University Pharma Assignment: pH, pKa, and Drug Absorption Analysis
VerifiedAdded on 2022/08/21
|11
|2339
|21
Report
AI Summary
This report critically analyzes the impact of pH and pKa on drug absorption within the gastrointestinal tract, focusing on the oral administration of drugs. It utilizes the Henderson-Hasselbalch equation to understand the relationship between pH, pKa, and drug ionization, which influences a drug's ability to cross cell membranes. The report examines the behavior of weak acids and bases, such as Indomethacin and ephedrine, in different pH environments and explains how ionization affects their absorption rates in the stomach and intestines. Furthermore, it includes chemical structures and equations to illustrate the ionization of drugs at different pH levels and compares the absorption patterns of Indomethacin, ephedrine, and morphine. The study also addresses the practical application of these principles by calculating the ionization ratios of ephedrine in blood and linking these concepts to the overall understanding of drug absorption and its implications in pharmaceutical science.

Pharma 1
Title:
Assignment Name:
Student Name:
Course Name and Number:
Professor:
Date:
Title:
Assignment Name:
Student Name:
Course Name and Number:
Professor:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharma 2
Question 1: Critically analyze the effect of pH and pKa on drug absorption?
pH and pKa are important parameters contributing to the drug absorptions in the
gastrointestinal tract of the human (oral administration of drugs). It is common to model the GI
tract in the laboratory by using aqueous solutions or buffers having different pH in contact with
ethyl acetate. The aqueous solutions of different pH in contact of ethyl acetate can depict the
aqueous medium and the lipid component lining different parts of the GI (Abuhelwa et al. 2017,
Kataoka et al. 2016).
The Henderson–Hasselbalch equation is important to draw a relation of the pH of a
solution comprising a mixture of two components (De Levie 2003), pKa and the concentrations
of those components in the solution. This equation is used primarily to measure the pH of
solutions produced by adding a known quantity of acids and their conjugated bases (De Levie
2003, Kataoka et al. 2016). This can also be achieved by neutralizing parts of the acid using a
known amount of a strong base.
The majority of drugs are weak organic bases or acids that are condensed and ionized in
an aqueous environment. The non-ionized form is usually lipid-soluble (lipophilic) and readily
diffuses across cell membranes. The ionized form has low lipid solubility (high water solubility -
hydrophilic) with high electrical resistance and therefore cannot easily move across cell
membranes (Sjögren et al. 2015 ).
The ratio of the non-ionized form of the drug available in the solution is measured by the
solution pH along with the pKa of the drug, which is the acid dissociation constant. This,
therefore, indicates the penetration ability of the drug through the cell membranes. The pka
represents the pH where an equal amount of non-ionized as well as ionized drug forms are
Question 1: Critically analyze the effect of pH and pKa on drug absorption?
pH and pKa are important parameters contributing to the drug absorptions in the
gastrointestinal tract of the human (oral administration of drugs). It is common to model the GI
tract in the laboratory by using aqueous solutions or buffers having different pH in contact with
ethyl acetate. The aqueous solutions of different pH in contact of ethyl acetate can depict the
aqueous medium and the lipid component lining different parts of the GI (Abuhelwa et al. 2017,
Kataoka et al. 2016).
The Henderson–Hasselbalch equation is important to draw a relation of the pH of a
solution comprising a mixture of two components (De Levie 2003), pKa and the concentrations
of those components in the solution. This equation is used primarily to measure the pH of
solutions produced by adding a known quantity of acids and their conjugated bases (De Levie
2003, Kataoka et al. 2016). This can also be achieved by neutralizing parts of the acid using a
known amount of a strong base.
The majority of drugs are weak organic bases or acids that are condensed and ionized in
an aqueous environment. The non-ionized form is usually lipid-soluble (lipophilic) and readily
diffuses across cell membranes. The ionized form has low lipid solubility (high water solubility -
hydrophilic) with high electrical resistance and therefore cannot easily move across cell
membranes (Sjögren et al. 2015 ).
