Using E. coli for Petrol Production from Lignocellulosic Hydrolysate
VerifiedAdded on 2023/06/09
|13
|2510
|261
Report
AI Summary
This report focuses on engineering Escherichia coli (E. coli) for the environmentally friendly production of petrol, utilizing lignocellulosic hydrolysate as a feedstock. The introduction covers the background of petrol and the chemical characteristics of lignocellulosic hydrolysate. The core of the report details the metabolic engineering strategies required to modify E. coli's pathways for efficient petrol production at the DNA level. It includes discussions on how to regulate precursor availability, eliminate oxidation pathways, and overexpress relevant genes. The use of immobilized E. coli cells in bioreactors is also highlighted. The report discusses the application of the bacterium E. coli for the synthesis of fatty acids and omega-3 fatty acids. Methods like enzyme engineering and cell immobilization are discussed with their impact on production yields. Finally, the report emphasizes the importance of maintaining optimal conditions for enzymes, including temperature, pH, substrate, and concentration gradients to achieve maximum yield.

Biomedical Engineering and Chemical Engineering 1
BIOMEDICAL ENGINEERING AND CHEMICAL ENGINEERING
By Name
Course
Instructor
Institution
Location
Date
BIOMEDICAL ENGINEERING AND CHEMICAL ENGINEERING
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biomedical Engineering and Chemical Engineering 2
Introduction
The study of Escherichia coli’s membrane lipids has greatly contributed to the knowledge of
manufacturing and lipids membranes in particular. Besides, to other various advantages
associated with this bacterium, the E. coli lipid composition amongst the widely known which
consist only main phospholipids species and three fatty acids (Tu et al., 2013).The bacterium is
easily grown and cultured and much inexpensive within the laboratory setting.it has been
extensively researched for almost five decades.it can take twenty minutes to reproduce E. coli
under conditions which are favorable.E. coli is known to be Gram-negative and can synthesis
ATP normally, however, has the ability to fermentation during anaerobic respiration. The cells
are rod-shaped about 2.0 micrometer long and0.25-1.0 micrometer in diameter h with volume
cell of about 0.6-0.7 cubic micrometer.
Fatty acids can be produced in the biosynthesis of lignocellulosic hydrolysate as hydrocarbon
intermediates. E. coli cell line that has been engineered can produce up to 4.5g/L/day total
cumulative fatty acid in fermentation fed-batch (Kung, Runguphan and Keasling, 2012). The
omega-3 fatty acids are very important to health in cancers, cardiovascular diseases and other
cognitive disorder and physiological problems can also lower the high pressures by largely
activating calcium ions conductance and potassium ion activation voltage channels which can
lead to vasodilation.Omega-3 is also involved in the process of neuronal repair after traumatized
brain injury. Also, they are vital for the development of the fetal brain. The For years fish has
been the chief source of omega-3 fatty acids, but due to overfishing and marine pollution has
made people think about other alternative sources of omega-3 fatty acids. The marine microbes
are rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which are the main
Introduction
The study of Escherichia coli’s membrane lipids has greatly contributed to the knowledge of
manufacturing and lipids membranes in particular. Besides, to other various advantages
associated with this bacterium, the E. coli lipid composition amongst the widely known which
consist only main phospholipids species and three fatty acids (Tu et al., 2013).The bacterium is
easily grown and cultured and much inexpensive within the laboratory setting.it has been
extensively researched for almost five decades.it can take twenty minutes to reproduce E. coli
under conditions which are favorable.E. coli is known to be Gram-negative and can synthesis
ATP normally, however, has the ability to fermentation during anaerobic respiration. The cells
are rod-shaped about 2.0 micrometer long and0.25-1.0 micrometer in diameter h with volume
cell of about 0.6-0.7 cubic micrometer.
Fatty acids can be produced in the biosynthesis of lignocellulosic hydrolysate as hydrocarbon
intermediates. E. coli cell line that has been engineered can produce up to 4.5g/L/day total
cumulative fatty acid in fermentation fed-batch (Kung, Runguphan and Keasling, 2012). The
omega-3 fatty acids are very important to health in cancers, cardiovascular diseases and other
cognitive disorder and physiological problems can also lower the high pressures by largely
activating calcium ions conductance and potassium ion activation voltage channels which can
lead to vasodilation.Omega-3 is also involved in the process of neuronal repair after traumatized
brain injury. Also, they are vital for the development of the fetal brain. The For years fish has
been the chief source of omega-3 fatty acids, but due to overfishing and marine pollution has
made people think about other alternative sources of omega-3 fatty acids. The marine microbes
are rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which are the main

Biomedical Engineering and Chemical Engineering 3
blocks compounds of omega-3 fatty acids. Transgene derivation and intensive metabolic
engineering of microbes that produce fatty acids have come to be very important in the
production of omega-3 fatty acids.
