane/enoxaparin injection amongst adult patients with deep venous thrombosis (DVT)?
The initial step in this literature review was development of the aforementioned research
question based on the PICOT framework (Speckman & Friedly, 2019). Below given are the
concepts that correspond to the components of this framework:
P- Adult patients with DVT
Heparin injection
C- Clexane injection
O- Reduce the reformation of thrombus
T- Primary research
With the aim of identifying evidences for exploring the comparative effectiveness of heparin
injection and clexane injection amongst DVT patients, regarding the re-formation of thrombus,
an extensive literature review was conducted, based on the electronic databases of Cumulative
Index to Nursing and Allied Health Literature (CINAHL) (Hopia & Heikkilä, 2019). The
following keywords were used in these databases for searching articles related to the
aforementioned research question: deep venous thrombosis, clexane/enoxaparin, heparin, and
thrombus. Variations and modifications of the keywords (as listed in appendix 1) were also
entered in the database, together with the primary words, and were combined by using the
boolean operators. Use of the boolean operator ‘AND’ narrowed down the search by extracting
all articles containing the keywords that were joined together, which eventually appeared in the
Research question
Does the use of heparin injection decrease the re-formation of thrombus, in comparison
to clexane/enoxaparin injection amongst adult patients with deep venous thrombosis (DVT)?
The initial step in this literature review was development of the aforementioned research
question based on the PICOT framework (Speckman & Friedly, 2019). Below given are the
concepts that correspond to the components of this framework:
P- Adult patients with DVT
Heparin injection
C- Clexane injection
O- Reduce the reformation of thrombus
T- Primary research
With the aim of identifying evidences for exploring the comparative effectiveness of heparin
injection and clexane injection amongst DVT patients, regarding the re-formation of thrombus,
an extensive literature review was conducted, based on the electronic databases of Cumulative
Index to Nursing and Allied Health Literature (CINAHL) (Hopia & Heikkilä, 2019). The
following keywords were used in these databases for searching articles related to the
aforementioned research question: deep venous thrombosis, clexane/enoxaparin, heparin, and
thrombus. Variations and modifications of the keywords (as listed in appendix 1) were also
entered in the database, together with the primary words, and were combined by using the
boolean operators. Use of the boolean operator ‘AND’ narrowed down the search by extracting
all articles containing the keywords that were joined together, which eventually appeared in the

2HEALTHCARE
result list. In contrast, the ‘OR’ operator broadened the literature search by producing any of the
search terms or phrases that were connected (Scells, Zuccon & Koopman, 2019).
The results of literature search were then limited using predetermined criteria such as, peer
reviewed articles, publication in English language, and date of publication between 2005 and
2020. The results obtained from the electronic databases were also filtered with the aim of
prioritising articles that work based on primary research design (Eriksen & Frandsen, 2018).
This was done using the filter of publication type and ‘randomised control trial’ and ‘clinical
trial’ were selected from the CINAHL database. Exclusion criteria resulted in removal of articles
that were based on animal studies, case study, systematic review, book chapters, and clinical
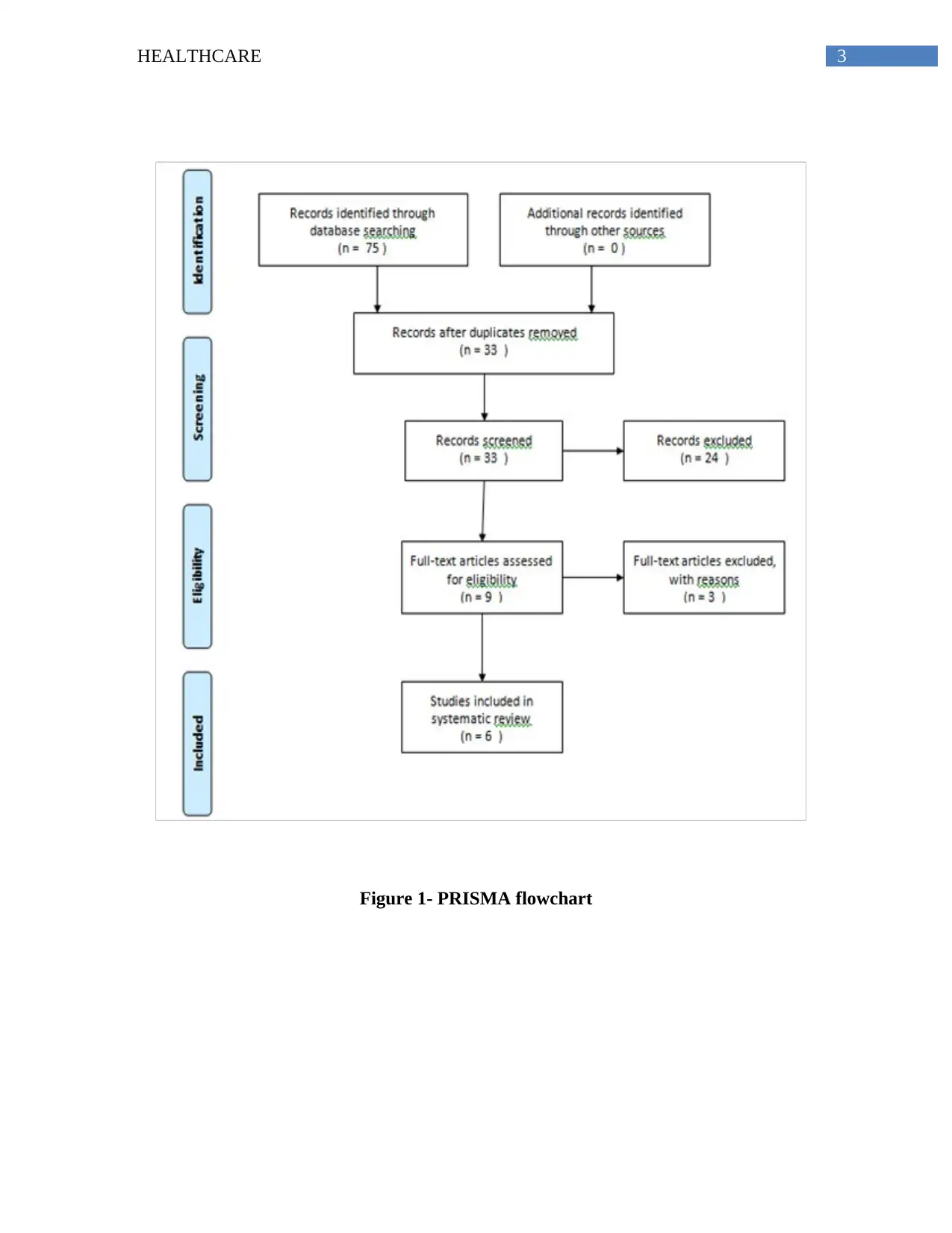
guidelines. This resulted in selection of an estimated 75 articles that were then subjected to
manual testing as presented in the PRISMA flowchart.
Articles were only incorporated in the literature review if they evaluated both the
