Assignment 5: Evaluating E-Cigarettes vs. Nicotine Replacement Therapy
VerifiedAdded on 2022/09/12
|3
|1214
|19
Homework Assignment
AI Summary
This assignment analyzes a randomized trial published in the New England Journal of Medicine (Hajek et al., 2019) comparing the effectiveness of e-cigarettes versus nicotine replacement therapy for smoking cessation. The student addresses key research questions, identifying the dependent and independent variables, and describing the experimental research design and sampling methods. The assignment details the interventions, control conditions, and ethical considerations of the study. It assesses the randomization procedures, data collection points, and blinding methods used. The results, including the 1-year abstinence rates and limitations, are discussed, along with the study's implications for public health and policy. The student concludes by evaluating the importance of the outcomes measured and the study's overall contribution to the field.
Assignment 5 Template
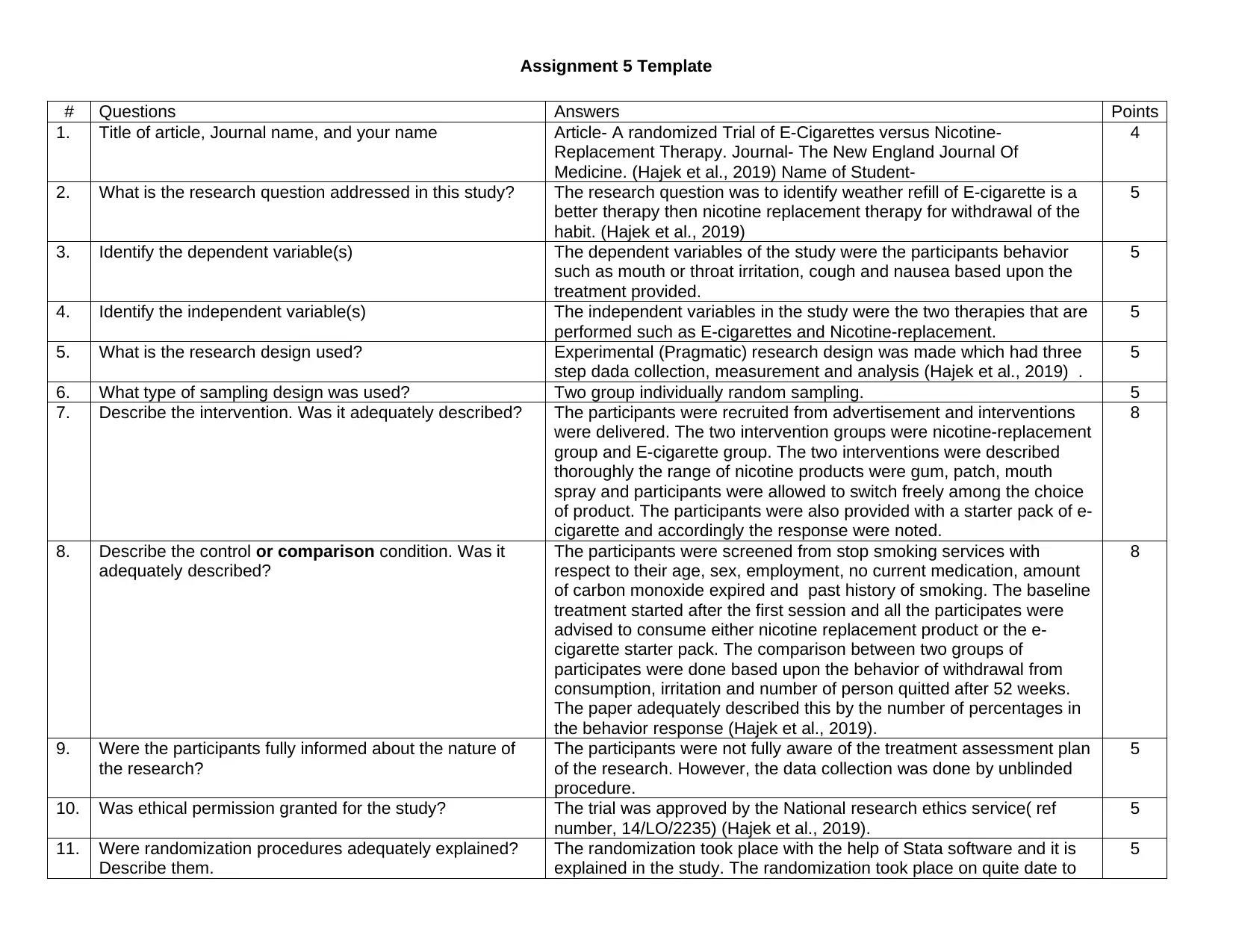
# Questions Answers Points
1. Title of article, Journal name, and your name Article- A randomized Trial of E-Cigarettes versus Nicotine-
Replacement Therapy. Journal- The New England Journal Of
Medicine. (Hajek et al., 2019) Name of Student-
4
2. What is the research question addressed in this study? The research question was to identify weather refill of E-cigarette is a
better therapy then nicotine replacement therapy for withdrawal of the
habit. (Hajek et al., 2019)
5
3. Identify the dependent variable(s) The dependent variables of the study were the participants behavior
such as mouth or throat irritation, cough and nausea based upon the
treatment provided.
5
4. Identify the independent variable(s) The independent variables in the study were the two therapies that are
performed such as E-cigarettes and Nicotine-replacement.
5
5. What is the research design used? Experimental (Pragmatic) research design was made which had three
step dada collection, measurement and analysis (Hajek et al., 2019) .
5
6. What type of sampling design was used? Two group individually random sampling. 5
7. Describe the intervention. Was it adequately described? The participants were recruited from advertisement and interventions
were delivered. The two intervention groups were nicotine-replacement
group and E-cigarette group. The two interventions were described
thoroughly the range of nicotine products were gum, patch, mouth