Classical Metabolic engineering
Definition: it is a technique of genetically optimization and processes regulation within the cells
to the production of substance targeted for the increase. This is another method of increasing
omega-3 fatty acids production (Becker, J. and Wittmann, 2012).
The process involved synthesis interactive and analysis whereby the ever-increasing
manufactured strains are designed and made on the basis of the past knowledge. A strategy was
applied for improvement of production of fatty acids. The formed strategy included regulation of
the availability in precursor’s malonyl-ACP, as well as oxidation elimination of the pathway
genes, fade, and fadD to stop prevent degradation of fatty acids. Many expressions of the chain
elongation genes fabZ, fabA and fabG that encode for the fatty acids biosynthesis pathway as
well. Besides that, over expression of native .E. coli thiosterasestes tesA and tesB and
heterologous thiosterasestes from glycerol and ethanol which can give good yields in fatty acids
production. More often the positive interventions were important to boost the production of fatty
acids productions. Natural occurrence of fatty acids transcription sensing factors and the
synthesis regulation and fatty acids degradation at the transcription level. FabR opposes
synthesis of FA by fabB and FAB genes repressions well as FadR factor of transcription.
blocks compounds of omega-3 fatty acids. Transgene derivation and intensive metabolic
engineering of microbes that produce fatty acids have come to be very important in the
production of omega-3 fatty acids.
Classical Metabolic engineering
Definition: it is a technique of genetically optimization and processes regulation within the cells
to the production of substance targeted for the increase. This is another method of increasing
omega-3 fatty acids production (Becker, J. and Wittmann, 2012).
The process involved synthesis interactive and analysis whereby the ever-increasing
manufactured strains are designed and made on the basis of the past knowledge. A strategy was
applied for improvement of production of fatty acids. The formed strategy included regulation of
the availability in precursor’s malonyl-ACP, as well as oxidation elimination of the pathway
genes, fade, and fadD to stop prevent degradation of fatty acids. Many expressions of the chain
elongation genes fabZ, fabA and fabG that encode for the fatty acids biosynthesis pathway as
well. Besides that, over expression of native .E. coli thiosterasestes tesA and tesB and
heterologous thiosterasestes from glycerol and ethanol which can give good yields in fatty acids
production. More often the positive interventions were important to boost the production of fatty
acids productions. Natural occurrence of fatty acids transcription sensing factors and the
synthesis regulation and fatty acids degradation at the transcription level. FabR opposes
synthesis of FA by fabB and FAB genes repressions well as FadR factor of transcription.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
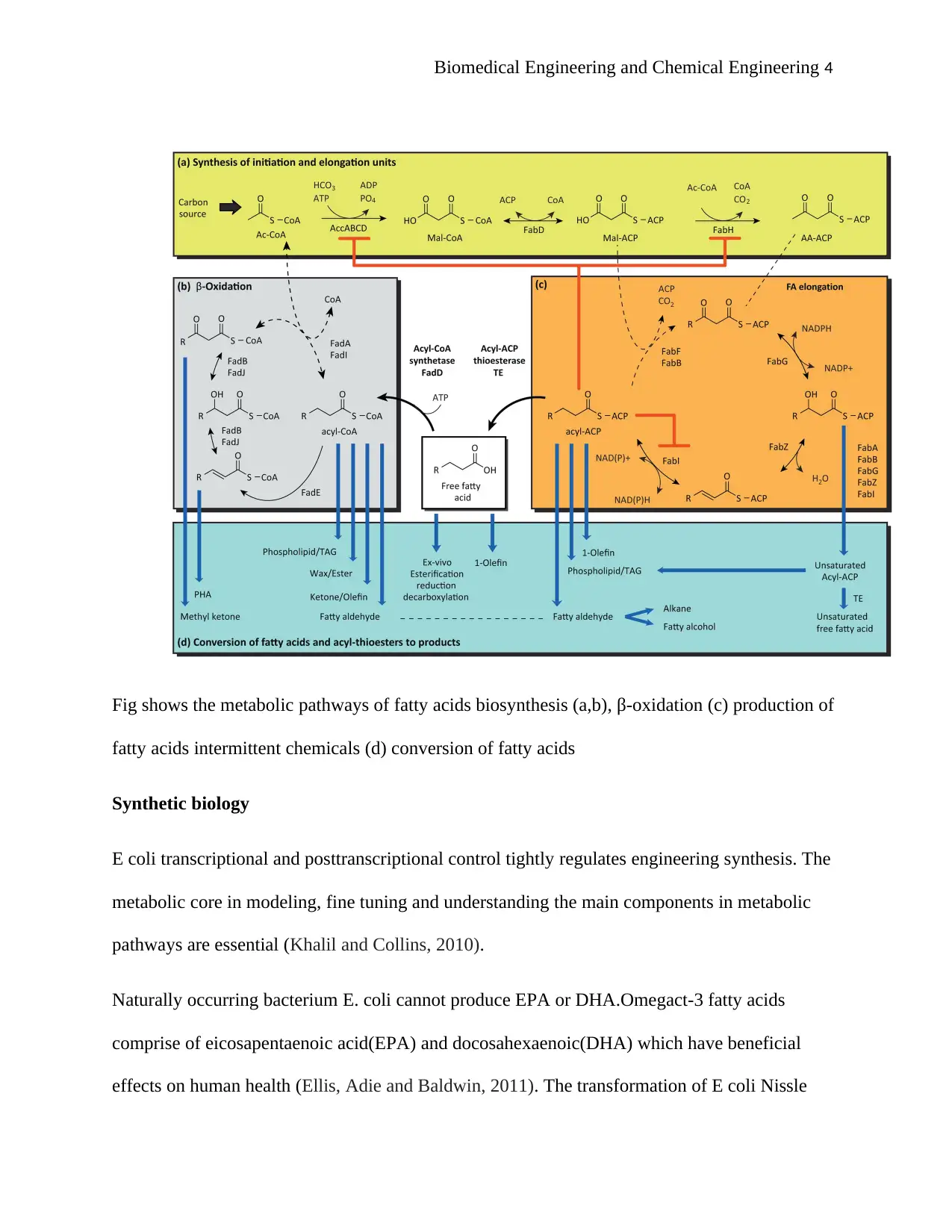
Biomedical Engineering and Chemical Engineering 4
