Randomized Trials and Efficacy Homework Assignment, NUR 735, Analysis
VerifiedAdded on 2022/08/26
|8
|1541
|40
Homework Assignment
AI Summary
This document presents a comprehensive homework assignment solution for NUR 735, focusing on randomized trials and efficacy in healthcare research. The assignment begins with defining target, actual, and study populations in the context of a Type II diabetes study in children and adolescents. It then explores inclusion and exclusion criteria in a hypothetical cohort study on meat consumption and colorectal cancer. The solution further analyzes the advantages and disadvantages of using clinical and community populations for epidemiologic studies and differentiates between internal and external validity. The assignment delves into statistical errors, accuracy, and precision, providing calculations and suggesting methods to improve study accuracy. It discusses the concept of an ideal comparison group in cohort studies and the role of placebos in research. The solution also covers the objectives of randomization, identifies an unethical randomized controlled trial scenario, and explains the significance of Randomized Controlled Trials (RCTs) as a gold standard. Finally, it provides examples illustrating large and small differences observed in research studies. The document is a valuable resource for students studying research methodologies.

Running head: QUESTION & ANSWER
Question & Answer
Name of the student
Name of the university
Author’s name
Question & Answer
Name of the student
Name of the university
Author’s name
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1QUESTION & ANSWER
Q1. The objective of the study is to conduct a study on the children and adolescents who have
Type II diabetes residing in Topeka, Kansas during the 2019 calendar year. For this study all the
children both boys and girls of 6 to 18 years of age will be selected whoever have diabetes as the
target population. Later different tests will be conducted such as Glycated hemoglobin (A1C) test
and other blood tests on the children to filter out the actual population.
Q2. Inclusion Criteria – This are the criteria or characteristics which is necessary to be possessed
by the prospective subjects if they are to be included in the research study.
Exclusion criteria - This are the criteria or characteristics which should not be possessed by the
prospective subjects if they are to be included in the research study.
For a hypothetical cohort study of the effects of meat consumption on the incidence of colorectal
cancer –
Inclusion Criteria –
Only those evidence based papers have been selected which were published in English and
were published between 2014 and 2019.
The papers should involve keywords like red meat, colorectal cancer, diet pattern.
The sample population should involve men and women between the age of 30 to 40.
Exclusion criteria -
Research papers which have been published in other languages other than English and are
older than 5 years were excluded.
If keywords like vegetables, breast cancer, lung cancer or any other type of cancer are
included in the research paper then that paper will be excluded.
Q1. The objective of the study is to conduct a study on the children and adolescents who have
Type II diabetes residing in Topeka, Kansas during the 2019 calendar year. For this study all the
children both boys and girls of 6 to 18 years of age will be selected whoever have diabetes as the
target population. Later different tests will be conducted such as Glycated hemoglobin (A1C) test
and other blood tests on the children to filter out the actual population.
Q2. Inclusion Criteria – This are the criteria or characteristics which is necessary to be possessed
by the prospective subjects if they are to be included in the research study.
Exclusion criteria - This are the criteria or characteristics which should not be possessed by the
prospective subjects if they are to be included in the research study.
For a hypothetical cohort study of the effects of meat consumption on the incidence of colorectal
cancer –
Inclusion Criteria –
Only those evidence based papers have been selected which were published in English and
were published between 2014 and 2019.
The papers should involve keywords like red meat, colorectal cancer, diet pattern.
The sample population should involve men and women between the age of 30 to 40.
Exclusion criteria -
Research papers which have been published in other languages other than English and are
older than 5 years were excluded.
If keywords like vegetables, breast cancer, lung cancer or any other type of cancer are
included in the research paper then that paper will be excluded.