intervention and its comparison amongst patients suffering from deep vein thrombosis. The
citations that had been manually filtered also facilitated in elimination of articles, which were
duplicates, not applicable to the research question, or were based on meta-analysis, or
investigated some other impacts of heparin and clexane or enoxaparin on the patients. This was
followed by conducting of manual search of the bibliography of the extracted articles, for
identification of the evidences, which might have been missed from the search results produced
by the electronic database (McGowan et al., 2016). At the end the search strategy, only 6 articles
were included in the final sample, based on their relevance to the topic.
result list. In contrast, the ‘OR’ operator broadened the literature search by producing any of the
search terms or phrases that were connected (Scells, Zuccon & Koopman, 2019).
The results of literature search were then limited using predetermined criteria such as, peer
reviewed articles, publication in English language, and date of publication between 2005 and
2020. The results obtained from the electronic databases were also filtered with the aim of
prioritising articles that work based on primary research design (Eriksen & Frandsen, 2018).
This was done using the filter of publication type and ‘randomised control trial’ and ‘clinical
trial’ were selected from the CINAHL database. Exclusion criteria resulted in removal of articles
that were based on animal studies, case study, systematic review, book chapters, and clinical
guidelines. This resulted in selection of an estimated 75 articles that were then subjected to
manual testing as presented in the PRISMA flowchart.
Articles were only incorporated in the literature review if they evaluated both the
intervention and its comparison amongst patients suffering from deep vein thrombosis. The
citations that had been manually filtered also facilitated in elimination of articles, which were
duplicates, not applicable to the research question, or were based on meta-analysis, or
investigated some other impacts of heparin and clexane or enoxaparin on the patients. This was
followed by conducting of manual search of the bibliography of the extracted articles, for
identification of the evidences, which might have been missed from the search results produced
by the electronic database (McGowan et al., 2016). At the end the search strategy, only 6 articles
were included in the final sample, based on their relevance to the topic.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
3HEALTHCARE
Figure 1- PRISMA flowchart
Figure 1- PRISMA flowchart
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4HEALTHCARE
Justification
The CINAHL database was selected since it is an index of prominent journal articles on
the domains of allied health, nursing, healthcare, and biomedicine, and it contains articles that
had been published from 1937 till the present date. Another reason behind selection of CINAHL
was that it contains the full text versions of not less than 1,200 journals (Glanville, Dooley,
Wisniewski, Foxlee & Noel‐Storr, 2019). Randomised control trials have been identified as the
best design for drawing conclusions to questions with two different interventions, since they help
in establishing effectiveness of one intervention over the other (Ishtiaq, 2019). Cohort studies
were also selected since it helps to establish associations between health outcomes and risk
factors, by investigating amongst a group of individuals, who share a defining feature. As
described in the previous sections, search terms and keywords were combined and entered in the
electronic databases since they represented the principal components of the phenomenon,
currently under investigation and provided clear description of the topics. Use of MeSH subject
headings also prevented missing of prospective studies that might have proved beneficial for
drawing conclusions to the research question (Smalheiser & Bonifield, 2016).