Fig shows the metabolic pathways of fatty acids biosynthesis (a,b), β-oxidation (c) production of
fatty acids intermittent chemicals (d) conversion of fatty acids
Synthetic biology
E coli transcriptional and posttranscriptional control tightly regulates engineering synthesis. The
metabolic core in modeling, fine tuning and understanding the main components in metabolic
pathways are essential (Khalil and Collins, 2010).
Naturally occurring bacterium E. coli cannot produce EPA or DHA.Omegact-3 fatty acids
comprise of eicosapentaenoic acid(EPA) and docosahexaenoic(DHA) which have beneficial
effects on human health (Ellis, Adie and Baldwin, 2011). The transformation of E coli Nissle
Fig shows the metabolic pathways of fatty acids biosynthesis (a,b), β-oxidation (c) production of
fatty acids intermittent chemicals (d) conversion of fatty acids
Synthetic biology
E coli transcriptional and posttranscriptional control tightly regulates engineering synthesis. The
metabolic core in modeling, fine tuning and understanding the main components in metabolic
pathways are essential (Khalil and Collins, 2010).
Naturally occurring bacterium E. coli cannot produce EPA or DHA.Omegact-3 fatty acids
comprise of eicosapentaenoic acid(EPA) and docosahexaenoic(DHA) which have beneficial
effects on human health (Ellis, Adie and Baldwin, 2011). The transformation of E coli Nissle
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biomedical Engineering and Chemical Engineering 5
1917 with a plasmid carrying DHA/EPA Cluster of genes which are isolated from bacterium
Shewanell baltica MAC1 comprising the genes pfaE, pfaD, pfaC, pfaB and which is found in
marine habitats. It has the ability to produce a significant amount of omega-3 fatty acid when
using lignocellulosic hydrolysate as feedstock. More especially transgenic E. coli Nissle EPA
was produced growing.
Analyzing transcriptomic by the use of reverse transcription, qPCR exhibited up-regulation gene
cluster entirely in E. coli Nissle. The E. coli cells can be cultured in bioreactors, and EPA/DHA
is extracted from the culture so that to produce a safe and alternative source of EPA/DHA. This
method can be used in the production of nutraceutical, agricultural products, cosmetics and
pharmaceuticals which contain omega-3 fatty acids.
The recombinant of E coli Nissle cell which comprises one or more genes as follows: pfaA,
pfaB, pfaC, pfaD and pfaE, and the cell can produce one or more omega-3 fatty acids. In one
embedment, the cell can be transformed into by a nucleic acid comprises a gene cluster pfaA,
pfaB, pfaC ,pfaD, and pfaE by culturing at a temperature between 5 degree Celsius to thirty
degrees Celsius, for optimal production of omega-3 fatty acids.
1917 with a plasmid carrying DHA/EPA Cluster of genes which are isolated from bacterium
Shewanell baltica MAC1 comprising the genes pfaE, pfaD, pfaC, pfaB and which is found in
marine habitats. It has the ability to produce a significant amount of omega-3 fatty acid when
using lignocellulosic hydrolysate as feedstock. More especially transgenic E. coli Nissle EPA
was produced growing.