The ratio of the non-ionized form of the drug available in the solution is measured by the
solution pH along with the pKa of the drug, which is the acid dissociation constant. This,
therefore, indicates the penetration ability of the drug through the cell membranes. The pka
represents the pH where an equal amount of non-ionized as well as ionized drug forms are

Pharma 3
present in the solution. If the pH is lower compared to the drug pKa, the non-ionized form of the
weak acid prevails, whereas, in the case of a drug that is a weak base, the ionized form
dominates (Sjögren et al. 2015).
The ratio of non-ionized to ionized forms in plasma (having pH 7.4) to a weak acid (such
as with a pKa 4.4) is 1: 1000 (Indomethacin). But, the ratio in gastric solution (having pH 1.4) is
reversed (1000: 1). A weak acid drug does not ionize in the stomach, promoting diffusion via the
gastric mucosa route. The result is reversed for a weak base with pKa 9.6 when maximum drugs
get ionized in the stomach (ephedrine) (Polster et al. 2010).
Weakly acidic drugs (such as Indomethacin) are more easily absorbed from the
membranes of the stomach as it is a more acidic medium when compared to drugs that are
weakly basic (such as ephedrine). Because of the large surface area and more permeability of the
membrane, maximum drug absorption takes place in the small intestine regardless of a drug
being acidic or basic (Holm et al. 2013, Padwal et al. 2010).
Question 2: Explain the result in Table 1 with Indomethacin, ephedrine and
Morphine in terms of pKa and site of absorption?
For ionized drugs such as Indomethacin and Ephedrine, their solubility in water is
influenced by pH. The pH in the stomach ranges from 1.2 to 3 whereas that in the intestine hover
around 8. Ephedrine, the weak base is almost completely ionized in the gastric pH of 1.2 to 3.
Therefore, it is highly soluble in water when present in stomach and less soluble in lipid or fat
(ethyl acetate). Thus, it is not absorbed from stomach, which contains lipid lining in the inner
membrane and no visible spot of this drug found for this drug at pH 1, but found at pH 8. The
weak base Ephedrine is not completely ionized (partially ionized) at intestinal pH 8 and is less
present in the solution. If the pH is lower compared to the drug pKa, the non-ionized form of the
weak acid prevails, whereas, in the case of a drug that is a weak base, the ionized form
dominates (Sjögren et al. 2015).
The ratio of non-ionized to ionized forms in plasma (having pH 7.4) to a weak acid (such
as with a pKa 4.4) is 1: 1000 (Indomethacin). But, the ratio in gastric solution (having pH 1.4) is
reversed (1000: 1). A weak acid drug does not ionize in the stomach, promoting diffusion via the
gastric mucosa route. The result is reversed for a weak base with pKa 9.6 when maximum drugs
get ionized in the stomach (ephedrine) (Polster et al. 2010).
Weakly acidic drugs (such as Indomethacin) are more easily absorbed from the
membranes of the stomach as it is a more acidic medium when compared to drugs that are
weakly basic (such as ephedrine). Because of the large surface area and more permeability of the
membrane, maximum drug absorption takes place in the small intestine regardless of a drug
being acidic or basic (Holm et al. 2013, Padwal et al. 2010).
Question 2: Explain the result in Table 1 with Indomethacin, ephedrine and
Morphine in terms of pKa and site of absorption?
For ionized drugs such as Indomethacin and Ephedrine, their solubility in water is
influenced by pH. The pH in the stomach ranges from 1.2 to 3 whereas that in the intestine hover
around 8. Ephedrine, the weak base is almost completely ionized in the gastric pH of 1.2 to 3.
Therefore, it is highly soluble in water when present in stomach and less soluble in lipid or fat
(ethyl acetate). Thus, it is not absorbed from stomach, which contains lipid lining in the inner
membrane and no visible spot of this drug found for this drug at pH 1, but found at pH 8. The
weak base Ephedrine is not completely ionized (partially ionized) at intestinal pH 8 and is less
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pharma 4
soluble in water and has a higher ethyl acetate solubility when compared to pH 1. So it is
absorbed more from the intestine (CHEN and WANG 2010, Limberger et al. 2013, Polster et al.