Selection of an appropriate research question forms of core element of both qualitative
and quantitative research. The PICOT framework or process refers to a mnemonic that is
particularly used in evidence based medicine, with the aim of framing and drawing relevant
conclusions to healthcare or clinical question. The acronym stands for
patient/problem/population, intervention, comparison/control, and outcomes (Scells et al., 2017).
The PICOT framework also provided intelligibility of the thought process, and facilitated
assessing if all the articles that had been retrieved from the databases were pertinent to the
question. Limiting the patient population to those suffering from DVT resulted in exclusion of
Justification
The CINAHL database was selected since it is an index of prominent journal articles on
the domains of allied health, nursing, healthcare, and biomedicine, and it contains articles that
had been published from 1937 till the present date. Another reason behind selection of CINAHL
was that it contains the full text versions of not less than 1,200 journals (Glanville, Dooley,
Wisniewski, Foxlee & Noel‐Storr, 2019). Randomised control trials have been identified as the
best design for drawing conclusions to questions with two different interventions, since they help
in establishing effectiveness of one intervention over the other (Ishtiaq, 2019). Cohort studies
were also selected since it helps to establish associations between health outcomes and risk
factors, by investigating amongst a group of individuals, who share a defining feature. As
described in the previous sections, search terms and keywords were combined and entered in the
electronic databases since they represented the principal components of the phenomenon,
currently under investigation and provided clear description of the topics. Use of MeSH subject
headings also prevented missing of prospective studies that might have proved beneficial for
drawing conclusions to the research question (Smalheiser & Bonifield, 2016).
Selection of an appropriate research question forms of core element of both qualitative
and quantitative research. The PICOT framework or process refers to a mnemonic that is
particularly used in evidence based medicine, with the aim of framing and drawing relevant
conclusions to healthcare or clinical question. The acronym stands for
patient/problem/population, intervention, comparison/control, and outcomes (Scells et al., 2017).
The PICOT framework also provided intelligibility of the thought process, and facilitated
assessing if all the articles that had been retrieved from the databases were pertinent to the
question. Limiting the patient population to those suffering from DVT resulted in exclusion of

5HEALTHCARE
articles with patients subjected to any surgery or other clinical procedures, who had been
assessed for the impacts of heparin or clexane.
Inclusion criteria consist of characteristics that should be present in potential articles that
are to be incorporated in a review (McGowan et al., 2016). This is in contrast to exclusion
criteria that consist of characteristics, which result in ineligibility of potential articles from being
integrated in a study. Articles that had been published within the last 15 years were considered
eligible during this literature review since they supported evidence-based clinical decision
making. While conducting an extensive search on biomedical literature, animal studies were also
encountered. However, they were excluded from the results, since they do not directly correlate
to the human population. Additionally, secondary research we are also excluded since it is
difficult to know about accuracy of the presented information, and publication bias can lead to
exaggeration of positive results, since the findings typically do not include negative or neutral
results (Quinlan, Babin, Carr & Griffin, 2019).
My present understanding about conducting a literature review is based on the fact that
scholarly evidences must be correctly identified, critically appraised, and correlated with the
research question. Use of appropriate search terms helps us in locating articles that are exactly
relevant to the topic of interest. In addition, the use of a predetermined inclusion and exclusion
criteria also help us to save time and effort that would have been lost while going through
irrelevant articles. Imposing filters and limiters while searching for scholarly evidences also add
to the study rigour and help in elimination of articles that do not provide definite conclusions to
the research question. Adopting a systematic approach also eliminated the risk of bias, and
helped in appropriate reporting of the search results, which could be easily replicated by any
other researcher.
articles with patients subjected to any surgery or other clinical procedures, who had been
assessed for the impacts of heparin or clexane.
Inclusion criteria consist of characteristics that should be present in potential articles that
are to be incorporated in a review (McGowan et al., 2016). This is in contrast to exclusion
criteria that consist of characteristics, which result in ineligibility of potential articles from being
integrated in a study. Articles that had been published within the last 15 years were considered
eligible during this literature review since they supported evidence-based clinical decision
making. While conducting an extensive search on biomedical literature, animal studies were also
encountered. However, they were excluded from the results, since they do not directly correlate
to the human population. Additionally, secondary research we are also excluded since it is
difficult to know about accuracy of the presented information, and publication bias can lead to
exaggeration of positive results, since the findings typically do not include negative or neutral
results (Quinlan, Babin, Carr & Griffin, 2019).