Analyzing transcriptomic by the use of reverse transcription, qPCR exhibited up-regulation gene
cluster entirely in E. coli Nissle. The E. coli cells can be cultured in bioreactors, and EPA/DHA
is extracted from the culture so that to produce a safe and alternative source of EPA/DHA. This
method can be used in the production of nutraceutical, agricultural products, cosmetics and
pharmaceuticals which contain omega-3 fatty acids.
The recombinant of E coli Nissle cell which comprises one or more genes as follows: pfaA,
pfaB, pfaC, pfaD and pfaE, and the cell can produce one or more omega-3 fatty acids. In one
embedment, the cell can be transformed into by a nucleic acid comprises a gene cluster pfaA,
pfaB, pfaC ,pfaD, and pfaE by culturing at a temperature between 5 degree Celsius to thirty
degrees Celsius, for optimal production of omega-3 fatty acids.
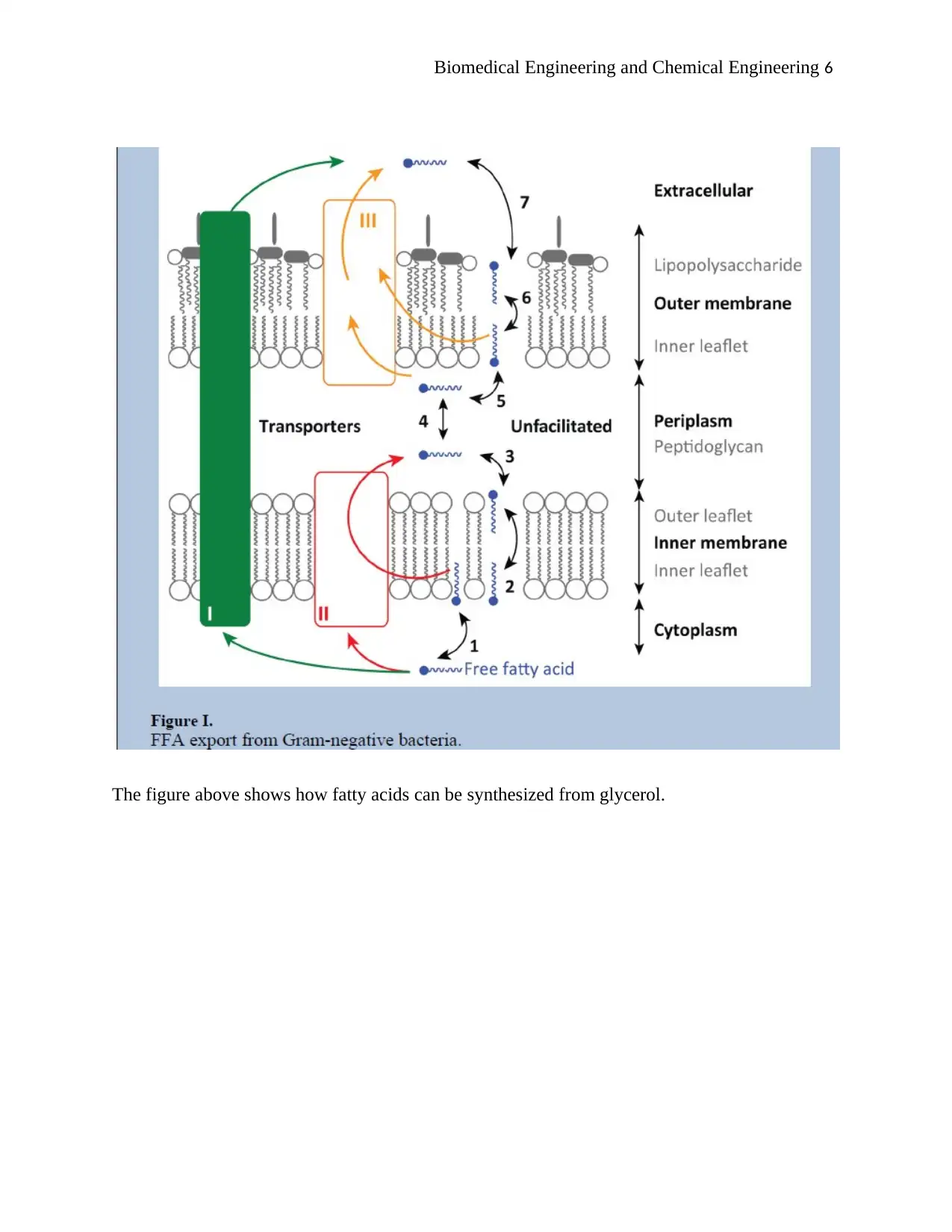
Biomedical Engineering and Chemical Engineering 6
The figure above shows how fatty acids can be synthesized from glycerol.