spray and participants were allowed to switch freely among the choice
of product. The participants were also provided with a starter pack of e-
cigarette and accordingly the response were noted.
8
8. Describe the control or comparison condition. Was it
adequately described?
The participants were screened from stop smoking services with
respect to their age, sex, employment, no current medication, amount
of carbon monoxide expired and past history of smoking. The baseline
treatment started after the first session and all the participates were
advised to consume either nicotine replacement product or the e-
cigarette starter pack. The comparison between two groups of
participates were done based upon the behavior of withdrawal from
consumption, irritation and number of person quitted after 52 weeks.
The paper adequately described this by the number of percentages in
the behavior response (Hajek et al., 2019).
8
9. Were the participants fully informed about the nature of
the research?
The participants were not fully aware of the treatment assessment plan
of the research. However, the data collection was done by unblinded
procedure.
5
10. Was ethical permission granted for the study? The trial was approved by the National research ethics service( ref
number, 14/LO/2235) (Hajek et al., 2019).
5
11. Were randomization procedures adequately explained?
Describe them.
The randomization took place with the help of Stata software and it is
explained in the study. The randomization took place on quite date to
5
# Questions Answers Points
1. Title of article, Journal name, and your name Article- A randomized Trial of E-Cigarettes versus Nicotine-
Replacement Therapy. Journal- The New England Journal Of
Medicine. (Hajek et al., 2019) Name of Student-
4
2. What is the research question addressed in this study? The research question was to identify weather refill of E-cigarette is a
better therapy then nicotine replacement therapy for withdrawal of the
habit. (Hajek et al., 2019)
5
3. Identify the dependent variable(s) The dependent variables of the study were the participants behavior
such as mouth or throat irritation, cough and nausea based upon the
treatment provided.
5
4. Identify the independent variable(s) The independent variables in the study were the two therapies that are
performed such as E-cigarettes and Nicotine-replacement.
5
5. What is the research design used? Experimental (Pragmatic) research design was made which had three
step dada collection, measurement and analysis (Hajek et al., 2019) .