My present understanding about conducting a literature review is based on the fact that
scholarly evidences must be correctly identified, critically appraised, and correlated with the
research question. Use of appropriate search terms helps us in locating articles that are exactly
relevant to the topic of interest. In addition, the use of a predetermined inclusion and exclusion
criteria also help us to save time and effort that would have been lost while going through
irrelevant articles. Imposing filters and limiters while searching for scholarly evidences also add
to the study rigour and help in elimination of articles that do not provide definite conclusions to
the research question. Adopting a systematic approach also eliminated the risk of bias, and
helped in appropriate reporting of the search results, which could be easily replicated by any
other researcher.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6HEALTHCARE
References
Eriksen, M. B., & Frandsen, T. F. (2018). The impact of patient, intervention, comparison,
outcome (PICO) as a search strategy tool on literature search quality: a systematic
review. Journal of the Medical Library Association: JMLA, 106(4), 420.
dx.doi.org/10.5195/jmla.2018.345
Glanville, J., Dooley, G., Wisniewski, S., Foxlee, R., & Noel‐Storr, A. (2019). Development of a
search filter to identify reports of controlled clinical trials within CINAHL Plus. Health
Information & Libraries Journal, 36(1), 73-90. https://doi.org/10.1111/hir.12251
Hopia, H., & Heikkilä, J. (2019). Nursing research priorities based on CINAHL database: A
scoping review. Nursing Open. https://doi.org/10.1002/nop2.428
Ishtiaq, M. (2019). Book Review Creswell, JW (2014). Research Design: Qualitative,
Quantitative and Mixed Methods Approaches . Thousand Oaks, CA: Sage. English
Language Teaching, 12(5), 40. DOI: 10.5539/elt.v12n5p40
McGowan, J., Sampson, M., Salzwedel, D. M., Cogo, E., Foerster, V., & Lefebvre, C. (2016).
PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of
clinical epidemiology, 75, 40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
Quinlan, C., Babin, B., Carr, J., & Griffin, M. (2019). Business research methods. South Western
Cengage. https://dora.dmu.ac.uk/handle/2086/16559
Scells, H., Zuccon, G., & Koopman, B. (2019, May). Automatic boolean query refinement for
systematic review literature search. In The World Wide Web Conference (pp. 1646-1656).
https://doi.org/10.1145/3308558.3313544
References
Eriksen, M. B., & Frandsen, T. F. (2018). The impact of patient, intervention, comparison,
outcome (PICO) as a search strategy tool on literature search quality: a systematic
review. Journal of the Medical Library Association: JMLA, 106(4), 420.
dx.doi.org/10.5195/jmla.2018.345
Glanville, J., Dooley, G., Wisniewski, S., Foxlee, R., & Noel‐Storr, A. (2019). Development of a
search filter to identify reports of controlled clinical trials within CINAHL Plus. Health
Information & Libraries Journal, 36(1), 73-90. https://doi.org/10.1111/hir.12251
Hopia, H., & Heikkilä, J. (2019). Nursing research priorities based on CINAHL database: A
scoping review. Nursing Open. https://doi.org/10.1002/nop2.428
Ishtiaq, M. (2019). Book Review Creswell, JW (2014). Research Design: Qualitative,
Quantitative and Mixed Methods Approaches . Thousand Oaks, CA: Sage. English
Language Teaching, 12(5), 40. DOI: 10.5539/elt.v12n5p40
McGowan, J., Sampson, M., Salzwedel, D. M., Cogo, E., Foerster, V., & Lefebvre, C. (2016).
PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of
clinical epidemiology, 75, 40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
Quinlan, C., Babin, B., Carr, J., & Griffin, M. (2019). Business research methods. South Western
Cengage. https://dora.dmu.ac.uk/handle/2086/16559
Scells, H., Zuccon, G., & Koopman, B. (2019, May). Automatic boolean query refinement for
systematic review literature search. In The World Wide Web Conference (pp. 1646-1656).
https://doi.org/10.1145/3308558.3313544
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7HEALTHCARE
Scells, H., Zuccon, G., Koopman, B., Deacon, A., Azzopardi, L., & Geva, S. (2017, November).