2010).
As a weak acid, Indomethacin is sparingly or not at all ionized at pH less than 3 and has high
solubility in ethyl acetate but has low water solubility. So it is not absorbed at pH 3 from the
stomach. Therefore, visible spot for Indomethacin was found at pH 1, but not found at pH 8
(Jung et al. 2010, Sugihara et al. 2012).
This further describes the more rapid absorption of weak acids in the stomach compared to the
weak bases. Weak acids that are unionized can get dissolved in lipids present in the stomach
lining and thus diffuse in across the bloodstream. On the other hand, the aqueous parts of the
stomach retain the ionized weak bases (Andari Sawaya et al. 2012).
This also illustrates the reason behind the absorption of weak bases faster than weak acids from
the intestine. Weak bases at pH 8 (the intestinal pH) are not ionized properly like the weak acids
and thus more readily dissolve in the lipid materials present in the intestinal wall (Estudante et al.
2013).
The drug Morphine always remains in ethyl acetate in high concentration irrespective of pH
(Balch and Trescot 2010, Pypendop et al. 2014). It does not ionize before the pH is much above
8 and it exists as a neutral molecule under all physiological conditions (Prasanna et al. 2012).
The solubility of Morphine in ethyl acetate is much higher than that in water. Therefore, it flows
easily from water to ethyl acetate. These drug types are absorbed in both the intestine and
stomach (so visible spot in both the pH 1 and 8). This explains the results shown in Table 1.
soluble in water and has a higher ethyl acetate solubility when compared to pH 1. So it is
absorbed more from the intestine (CHEN and WANG 2010, Limberger et al. 2013, Polster et al.
2010).
As a weak acid, Indomethacin is sparingly or not at all ionized at pH less than 3 and has high
solubility in ethyl acetate but has low water solubility. So it is not absorbed at pH 3 from the
stomach. Therefore, visible spot for Indomethacin was found at pH 1, but not found at pH 8
(Jung et al. 2010, Sugihara et al. 2012).
This further describes the more rapid absorption of weak acids in the stomach compared to the
weak bases. Weak acids that are unionized can get dissolved in lipids present in the stomach
lining and thus diffuse in across the bloodstream. On the other hand, the aqueous parts of the
stomach retain the ionized weak bases (Andari Sawaya et al. 2012).
This also illustrates the reason behind the absorption of weak bases faster than weak acids from
the intestine. Weak bases at pH 8 (the intestinal pH) are not ionized properly like the weak acids
and thus more readily dissolve in the lipid materials present in the intestinal wall (Estudante et al.
2013).
The drug Morphine always remains in ethyl acetate in high concentration irrespective of pH
(Balch and Trescot 2010, Pypendop et al. 2014). It does not ionize before the pH is much above
8 and it exists as a neutral molecule under all physiological conditions (Prasanna et al. 2012).
The solubility of Morphine in ethyl acetate is much higher than that in water. Therefore, it flows
easily from water to ethyl acetate. These drug types are absorbed in both the intestine and
stomach (so visible spot in both the pH 1 and 8). This explains the results shown in Table 1.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharma 5
Question 3: Explain in your own words what will happen to the chemical
structure of Indomethacin when it is in solution at pH 1.0. Compare this to
what will happen to the chemical structure of Indomethacin at pH 8.0.
Highlight your answers by drawing appropriate chemical structures for
Indomethacin at each of the pH values.
At pH 1 Indomethacin will be not be ionized (or sparingly ionized) as it has a pKa of 4.5.
At slightly acidic or neutral pH, the structure remains stable. It will decompose completely in
strong alkaline medium i.e. at pH 8 (Denton and Rostron 2013).
Structure of Indomethacin at pH 1
Question 3: Explain in your own words what will happen to the chemical
structure of Indomethacin when it is in solution at pH 1.0. Compare this to
what will happen to the chemical structure of Indomethacin at pH 8.0.
Highlight your answers by drawing appropriate chemical structures for
Indomethacin at each of the pH values.