5
6. What type of sampling design was used? Two group individually random sampling. 5
7. Describe the intervention. Was it adequately described? The participants were recruited from advertisement and interventions
were delivered. The two intervention groups were nicotine-replacement
group and E-cigarette group. The two interventions were described
thoroughly the range of nicotine products were gum, patch, mouth
spray and participants were allowed to switch freely among the choice
of product. The participants were also provided with a starter pack of e-
cigarette and accordingly the response were noted.
8
8. Describe the control or comparison condition. Was it
adequately described?
The participants were screened from stop smoking services with
respect to their age, sex, employment, no current medication, amount
of carbon monoxide expired and past history of smoking. The baseline
treatment started after the first session and all the participates were
advised to consume either nicotine replacement product or the e-
cigarette starter pack. The comparison between two groups of
participates were done based upon the behavior of withdrawal from
consumption, irritation and number of person quitted after 52 weeks.
The paper adequately described this by the number of percentages in
the behavior response (Hajek et al., 2019).
8
9. Were the participants fully informed about the nature of
the research?
The participants were not fully aware of the treatment assessment plan
of the research. However, the data collection was done by unblinded
procedure.
5
10. Was ethical permission granted for the study? The trial was approved by the National research ethics service( ref
number, 14/LO/2235) (Hajek et al., 2019).
5
11. Were randomization procedures adequately explained?
Describe them.
The randomization took place with the help of Stata software and it is
explained in the study. The randomization took place on quite date to
5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
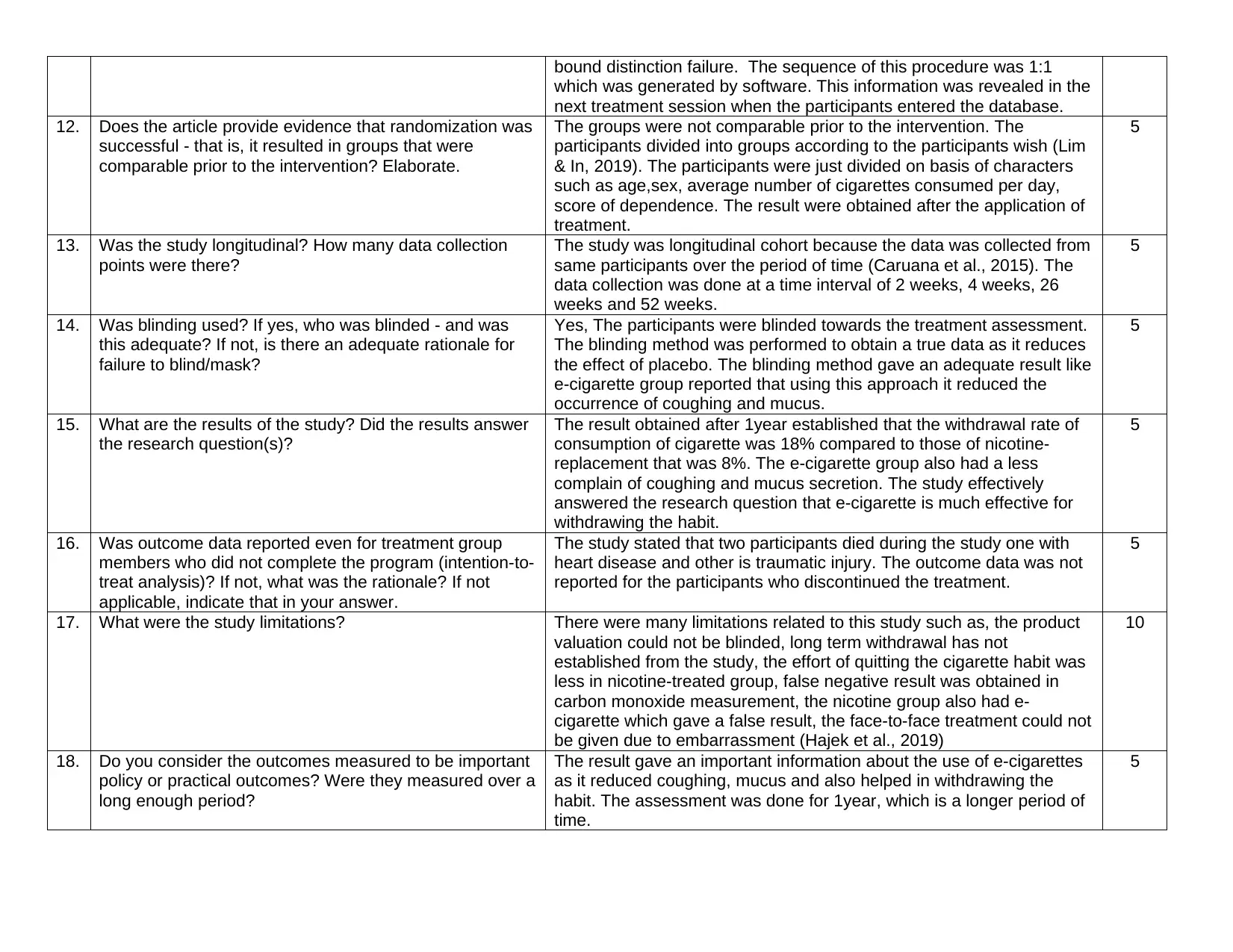
bound distinction failure. The sequence of this procedure was 1:1
which was generated by software. This information was revealed in the
next treatment session when the participants entered the database.
12. Does the article provide evidence that randomization was
successful - that is, it resulted in groups that were
comparable prior to the intervention? Elaborate.
The groups were not comparable prior to the intervention. The
participants divided into groups according to the participants wish (Lim
& In, 2019). The participants were just divided on basis of characters