Integrating the framing of clinical questions via PICO into the retrieval of medical
literature for systematic reviews. In Proceedings of the 2017 ACM on Conference on
Information and Knowledge Management (pp. 2291-2294).
https://doi.org/10.1145/3132847.3133080
Smalheiser, N. R., & Bonifield, G. (2016). Two Similarity Metrics for Medical Subject Headings
(MeSH):: An Aid to Biomedical Text Mining and Author Name Disambiguation. Journal
of biomedical discovery and collaboration, 7. doi: 10.5210/disco.v7i0.6654
Speckman, R. A., & Friedly, J. L. (2019). Asking Structured, Answerable Clinical Questions
Using the Population, Intervention/Comparator, Outcome (PICO)
Framework. Pm&r, 11(5), 548-553. https://doi.org/10.1002/pmrj.12116
Scells, H., Zuccon, G., Koopman, B., Deacon, A., Azzopardi, L., & Geva, S. (2017, November).
Integrating the framing of clinical questions via PICO into the retrieval of medical
literature for systematic reviews. In Proceedings of the 2017 ACM on Conference on
Information and Knowledge Management (pp. 2291-2294).
https://doi.org/10.1145/3132847.3133080
Smalheiser, N. R., & Bonifield, G. (2016). Two Similarity Metrics for Medical Subject Headings
(MeSH):: An Aid to Biomedical Text Mining and Author Name Disambiguation. Journal
of biomedical discovery and collaboration, 7. doi: 10.5210/disco.v7i0.6654
Speckman, R. A., & Friedly, J. L. (2019). Asking Structured, Answerable Clinical Questions
Using the Population, Intervention/Comparator, Outcome (PICO)
Framework. Pm&r, 11(5), 548-553. https://doi.org/10.1002/pmrj.12116
8HEALTHCARE
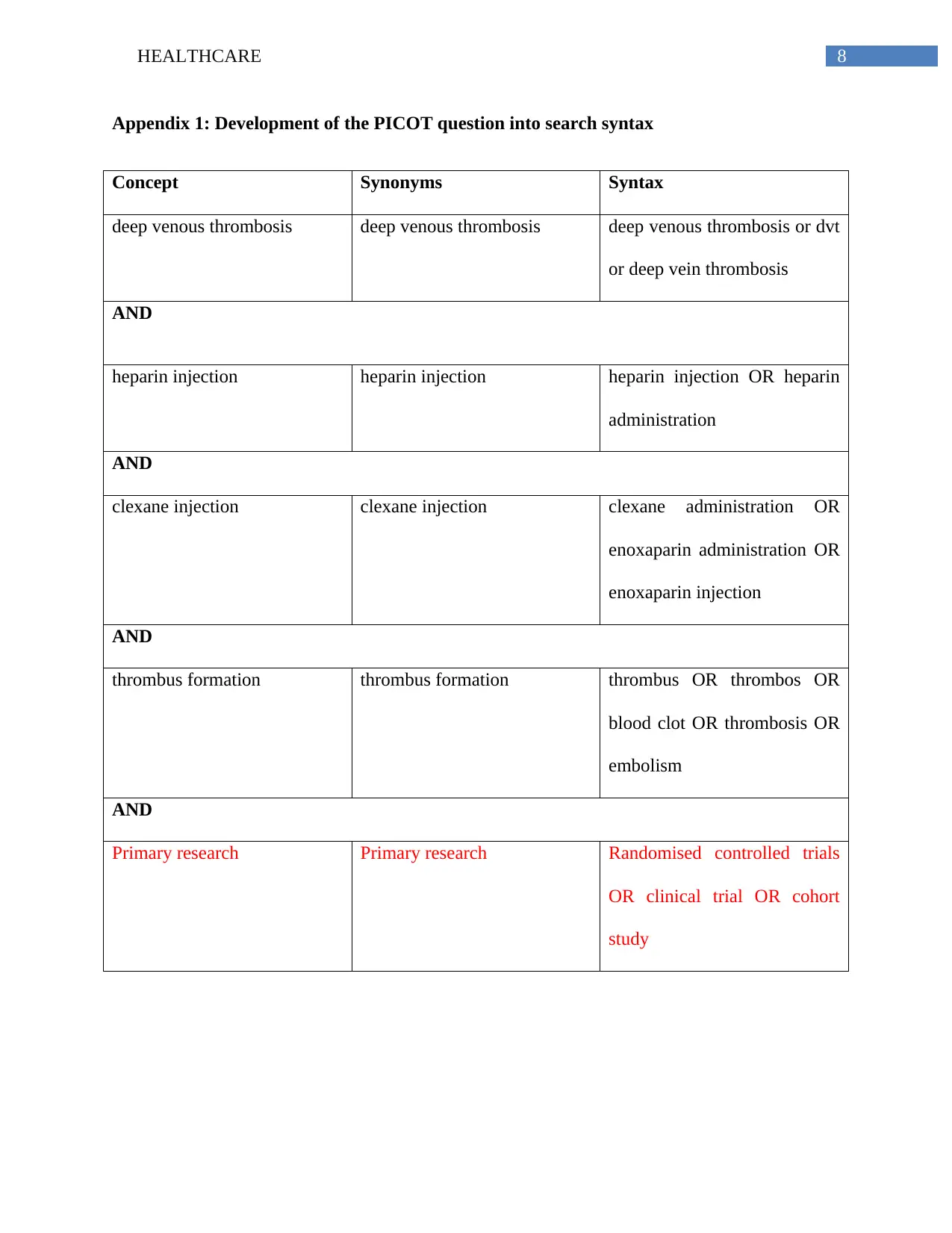
Appendix 1: Development of the PICOT question into search syntax
Concept Synonyms Syntax
deep venous thrombosis deep venous thrombosis deep venous thrombosis or dvt
or deep vein thrombosis
AND
heparin injection heparin injection heparin injection OR heparin
administration
AND
clexane injection clexane injection clexane administration OR
enoxaparin administration OR
enoxaparin injection
AND
thrombus formation thrombus formation thrombus OR thrombos OR
blood clot OR thrombosis OR
embolism
AND
Primary research Primary research Randomised controlled trials
OR clinical trial OR cohort
study