At pH 1 Indomethacin will be not be ionized (or sparingly ionized) as it has a pKa of 4.5.
At slightly acidic or neutral pH, the structure remains stable. It will decompose completely in
strong alkaline medium i.e. at pH 8 (Denton and Rostron 2013).
Structure of Indomethacin at pH 1

Pharma 6
Structure of Indomethacin at pH 8
Question 4: Explain the result with ephedrine at pH 8.0. Include a chemical
equation which shows the conjugate acid and/or base formed by ephedrine at
pH 8.0. Draw and label the predominant structures of ephedrine at pH 1, pH
7 and pH 10.
The weak base Ephedrine is not completely ionized (partially ionized) at intestinal pH 8 and is
less soluble in water and has a higher ethyl acetate solubility. On the other hand it is almost
completely ionized in the gastric pH of 1.2 to 3. Therefore, it is highly soluble in water when
present in stomach and less soluble in lipid or fat (ethyl acetate). Thus, it is not absorbed from
stomach, which contains lipid lining in the inner membrane and no visible spot of this drug
found for this drug at pH 1, but found at pH 8 (Denton and Rostron 2013).
Structure of Indomethacin at pH 8
Question 4: Explain the result with ephedrine at pH 8.0. Include a chemical
equation which shows the conjugate acid and/or base formed by ephedrine at