The figure above shows how fatty acids can be synthesized from glycerol.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
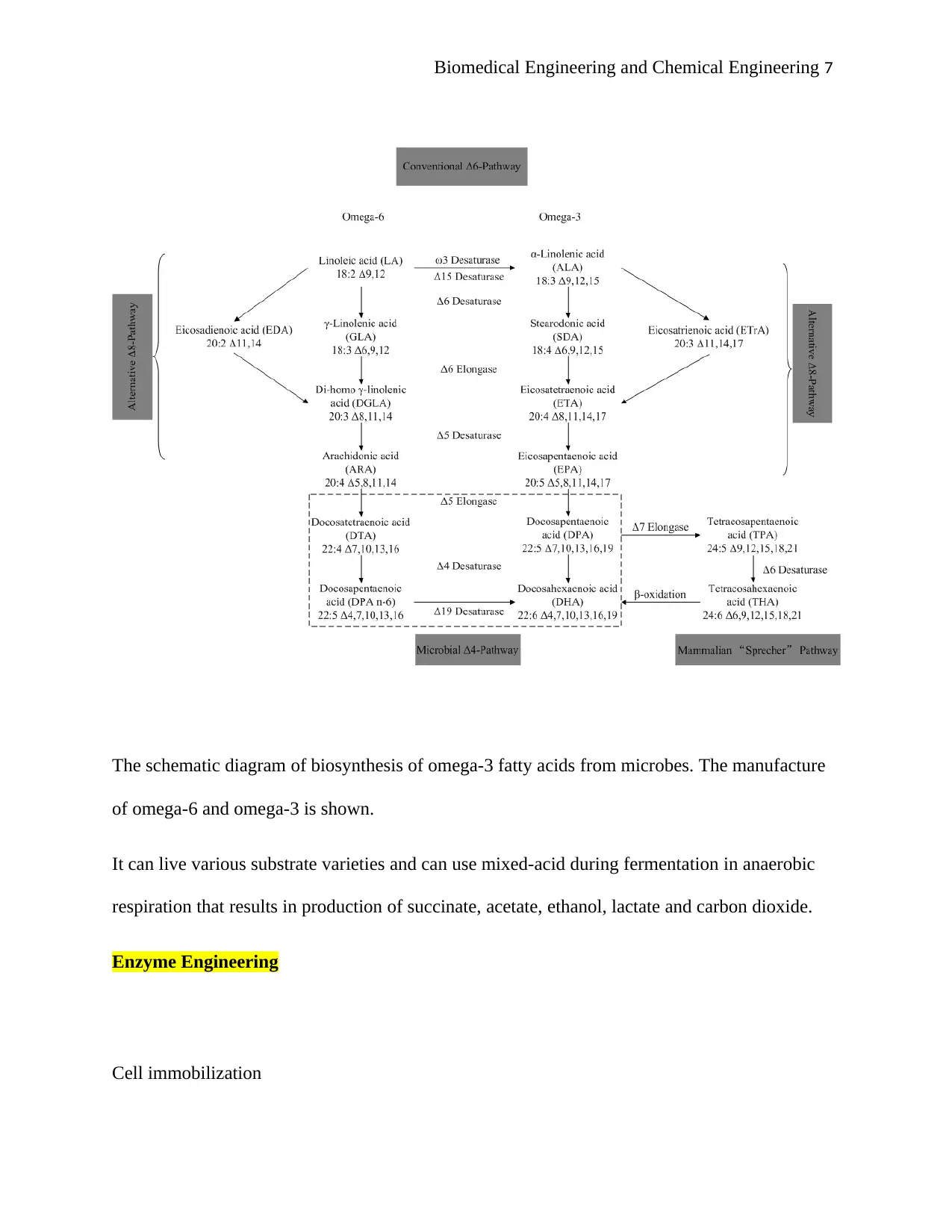
Biomedical Engineering and Chemical Engineering 7
The schematic diagram of biosynthesis of omega-3 fatty acids from microbes. The manufacture
of omega-6 and omega-3 is shown.
It can live various substrate varieties and can use mixed-acid during fermentation in anaerobic
respiration that results in production of succinate, acetate, ethanol, lactate and carbon dioxide.
Enzyme Engineering
Cell immobilization
The schematic diagram of biosynthesis of omega-3 fatty acids from microbes. The manufacture
of omega-6 and omega-3 is shown.
It can live various substrate varieties and can use mixed-acid during fermentation in anaerobic
respiration that results in production of succinate, acetate, ethanol, lactate and carbon dioxide.