such as age,sex, average number of cigarettes consumed per day,
score of dependence. The result were obtained after the application of
treatment.
5
13. Was the study longitudinal? How many data collection
points were there?
The study was longitudinal cohort because the data was collected from
same participants over the period of time (Caruana et al., 2015). The
data collection was done at a time interval of 2 weeks, 4 weeks, 26
weeks and 52 weeks.
5
14. Was blinding used? If yes, who was blinded - and was
this adequate? If not, is there an adequate rationale for
failure to blind/mask?
Yes, The participants were blinded towards the treatment assessment.
The blinding method was performed to obtain a true data as it reduces
the effect of placebo. The blinding method gave an adequate result like
e-cigarette group reported that using this approach it reduced the
occurrence of coughing and mucus.
5
15. What are the results of the study? Did the results answer
the research question(s)?
The result obtained after 1year established that the withdrawal rate of
consumption of cigarette was 18% compared to those of nicotine-
replacement that was 8%. The e-cigarette group also had a less
complain of coughing and mucus secretion. The study effectively
answered the research question that e-cigarette is much effective for
withdrawing the habit.
5
16. Was outcome data reported even for treatment group
members who did not complete the program (intention-to-
treat analysis)? If not, what was the rationale? If not
applicable, indicate that in your answer.
The study stated that two participants died during the study one with
heart disease and other is traumatic injury. The outcome data was not
reported for the participants who discontinued the treatment.
5
17. What were the study limitations? There were many limitations related to this study such as, the product
valuation could not be blinded, long term withdrawal has not
established from the study, the effort of quitting the cigarette habit was
less in nicotine-treated group, false negative result was obtained in
carbon monoxide measurement, the nicotine group also had e-
cigarette which gave a false result, the face-to-face treatment could not
be given due to embarrassment (Hajek et al., 2019)
10
18. Do you consider the outcomes measured to be important
policy or practical outcomes? Were they measured over a
long enough period?
The result gave an important information about the use of e-cigarettes
as it reduced coughing, mucus and also helped in withdrawing the
habit. The assessment was done for 1year, which is a longer period of
time.