2QUESTION & ANSWER
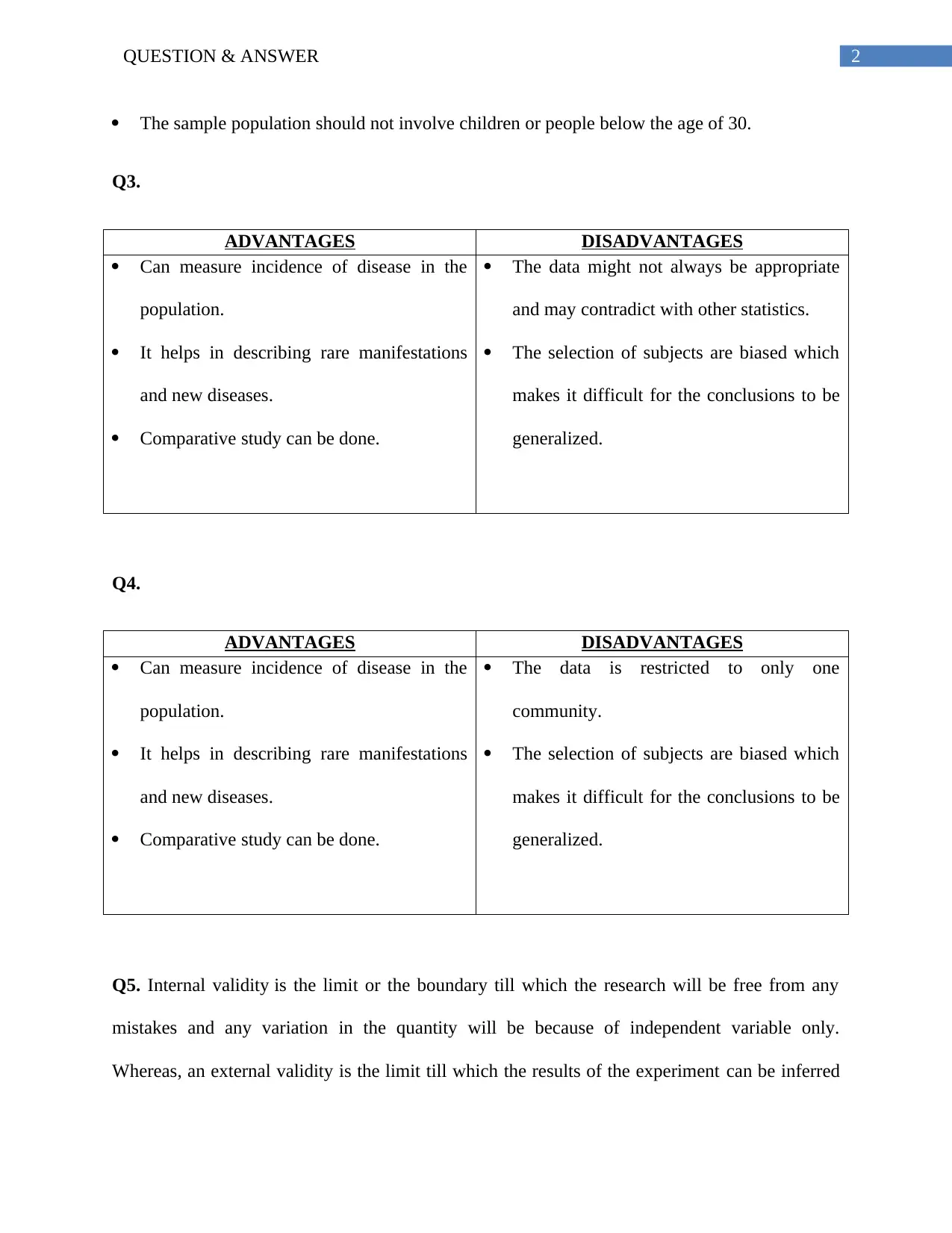
The sample population should not involve children or people below the age of 30.
Q3.
ADVANTAGES DISADVANTAGES
Can measure incidence of disease in the
population.
It helps in describing rare manifestations
and new diseases.
Comparative study can be done.
The data might not always be appropriate
and may contradict with other statistics.