Enzyme Engineering
Cell immobilization
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biomedical Engineering and Chemical Engineering 8
In this present work, the mutated E. coli cells were permeabilized and then immobilized on
glutaraldehyde mixed with chitosan for omega-3 production from glycerol. The fermentation
experiments were made with glycerol and ethanol in two liters as working volume containing
immobilized cells of a 100g chitosan coated with glutaraldehyde cross linkage. Images
confirmed from the Scanning Electron Microscopy that the bacteria cells were entrapped of
porous network of linked cross chitosan beads.
K80 lipase were used throughout the study. The strain was sub cultured in which the is media
that is broth by incubating a rotary shaker at 37 degrees Celsius at 150 rpm for 72h.Cultures of
batch put in 900ml of closed bottled glass of a 250ml with cap in blue color as a working
volume, with a sterilized Mueller Hinton Broth medium comprising of (starch 1.5% g/l. beef
infusion solids 2.0% g/l, and casein hydrolysate 17.5% g/l).The sterilization was done separately
for 20min at 120 degree Celsius. The spectrophotometric method was used to monitor cell
growth by measuring optic density of cultured cells at 600mn.They were harvested at active
growth phase culture broth through a transfer to 1.5ml micro-centrifuge tube as well as
centrifuged to keep the cells. The cells were finally suspended in sodium sterilize of 10ml and
kept at 4 degrees Celsius for later use. The immobilized K80 lipase was named during
preparation as ‘ImmK80’ and kept in store at 4 degrees Celsius until use.
Immobilization yield (ɳ) as well as retention (R) their calculations are as follows:
Where Pf is the quantity of protein of soluble lipase and Pi is the supernat later immobilization.
In this present work, the mutated E. coli cells were permeabilized and then immobilized on
glutaraldehyde mixed with chitosan for omega-3 production from glycerol. The fermentation
experiments were made with glycerol and ethanol in two liters as working volume containing
immobilized cells of a 100g chitosan coated with glutaraldehyde cross linkage. Images
confirmed from the Scanning Electron Microscopy that the bacteria cells were entrapped of
porous network of linked cross chitosan beads.
K80 lipase were used throughout the study. The strain was sub cultured in which the is media
that is broth by incubating a rotary shaker at 37 degrees Celsius at 150 rpm for 72h.Cultures of
batch put in 900ml of closed bottled glass of a 250ml with cap in blue color as a working
volume, with a sterilized Mueller Hinton Broth medium comprising of (starch 1.5% g/l. beef
infusion solids 2.0% g/l, and casein hydrolysate 17.5% g/l).The sterilization was done separately
for 20min at 120 degree Celsius. The spectrophotometric method was used to monitor cell
growth by measuring optic density of cultured cells at 600mn.They were harvested at active
growth phase culture broth through a transfer to 1.5ml micro-centrifuge tube as well as
centrifuged to keep the cells. The cells were finally suspended in sodium sterilize of 10ml and
kept at 4 degrees Celsius for later use. The immobilized K80 lipase was named during
preparation as ‘ImmK80’ and kept in store at 4 degrees Celsius until use.
Immobilization yield (ɳ) as well as retention (R) their calculations are as follows:
Where Pf is the quantity of protein of soluble lipase and Pi is the supernat later immobilization.

Biomedical Engineering and Chemical Engineering 9
However, Immobiliztion for complex protein production and for the proteins that undergo post
translational changes, prakarytic cells are not prefered for use.
When lipase K80 was immobilized onto a MADVB bead, in glyrol by the use of both hydrophobic
adsorption and cross-linking covalent.Due to that,the immobilized yield (R) was 77% which was
calculated by measuring the quantity of protein attached t a bead after 4 hours of incubation.The is
effcint thgatotal transesterification activies of he specific activity of free K80 was 16.0U/g protein while
18.1 is for ImmK80.Therefore immobilization process is efficent in transesterification,it is a good
proposal to use ImmoK80 in transesterifications reactions.
Hydrolytic activity
The measurement of hydrolytic activity was done by use of the pH stat method. Glycerol
emulsification was done prepared through emulsification process of the mixture containing 1%
glycerol, 20 mM NaCl, 1% Arabic gum and 5 mM CaCl2 for two minutes in a Waring blender at
the optimum speed. Afterwards 10mM NaOH was used to adjust PH of substrate emulsification
(20ml) top H 8.0, 10 microliters of K80 soluble was added. The release rate of fatty acids was
measured by the use of a pH titrator for 3min at 30 degrees Celsius. Lipase activity (U) was well-
defined by one unit as the quantity of enzyme needed to release 1 micromole of fatty acid per
minute.
Transesterification activity
However, Immobiliztion for complex protein production and for the proteins that undergo post
translational changes, prakarytic cells are not prefered for use.
When lipase K80 was immobilized onto a MADVB bead, in glyrol by the use of both hydrophobic
adsorption and cross-linking covalent.Due to that,the immobilized yield (R) was 77% which was
calculated by measuring the quantity of protein attached t a bead after 4 hours of incubation.The is
effcint thgatotal transesterification activies of he specific activity of free K80 was 16.0U/g protein while
18.1 is for ImmK80.Therefore immobilization process is efficent in transesterification,it is a good
proposal to use ImmoK80 in transesterifications reactions.