pH 8.0. Draw and label the predominant structures of ephedrine at pH 1, pH
7 and pH 10.
The weak base Ephedrine is not completely ionized (partially ionized) at intestinal pH 8 and is
less soluble in water and has a higher ethyl acetate solubility. On the other hand it is almost
completely ionized in the gastric pH of 1.2 to 3. Therefore, it is highly soluble in water when
present in stomach and less soluble in lipid or fat (ethyl acetate). Thus, it is not absorbed from
stomach, which contains lipid lining in the inner membrane and no visible spot of this drug
found for this drug at pH 1, but found at pH 8 (Denton and Rostron 2013).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
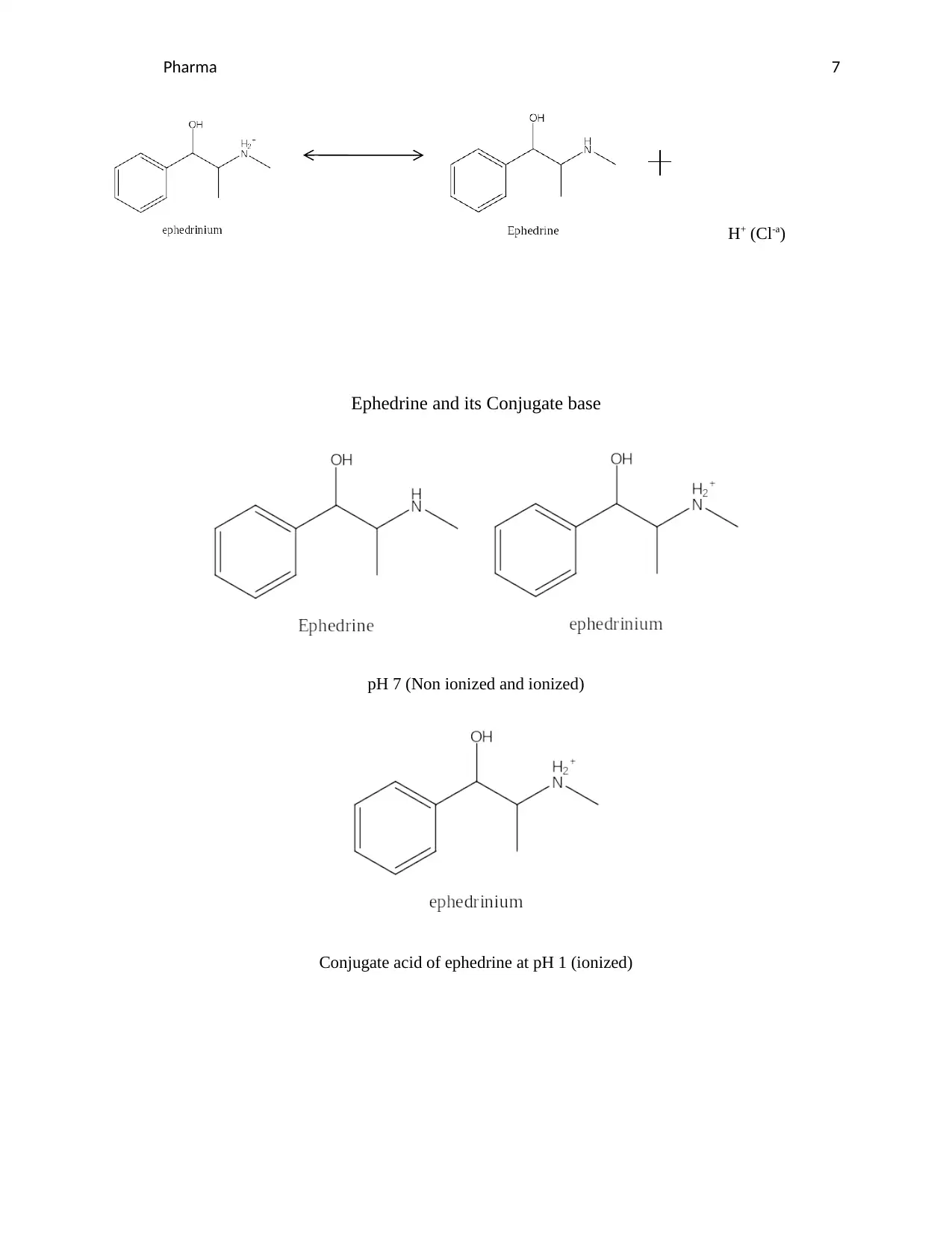
Pharma 7
H+ (Cl-a)
Ephedrine and its Conjugate base
pH 7 (Non ionized and ionized)
Conjugate acid of ephedrine at pH 1 (ionized)
H+ (Cl-a)
Ephedrine and its Conjugate base
pH 7 (Non ionized and ionized)
Conjugate acid of ephedrine at pH 1 (ionized)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
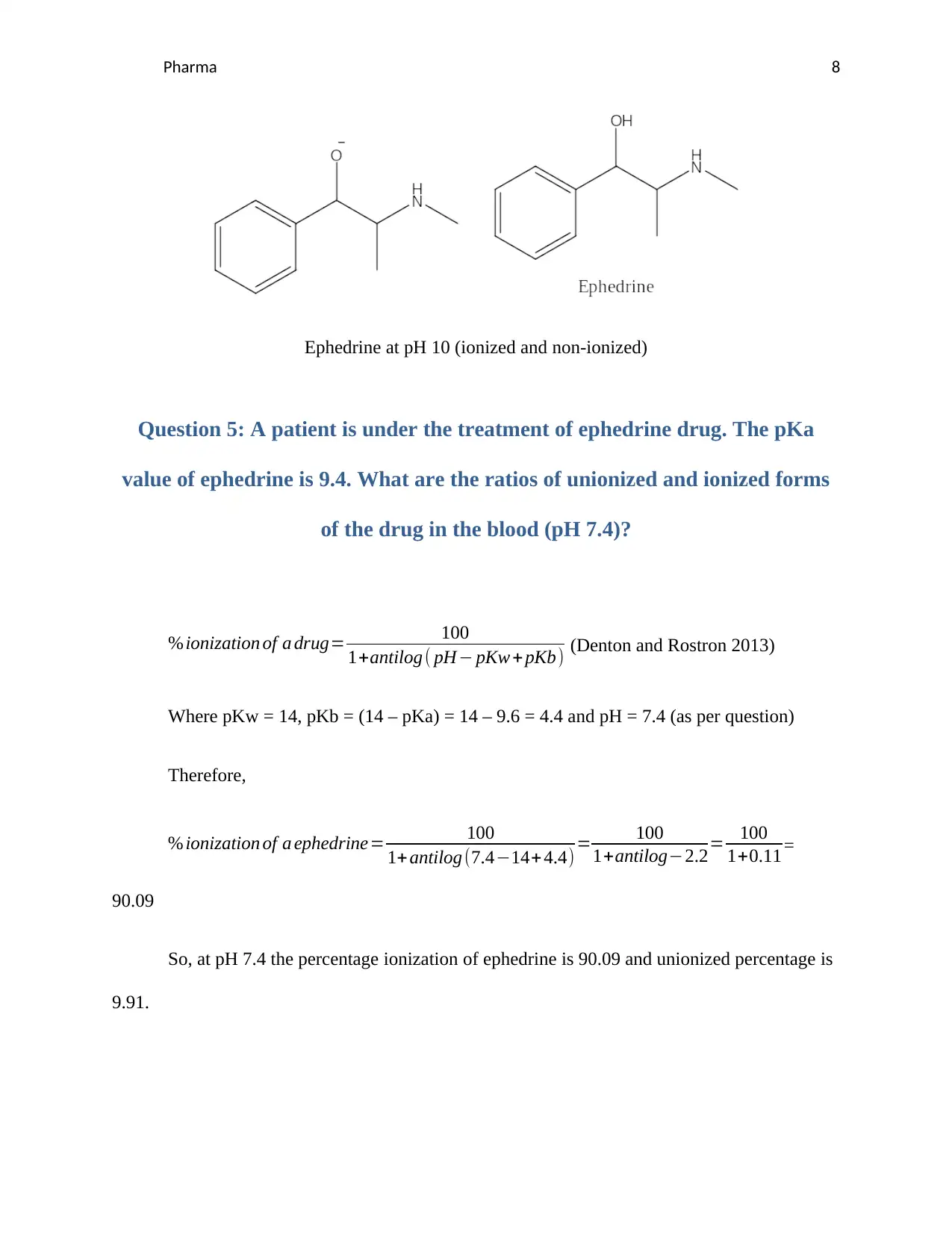
Pharma 8
Ephedrine at pH 10 (ionized and non-ionized)
Question 5: A patient is under the treatment of ephedrine drug. The pKa
value of ephedrine is 9.4. What are the ratios of unionized and ionized forms
of the drug in the blood (pH 7.4)?
% ionization of a drug= 100
1+antilog ( pH− pKw + pKb) (Denton and Rostron 2013)