The selection of subjects are biased which
makes it difficult for the conclusions to be
generalized.
Q4.
ADVANTAGES DISADVANTAGES
Can measure incidence of disease in the
population.
It helps in describing rare manifestations
and new diseases.
Comparative study can be done.
The data is restricted to only one
community.
The selection of subjects are biased which
makes it difficult for the conclusions to be
generalized.
Q5. Internal validity is the limit or the boundary till which the research will be free from any
mistakes and any variation in the quantity will be because of independent variable only.
Whereas, an external validity is the limit till which the results of the experiment can be inferred
The sample population should not involve children or people below the age of 30.
Q3.
ADVANTAGES DISADVANTAGES
Can measure incidence of disease in the
population.
It helps in describing rare manifestations
and new diseases.
Comparative study can be done.
The data might not always be appropriate
and may contradict with other statistics.
The selection of subjects are biased which
makes it difficult for the conclusions to be
generalized.
Q4.
ADVANTAGES DISADVANTAGES
Can measure incidence of disease in the
population.
It helps in describing rare manifestations
and new diseases.
Comparative study can be done.
The data is restricted to only one
community.
The selection of subjects are biased which
makes it difficult for the conclusions to be
generalized.
Q5. Internal validity is the limit or the boundary till which the research will be free from any
mistakes and any variation in the quantity will be because of independent variable only.
Whereas, an external validity is the limit till which the results of the experiment can be inferred
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
3QUESTION & ANSWER
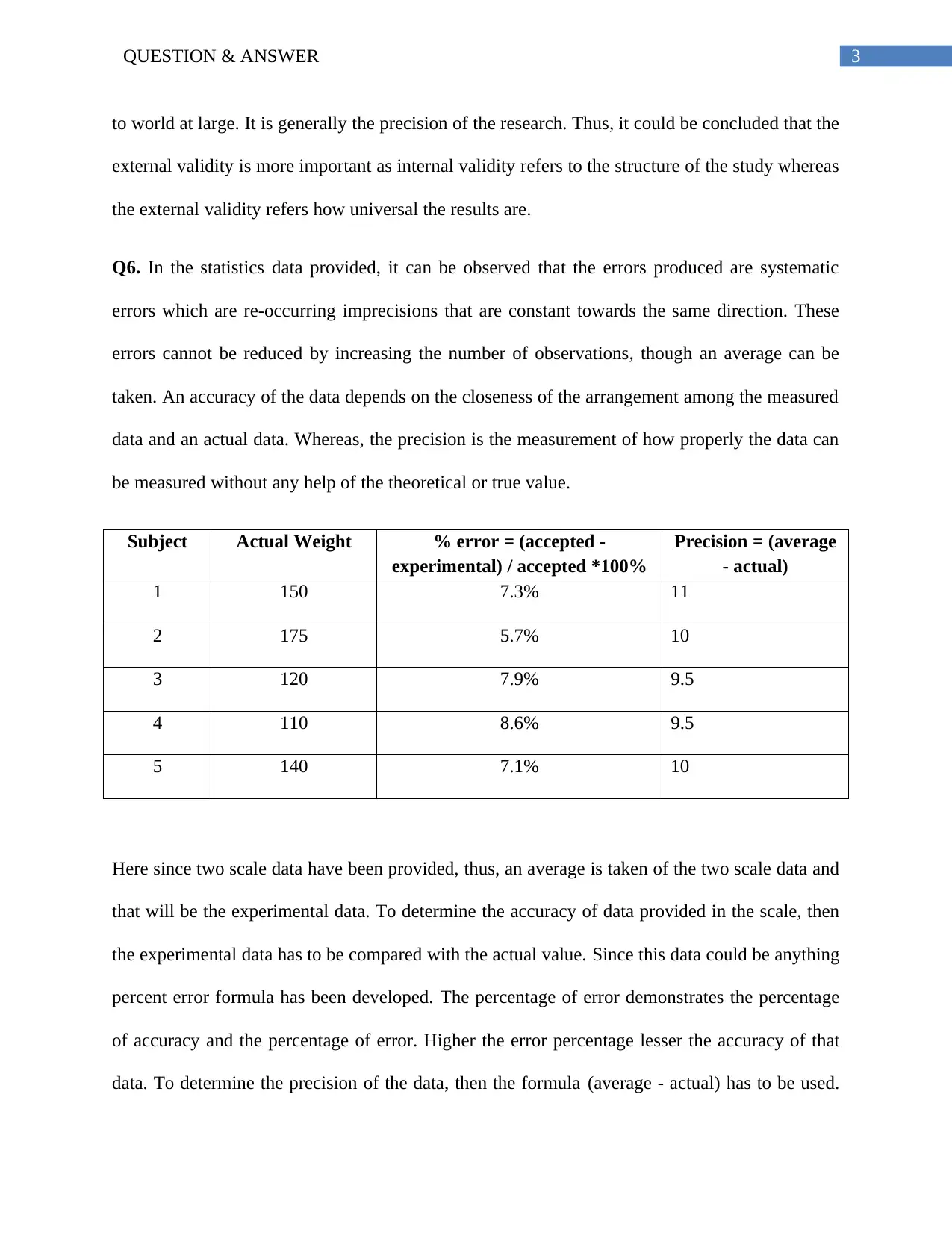
to world at large. It is generally the precision of the research. Thus, it could be concluded that the
external validity is more important as internal validity refers to the structure of the study whereas
the external validity refers how universal the results are.
Q6. In the statistics data provided, it can be observed that the errors produced are systematic
errors which are re-occurring imprecisions that are constant towards the same direction. These
errors cannot be reduced by increasing the number of observations, though an average can be
taken. An accuracy of the data depends on the closeness of the arrangement among the measured
data and an actual data. Whereas, the precision is the measurement of how properly the data can