Hydrolytic activity
The measurement of hydrolytic activity was done by use of the pH stat method. Glycerol
emulsification was done prepared through emulsification process of the mixture containing 1%
glycerol, 20 mM NaCl, 1% Arabic gum and 5 mM CaCl2 for two minutes in a Waring blender at
the optimum speed. Afterwards 10mM NaOH was used to adjust PH of substrate emulsification
(20ml) top H 8.0, 10 microliters of K80 soluble was added. The release rate of fatty acids was
measured by the use of a pH titrator for 3min at 30 degrees Celsius. Lipase activity (U) was well-
defined by one unit as the quantity of enzyme needed to release 1 micromole of fatty acid per
minute.
Transesterification activity
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Biomedical Engineering and Chemical Engineering
10
Enzyme samples were added 0.5ml of 10mM p-nitrophenol palmitate in hexane and 30
microliters of ethanol. The experiment was done at 30 degrees Celsius in 10min with 230
revolutions per minute r resting of lipase, 25 microliters of clear supernat was reserved and
mixed with 1 microliter of 0.1 M NaOH. The released p-nitrophenol was removed using the
aqueous alkaline stage.
Effects of pH and Temperature on Free and ImmK80 lipases
To get the impacts of, activity of lipase was measured at different temperatures between 20 to 80
degrees Celsius by the use of pH stat method. To analyze stability in temperature, pre-incubation
of enzymes was done for 30min at 20-60 degrees Celsius, afterwards the activity remainder was
assayed at 30 degrees Celsius. Activities of the enzymes at different pH values were measured to
get optimal ph. The given buffers were useful in characterizing the pH impacts: pH 4 - pH
6 ,sodium acetate or acetic acid; pH 6 -pH 8, K2HPO4; pH 8 - pH 9, Tris-HCl; pH 9-pH 11,
glycine –KOH; pH 11 to pH 12,K3PO4.For confirmation of stability in pH the enzymes were
incubated for30 min the mentioned previously pH buffers at 30 degrees Celsius and next
adjusted to pH 8.0; the enzyme remaining activity was examined under circumstances following
the pH modification.
Lipase –Catalyzed Transesterification
The transesterification of enzymes for glycerol was done as follows. Glycerol and ethanol were
mixed in a molar ratio of 1:3,whereby glycerol of 1ml and ethanol of 0.186ml were placed in a
glass vial of 30ml.Different quantities of (0,132,298,and 508 ml) of diluted water was added to
obtain 0,10,20, and 30% hydrated mixtures of reactions, respectively. If of 0% reaction, then 508
10
Enzyme samples were added 0.5ml of 10mM p-nitrophenol palmitate in hexane and 30
microliters of ethanol. The experiment was done at 30 degrees Celsius in 10min with 230
revolutions per minute r resting of lipase, 25 microliters of clear supernat was reserved and
mixed with 1 microliter of 0.1 M NaOH. The released p-nitrophenol was removed using the
aqueous alkaline stage.
Effects of pH and Temperature on Free and ImmK80 lipases
To get the impacts of, activity of lipase was measured at different temperatures between 20 to 80
degrees Celsius by the use of pH stat method. To analyze stability in temperature, pre-incubation
of enzymes was done for 30min at 20-60 degrees Celsius, afterwards the activity remainder was
assayed at 30 degrees Celsius. Activities of the enzymes at different pH values were measured to
get optimal ph. The given buffers were useful in characterizing the pH impacts: pH 4 - pH
6 ,sodium acetate or acetic acid; pH 6 -pH 8, K2HPO4; pH 8 - pH 9, Tris-HCl; pH 9-pH 11,
glycine –KOH; pH 11 to pH 12,K3PO4.For confirmation of stability in pH the enzymes were
incubated for30 min the mentioned previously pH buffers at 30 degrees Celsius and next
adjusted to pH 8.0; the enzyme remaining activity was examined under circumstances following
the pH modification.
Lipase –Catalyzed Transesterification
The transesterification of enzymes for glycerol was done as follows. Glycerol and ethanol were
mixed in a molar ratio of 1:3,whereby glycerol of 1ml and ethanol of 0.186ml were placed in a
glass vial of 30ml.Different quantities of (0,132,298,and 508 ml) of diluted water was added to
obtain 0,10,20, and 30% hydrated mixtures of reactions, respectively. If of 0% reaction, then 508
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biomedical Engineering and Chemical Engineering
11
microliters of hexane was added. The same unit of the ImmK80 or LyoK80 was later to the
mixture reaction. When Lyok80, about 25 glass beads were added for effective mixing. The
reaction of transesterification was done for period of 24h at 30 degrees.in an incubator shaking at
230rpm.Ethanol was added in a step of one to ImmK80 at a mediated reaction and three steps for
a LyoK80 reaction mediated. After reaction of enzymes, the filtration of reaction mixtures and
chromatography analysis using a thin layer and gas chromatography.