Where pKw = 14, pKb = (14 – pKa) = 14 – 9.6 = 4.4 and pH = 7.4 (as per question)
Therefore,
% ionization of a ephedrine= 100
1+ antilog (7.4−14+ 4.4) = 100
1+antilog−2.2= 100
1+0.11 =
90.09
So, at pH 7.4 the percentage ionization of ephedrine is 90.09 and unionized percentage is
9.91.
Ephedrine at pH 10 (ionized and non-ionized)
Question 5: A patient is under the treatment of ephedrine drug. The pKa
value of ephedrine is 9.4. What are the ratios of unionized and ionized forms
of the drug in the blood (pH 7.4)?
% ionization of a drug= 100
1+antilog ( pH− pKw + pKb) (Denton and Rostron 2013)
Where pKw = 14, pKb = (14 – pKa) = 14 – 9.6 = 4.4 and pH = 7.4 (as per question)
Therefore,
% ionization of a ephedrine= 100
1+ antilog (7.4−14+ 4.4) = 100
1+antilog−2.2= 100
1+0.11 =
90.09
So, at pH 7.4 the percentage ionization of ephedrine is 90.09 and unionized percentage is
9.91.

Pharma 9
Question 6: Compare on the effect of ionization of ephedrine and the site of
absorption.
Ephedrine as a weak base is fully ionized at low gastric pH of 1.2 to 3, so it is retained in the aqueous
solution and does not get absorbed by the lipid rich stomach walls. They are in turn absorbed in the
intestine where the pH is 8, resulting low ionization (and hence less soluble) (CHEN and WANG 2010,
Denton and Rostron 2013).