Appendix 1: Development of the PICOT question into search syntax
Concept Synonyms Syntax
deep venous thrombosis deep venous thrombosis deep venous thrombosis or dvt
or deep vein thrombosis
AND
heparin injection heparin injection heparin injection OR heparin
administration
AND
clexane injection clexane injection clexane administration OR
enoxaparin administration OR
enoxaparin injection
AND
thrombus formation thrombus formation thrombus OR thrombos OR
blood clot OR thrombosis OR
embolism
AND
Primary research Primary research Randomised controlled trials
OR clinical trial OR cohort
study
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9HEALTHCARE
Appendix 2: Articles obtained
Argenta, C., Ferreira, M. A. P., Sander, G. B., & Moreira, L. B. (2011). Short-term therapy with
enoxaparin or unfractionated heparin for venous thromboembolism in hospitalized
patients: utilization study and cost-minimization analysis. Value in Health, 14(5), S89-
S92. https://doi.org/10.1016/j.jval.2011.05.017
Arnold, J. D., Dart, B. W., Barker, D. E., Maxwell, R. A., Burkholder, H. C., Mejia, V. A., &
Smith, P. W. (2010). Gold Medal Forum Winner: unfractionated heparin three times a
day versus enoxaparin in the prevention of deep vein thrombosis in trauma patients. The
American surgeon, 76(6), 563.
https://search.proquest.com/openview/2aee27f5ab41fe36e03e19d6c1ecf7e5/1?pq-
origsite=gscholar&cbl=49079
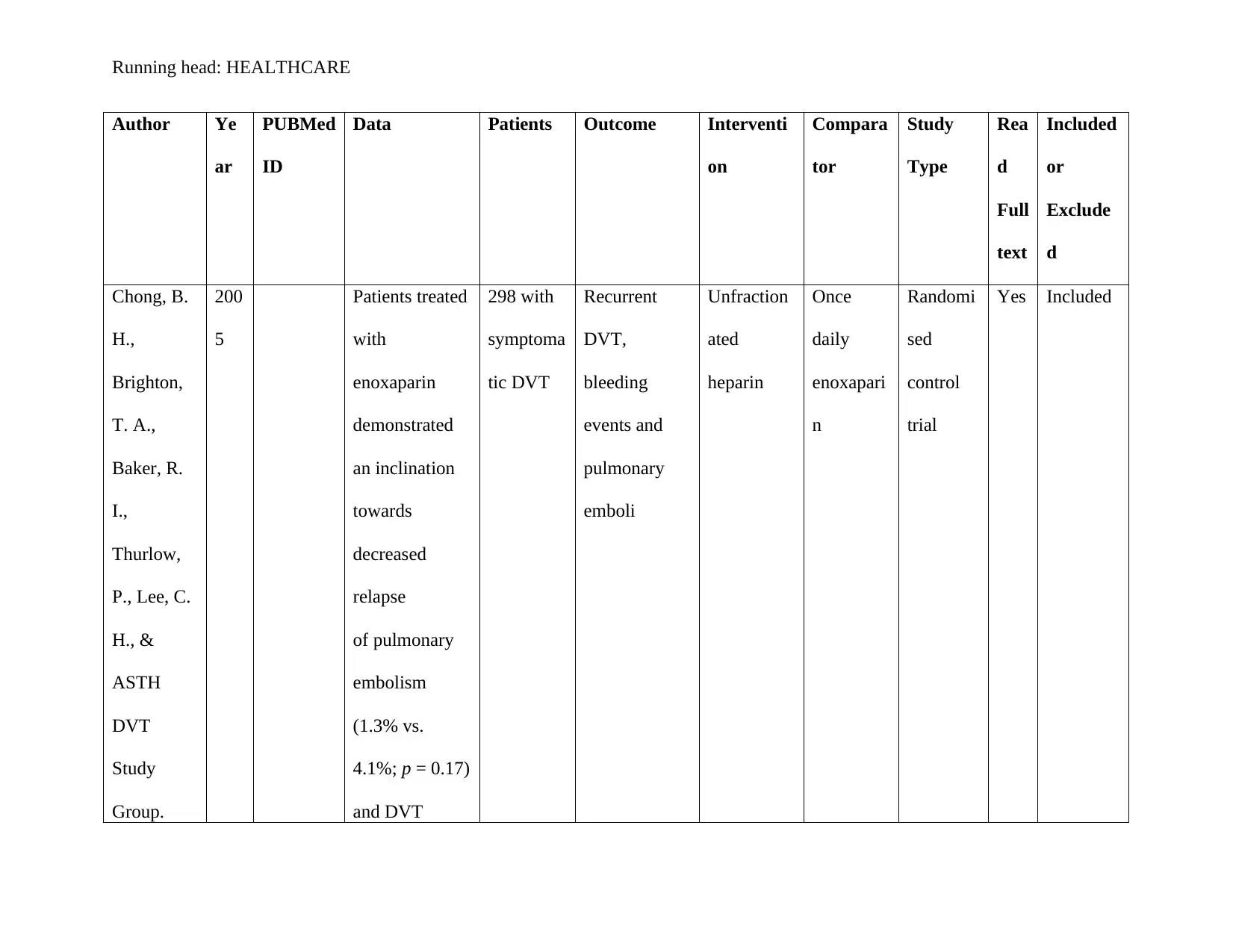
Chong, B. H., Brighton, T. A., Baker, R. I., Thurlow, P., Lee, C. H., & ASTH DVT Study
Group. (2005). Once-daily enoxaparin in the outpatient setting versus unfractionated
heparin in hospital for the treatment of symptomatic deep-vein thrombosis. Journal of
thrombosis and thrombolysis, 19(3), 173-181. https://doi.org/10.1007/s11239-005-1848-x
Kothari, S. N., Lambert, P. J., & Mathiason, M. A. (2007). A comparison of thromboembolic and
bleeding events following laparoscopic gastric bypass in patients treated with
prophylactic regimens of unfractionated heparin or enoxaparin. The American journal of
surgery, 194(6), 709-711. https://doi.org/10.1016/j.amjsurg.2007.08.018
Leykum, L., Pugh, J., Diuguid, D., & Papadopoulos, K. (2006). Cost utility of substituting
enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the
Appendix 2: Articles obtained
Argenta, C., Ferreira, M. A. P., Sander, G. B., & Moreira, L. B. (2011). Short-term therapy with
enoxaparin or unfractionated heparin for venous thromboembolism in hospitalized
patients: utilization study and cost-minimization analysis. Value in Health, 14(5), S89-
S92. https://doi.org/10.1016/j.jval.2011.05.017
Arnold, J. D., Dart, B. W., Barker, D. E., Maxwell, R. A., Burkholder, H. C., Mejia, V. A., &
Smith, P. W. (2010). Gold Medal Forum Winner: unfractionated heparin three times a
day versus enoxaparin in the prevention of deep vein thrombosis in trauma patients. The
American surgeon, 76(6), 563.
https://search.proquest.com/openview/2aee27f5ab41fe36e03e19d6c1ecf7e5/1?pq-
origsite=gscholar&cbl=49079
Chong, B. H., Brighton, T. A., Baker, R. I., Thurlow, P., Lee, C. H., & ASTH DVT Study
Group. (2005). Once-daily enoxaparin in the outpatient setting versus unfractionated
heparin in hospital for the treatment of symptomatic deep-vein thrombosis. Journal of
thrombosis and thrombolysis, 19(3), 173-181. https://doi.org/10.1007/s11239-005-1848-x
Kothari, S. N., Lambert, P. J., & Mathiason, M. A. (2007). A comparison of thromboembolic and
bleeding events following laparoscopic gastric bypass in patients treated with
prophylactic regimens of unfractionated heparin or enoxaparin. The American journal of
surgery, 194(6), 709-711. https://doi.org/10.1016/j.amjsurg.2007.08.018
Leykum, L., Pugh, J., Diuguid, D., & Papadopoulos, K. (2006). Cost utility of substituting
enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10HEALTHCARE
hospitalized medical patient. Journal of Hospital Medicine: An Official Publication of the
Society of Hospital Medicine, 1(3), 168-176. https://doi.org/10.1002/jhm.97
Olson, E. J., Bandle, J., Calvo, R. Y., Shackford, S. R., Dunne, C. E., Van Gent, J. M., ... & Sise,
C. B. (2015). Heparin versus enoxaparin for prevention of venous thromboembolism after
trauma: a randomized noninferiority trial. Journal of Trauma and Acute Care
Surgery, 79(6), 961-969. doi: 10.1097/TA.0000000000000750
Schobess, R., During, C., Bidlingmaier, C., Heinecke, A., Merkel, N., & Nowak-Gottl, U.