Conclusion
Metabolic engineering has greatly combined experimental/computational approach, and
biosynthesis has contributed immensely to production improvement of omega-3 fatty acids in E.
coli Nissle and can be extended to the cell factory development for the specific chemical to be
produced. Through this method, it is more effective to produce Omega-3 fatty acids than
harvesting from fish. When NADPH and ATP are reduced, also the fatty acid production
reduced. For the production of maximum yield, the conditions must be maintained for both
enzymes, Temperature, PH, substrate and concentration gradient.
11
microliters of hexane was added. The same unit of the ImmK80 or LyoK80 was later to the
mixture reaction. When Lyok80, about 25 glass beads were added for effective mixing. The
reaction of transesterification was done for period of 24h at 30 degrees.in an incubator shaking at
230rpm.Ethanol was added in a step of one to ImmK80 at a mediated reaction and three steps for
a LyoK80 reaction mediated. After reaction of enzymes, the filtration of reaction mixtures and
chromatography analysis using a thin layer and gas chromatography.
Conclusion
Metabolic engineering has greatly combined experimental/computational approach, and
biosynthesis has contributed immensely to production improvement of omega-3 fatty acids in E.
coli Nissle and can be extended to the cell factory development for the specific chemical to be
produced. Through this method, it is more effective to produce Omega-3 fatty acids than
harvesting from fish. When NADPH and ATP are reduced, also the fatty acid production
reduced. For the production of maximum yield, the conditions must be maintained for both
enzymes, Temperature, PH, substrate and concentration gradient.

Biomedical Engineering and Chemical Engineering
12
References
Becker, J. and Wittmann, C., 2012. Systems and synthetic metabolic engineering for amino acid
production–the heartbeat of industrial strain development. Current opinion in
biotechnology, 23(5), pp.718-726.
Brena, B., González-Pombo, P. and Batista-Viera, F., 2013. Immobilization of enzymes: a
literature survey. In Immobilization of enzymes and cells (pp. 15-31). Humana Press, Totowa,
NJ.
Brodelius, P., 2018. Immobilized plant cells. In Immobilized cells and organelles (pp. 27-56).
CRC Press.
Ellis, T., Adie, T. and Baldwin, G.S., 2011. DNA assembly for synthetic biology: from parts to
pathways and beyond. Integrative Biology, 3(2), pp.109-118.
French, R. and Petrowsky, M., 2014. Molecular model of self-diffusion in polar organic liquids:
implications for conductivity and fluidity in polar organic liquids and electrolytes. The Journal of
Physical Chemistry B, 118(9), pp.2422-2432.
Khalil, A.S. and Collins, J.J., 2010. Synthetic biology: applications come of age. Nature Reviews
Genetics, 11(5), p.367.
Kung, Y., Runguphan, W. and Keasling, J.D., 2012. From fields to fuels: recent advances in the
microbial production of biofuels. ACS synthetic biology, 1(11), pp.498-513.
Liu, S., 2016. Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design. Elsevier.
12
References
Becker, J. and Wittmann, C., 2012. Systems and synthetic metabolic engineering for amino acid
production–the heartbeat of industrial strain development. Current opinion in
biotechnology, 23(5), pp.718-726.
Brena, B., González-Pombo, P. and Batista-Viera, F., 2013. Immobilization of enzymes: a
literature survey. In Immobilization of enzymes and cells (pp. 15-31). Humana Press, Totowa,
NJ.
Brodelius, P., 2018. Immobilized plant cells. In Immobilized cells and organelles (pp. 27-56).
CRC Press.
Ellis, T., Adie, T. and Baldwin, G.S., 2011. DNA assembly for synthetic biology: from parts to
pathways and beyond. Integrative Biology, 3(2), pp.109-118.
French, R. and Petrowsky, M., 2014. Molecular model of self-diffusion in polar organic liquids:
implications for conductivity and fluidity in polar organic liquids and electrolytes. The Journal of
Physical Chemistry B, 118(9), pp.2422-2432.
Khalil, A.S. and Collins, J.J., 2010. Synthetic biology: applications come of age. Nature Reviews
Genetics, 11(5), p.367.
Kung, Y., Runguphan, W. and Keasling, J.D., 2012. From fields to fuels: recent advances in the
microbial production of biofuels. ACS synthetic biology, 1(11), pp.498-513.
Liu, S., 2016. Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design. Elsevier.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