References
Abuhelwa, A. Y., Williams, D. B., Upton, R. N. and Foster, D. J. (2017) Food, gastrointestinal pH, and
models of oral drug absorption. European Journal of Pharmaceutics and Biopharmaceutics, 112,
pp. 234-248.
Andari Sawaya, R., Jaffe, J., Friedenberg, L. and K Friedenberg, F. (2012) Vitamin, mineral, and drug
absorption following bariatric surgery. Current drug metabolism, 13(9), pp. 1345-1355.
Balch, R. J. and Trescot, A. (2010) Extended-release morphine sulfate in treatment of severe acute and
chronic pain. Journal of pain research, 3, pp. 191.
CHEN, Y.-p. and WANG, B. (2010) Study on Ephedrine Hydrochloride Intestinal Absorption in Rats [J].
Progress in Pharmaceutical Sciences, 7.
Question 6: Compare on the effect of ionization of ephedrine and the site of
absorption.
Ephedrine as a weak base is fully ionized at low gastric pH of 1.2 to 3, so it is retained in the aqueous
solution and does not get absorbed by the lipid rich stomach walls. They are in turn absorbed in the
intestine where the pH is 8, resulting low ionization (and hence less soluble) (CHEN and WANG 2010,
Denton and Rostron 2013).
References
Abuhelwa, A. Y., Williams, D. B., Upton, R. N. and Foster, D. J. (2017) Food, gastrointestinal pH, and
models of oral drug absorption. European Journal of Pharmaceutics and Biopharmaceutics, 112,
pp. 234-248.
Andari Sawaya, R., Jaffe, J., Friedenberg, L. and K Friedenberg, F. (2012) Vitamin, mineral, and drug
absorption following bariatric surgery. Current drug metabolism, 13(9), pp. 1345-1355.
Balch, R. J. and Trescot, A. (2010) Extended-release morphine sulfate in treatment of severe acute and
chronic pain. Journal of pain research, 3, pp. 191.
CHEN, Y.-p. and WANG, B. (2010) Study on Ephedrine Hydrochloride Intestinal Absorption in Rats [J].
Progress in Pharmaceutical Sciences, 7.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pharma 10
De Levie, R. (2003) The Henderson-Hasselbalch equation: its history and limitations. Journal of Chemical
Education, 80(2), pp. 146.
Denton, P. and Rostron, C. (2013) Pharmaceutics: The Science of Medicine Design, Oxford University
Press.
Estudante, M., Morais, J. G., Soveral, G. and Benet, L. Z. (2013) Intestinal drug transporters: an overview.
Advanced drug delivery reviews, 65(10), pp. 1340-1356.
Holm, R., Müllertz, A. and Mu, H. (2013) Bile salts and their importance for drug absorption.
International journal of pharmaceutics, 453(1), pp. 44-55.
Jung, M. S., Kim, J. S., Kim, M. S., Alhalaweh, A., Cho, W., Hwang, S. J. and Velaga, S. P. (2010)
Bioavailability of indomethacin saccharin cocrystals.‐ Journal of pharmacy and pharmacology,
62(11), pp. 1560-1568.
Kataoka, M., Fukahori, M., Ikemura, A., Kubota, A., Higashino, H., Sakuma, S. and Yamashita, S. (2016)
Effects of gastric pH on oral drug absorption: in vitro assessment using a dissolution/permeation
system reflecting the gastric dissolution process. European Journal of Pharmaceutics and
Biopharmaceutics, 101, pp. 103-111.
Limberger, R. P., Jacques, A. L. B., Schmitt, G. C. and Arbo, M. D. (2013) Pharmacological Effects of
Ephedrine 38.
Padwal, R., Brocks, D. and Sharma, A. (2010) A systematic review of drug absorption following bariatric
surgery and its theoretical implications. obesity reviews, 11(1), pp. 41-50.
Polster, C. S., Atassi, F., Wu, S.-J. and Sperry, D. C. (2010) Use of artificial stomach− duodenum model for
investigation of dosing fluid effect on clinical trial variability. Molecular pharmaceutics, 7(5), pp.