(2006). Long-term safety and efficacy data on childhood venous thrombosis treated with
a low molecular weight heparin: an open-label pilot study of once-daily versus twice-
daily enoxaparin administration. Haematologica, 91(12), 1701-1704.
http://www.haematologica.org/content/91/12/1701.short
Senaran, H., Acaroğlu, E., Özdemir, H. M., & Atilla, B. (2006). Enoxaparin and heparin
comparison of deep vein thrombosis prophylaxis in total hip replacement
patients. Archives of orthopaedic and trauma surgery, 126(1), 1-5.
https://doi.org/10.1007/s00402-005-0079-0
Sherman, D. G., Albers, G. W., Bladin, C., Fieschi, C., Gabbai, A. A., Kase, C. S., ... &
PREVAIL Investigators. (2007). The efficacy and safety of enoxaparin versus
unfractionated heparin for the prevention of venous thromboembolism after acute
ischaemic stroke (PREVAIL Study): an open-label randomised comparison. The
Lancet, 369(9570), 1347-1355. https://doi.org/10.1016/S0140-6736(07)60633-3
hospitalized medical patient. Journal of Hospital Medicine: An Official Publication of the
Society of Hospital Medicine, 1(3), 168-176. https://doi.org/10.1002/jhm.97
Olson, E. J., Bandle, J., Calvo, R. Y., Shackford, S. R., Dunne, C. E., Van Gent, J. M., ... & Sise,
C. B. (2015). Heparin versus enoxaparin for prevention of venous thromboembolism after
trauma: a randomized noninferiority trial. Journal of Trauma and Acute Care
Surgery, 79(6), 961-969. doi: 10.1097/TA.0000000000000750
Schobess, R., During, C., Bidlingmaier, C., Heinecke, A., Merkel, N., & Nowak-Gottl, U.
(2006). Long-term safety and efficacy data on childhood venous thrombosis treated with
a low molecular weight heparin: an open-label pilot study of once-daily versus twice-
daily enoxaparin administration. Haematologica, 91(12), 1701-1704.
http://www.haematologica.org/content/91/12/1701.short
Senaran, H., Acaroğlu, E., Özdemir, H. M., & Atilla, B. (2006). Enoxaparin and heparin
comparison of deep vein thrombosis prophylaxis in total hip replacement
patients. Archives of orthopaedic and trauma surgery, 126(1), 1-5.
https://doi.org/10.1007/s00402-005-0079-0
Sherman, D. G., Albers, G. W., Bladin, C., Fieschi, C., Gabbai, A. A., Kase, C. S., ... &
PREVAIL Investigators. (2007). The efficacy and safety of enoxaparin versus
unfractionated heparin for the prevention of venous thromboembolism after acute
ischaemic stroke (PREVAIL Study): an open-label randomised comparison. The
Lancet, 369(9570), 1347-1355. https://doi.org/10.1016/S0140-6736(07)60633-3
Running head: HEALTHCARE
Author Ye
ar
PUBMed
ID
Data Patients Outcome Interventi
on
Compara
tor
Study
Type
Rea
d
Full
text
Included
or
Exclude
d
Chong, B.
H.,
Brighton,
T. A.,
Baker, R.
I.,
Thurlow,
P., Lee, C.
H., &
ASTH
DVT
Study
Group.
200
5
Patients treated
with
enoxaparin
demonstrated
an inclination
towards
decreased
relapse
of pulmonary
embolism
(1.3% vs.
4.1%; p = 0.17)
and DVT
298 with
symptoma
tic DVT
Recurrent
DVT,
bleeding
events and
pulmonary
emboli
Unfraction
ated
heparin
Once
daily
enoxapari
n
Randomi
sed
control
trial
Yes Included
Author Ye
ar
PUBMed
ID
Data Patients Outcome Interventi
on
Compara
tor
Study
Type
Rea
d
Full
text
Included
or
Exclude
d
Chong, B.
H.,
Brighton,
T. A.,
Baker, R.
I.,
Thurlow,
P., Lee, C.
H., &
ASTH
DVT
Study
Group.
200
5
Patients treated
with
enoxaparin
demonstrated
an inclination
towards
decreased
relapse
of pulmonary
embolism
(1.3% vs.
4.1%; p = 0.17)
and DVT
298 with
symptoma
tic DVT
Recurrent
DVT,
bleeding
events and
pulmonary
emboli
Unfraction
ated
heparin
Once
daily
enoxapari
n
Randomi
sed
control
trial
Yes Included
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 33
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