5
which was generated by software. This information was revealed in the
next treatment session when the participants entered the database.
12. Does the article provide evidence that randomization was
successful - that is, it resulted in groups that were
comparable prior to the intervention? Elaborate.
The groups were not comparable prior to the intervention. The
participants divided into groups according to the participants wish (Lim
& In, 2019). The participants were just divided on basis of characters
such as age,sex, average number of cigarettes consumed per day,
score of dependence. The result were obtained after the application of
treatment.
5
13. Was the study longitudinal? How many data collection
points were there?
The study was longitudinal cohort because the data was collected from
same participants over the period of time (Caruana et al., 2015). The
data collection was done at a time interval of 2 weeks, 4 weeks, 26
weeks and 52 weeks.
5
14. Was blinding used? If yes, who was blinded - and was
this adequate? If not, is there an adequate rationale for
failure to blind/mask?
Yes, The participants were blinded towards the treatment assessment.
The blinding method was performed to obtain a true data as it reduces
the effect of placebo. The blinding method gave an adequate result like
e-cigarette group reported that using this approach it reduced the
occurrence of coughing and mucus.
5
15. What are the results of the study? Did the results answer
the research question(s)?
The result obtained after 1year established that the withdrawal rate of
consumption of cigarette was 18% compared to those of nicotine-
replacement that was 8%. The e-cigarette group also had a less
complain of coughing and mucus secretion. The study effectively
answered the research question that e-cigarette is much effective for
withdrawing the habit.
5
16. Was outcome data reported even for treatment group
members who did not complete the program (intention-to-
treat analysis)? If not, what was the rationale? If not
applicable, indicate that in your answer.
The study stated that two participants died during the study one with
heart disease and other is traumatic injury. The outcome data was not
reported for the participants who discontinued the treatment.
5
17. What were the study limitations? There were many limitations related to this study such as, the product
valuation could not be blinded, long term withdrawal has not
established from the study, the effort of quitting the cigarette habit was
less in nicotine-treated group, false negative result was obtained in
carbon monoxide measurement, the nicotine group also had e-
cigarette which gave a false result, the face-to-face treatment could not
be given due to embarrassment (Hajek et al., 2019)
10
18. Do you consider the outcomes measured to be important
policy or practical outcomes? Were they measured over a
long enough period?
The result gave an important information about the use of e-cigarettes
as it reduced coughing, mucus and also helped in withdrawing the
habit. The assessment was done for 1year, which is a longer period of
time.
5

References
Caruana, E. J., Roman, M., Hernández-Sánchez, J., & Solli, P. (2015). Longitudinal studies. Journal of thoracic disease, 7(11), E537–E540.
https://doi.org/10.3978/j.issn.2072-1439.2015.10.63
Hajek, P., Phillips-Waller, A., Przulj, D., Pesola, F., Myers Smith, K., Bisal, N., ... & Ross, L. (2019). A randomized trial of e-cigarettes versus nicotine-replacement
therapy. New England Journal of Medicine, 380(7), 629-637.
Lim, C. Y., & In, J. (2019). Randomization in clinical studies. Korean journal of anesthesiology, 72(3), 221–232. https://doi.org/10.4097/kja.19049
Caruana, E. J., Roman, M., Hernández-Sánchez, J., & Solli, P. (2015). Longitudinal studies. Journal of thoracic disease, 7(11), E537–E540.
https://doi.org/10.3978/j.issn.2072-1439.2015.10.63
Hajek, P., Phillips-Waller, A., Przulj, D., Pesola, F., Myers Smith, K., Bisal, N., ... & Ross, L. (2019). A randomized trial of e-cigarettes versus nicotine-replacement
therapy. New England Journal of Medicine, 380(7), 629-637.
Lim, C. Y., & In, J. (2019). Randomization in clinical studies. Korean journal of anesthesiology, 72(3), 221–232. https://doi.org/10.4097/kja.19049
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 3
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