1533-1538.
Prasanna, J. L., Deepthi, B. and Rao, N. R. (2012) Rectal drug delivery: A promising route for enhancing
drug absorption. Asian Journal of Research in Pharmaceutical Science, 2(4), pp. 143-149.
Pypendop, B. H., Ilkiw, J. and Shilo Benjamini, Y. (2014) Bioavailability of morphine, methadone,‐
hydromorphone, and oxymorphone following buccal administration in cats. Journal of veterinary
pharmacology and therapeutics, 37(3), pp. 295-300.
De Levie, R. (2003) The Henderson-Hasselbalch equation: its history and limitations. Journal of Chemical
Education, 80(2), pp. 146.
Denton, P. and Rostron, C. (2013) Pharmaceutics: The Science of Medicine Design, Oxford University
Press.
Estudante, M., Morais, J. G., Soveral, G. and Benet, L. Z. (2013) Intestinal drug transporters: an overview.
Advanced drug delivery reviews, 65(10), pp. 1340-1356.
Holm, R., Müllertz, A. and Mu, H. (2013) Bile salts and their importance for drug absorption.
International journal of pharmaceutics, 453(1), pp. 44-55.
Jung, M. S., Kim, J. S., Kim, M. S., Alhalaweh, A., Cho, W., Hwang, S. J. and Velaga, S. P. (2010)
Bioavailability of indomethacin saccharin cocrystals.‐ Journal of pharmacy and pharmacology,
62(11), pp. 1560-1568.
Kataoka, M., Fukahori, M., Ikemura, A., Kubota, A., Higashino, H., Sakuma, S. and Yamashita, S. (2016)
Effects of gastric pH on oral drug absorption: in vitro assessment using a dissolution/permeation
system reflecting the gastric dissolution process. European Journal of Pharmaceutics and
Biopharmaceutics, 101, pp. 103-111.
Limberger, R. P., Jacques, A. L. B., Schmitt, G. C. and Arbo, M. D. (2013) Pharmacological Effects of
Ephedrine 38.
Padwal, R., Brocks, D. and Sharma, A. (2010) A systematic review of drug absorption following bariatric
surgery and its theoretical implications. obesity reviews, 11(1), pp. 41-50.
Polster, C. S., Atassi, F., Wu, S.-J. and Sperry, D. C. (2010) Use of artificial stomach− duodenum model for
investigation of dosing fluid effect on clinical trial variability. Molecular pharmaceutics, 7(5), pp.
1533-1538.
Prasanna, J. L., Deepthi, B. and Rao, N. R. (2012) Rectal drug delivery: A promising route for enhancing
drug absorption. Asian Journal of Research in Pharmaceutical Science, 2(4), pp. 143-149.
Pypendop, B. H., Ilkiw, J. and Shilo Benjamini, Y. (2014) Bioavailability of morphine, methadone,‐
hydromorphone, and oxymorphone following buccal administration in cats. Journal of veterinary
pharmacology and therapeutics, 37(3), pp. 295-300.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharma 11
Sjögren, E., Dahlgren, D., Roos, C. and Lennernas, H. (2015) Human in vivo regional intestinal
permeability: quantitation using site-specific drug absorption data. Molecular pharmaceutics,
12(6), pp. 2026-2039.
Sugihara, H., Yamamoto, H., Kawashima, Y. and Takeuchi, H. (2012) Effectiveness of submicronized
chitosan-coated liposomes in oral absorption of indomethacin. Journal of liposome research,
22(1), pp. 72-79.
Sjögren, E., Dahlgren, D., Roos, C. and Lennernas, H. (2015) Human in vivo regional intestinal
permeability: quantitation using site-specific drug absorption data. Molecular pharmaceutics,
12(6), pp. 2026-2039.
Sugihara, H., Yamamoto, H., Kawashima, Y. and Takeuchi, H. (2012) Effectiveness of submicronized
chitosan-coated liposomes in oral absorption of indomethacin. Journal of liposome research,
22(1), pp. 72-79.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.