be measured without any help of the theoretical or true value.
Subject Actual Weight % error = (accepted -
experimental) / accepted *100%
Precision = (average
- actual)
1 150 7.3% 11
2 175 5.7% 10
3 120 7.9% 9.5
4 110 8.6% 9.5
5 140 7.1% 10
Here since two scale data have been provided, thus, an average is taken of the two scale data and
that will be the experimental data. To determine the accuracy of data provided in the scale, then
the experimental data has to be compared with the actual value. Since this data could be anything
percent error formula has been developed. The percentage of error demonstrates the percentage
of accuracy and the percentage of error. Higher the error percentage lesser the accuracy of that
data. To determine the precision of the data, then the formula (average - actual) has to be used.
to world at large. It is generally the precision of the research. Thus, it could be concluded that the
external validity is more important as internal validity refers to the structure of the study whereas
the external validity refers how universal the results are.
Q6. In the statistics data provided, it can be observed that the errors produced are systematic
errors which are re-occurring imprecisions that are constant towards the same direction. These
errors cannot be reduced by increasing the number of observations, though an average can be
taken. An accuracy of the data depends on the closeness of the arrangement among the measured
data and an actual data. Whereas, the precision is the measurement of how properly the data can
be measured without any help of the theoretical or true value.
Subject Actual Weight % error = (accepted -
experimental) / accepted *100%
Precision = (average
- actual)
1 150 7.3% 11
2 175 5.7% 10
3 120 7.9% 9.5
4 110 8.6% 9.5
5 140 7.1% 10
Here since two scale data have been provided, thus, an average is taken of the two scale data and
that will be the experimental data. To determine the accuracy of data provided in the scale, then
the experimental data has to be compared with the actual value. Since this data could be anything
percent error formula has been developed. The percentage of error demonstrates the percentage
of accuracy and the percentage of error. Higher the error percentage lesser the accuracy of that
data. To determine the precision of the data, then the formula (average - actual) has to be used.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4QUESTION & ANSWER
The average will of the two scales (scale 1 + scale 2) (Sophia, 2020). Lack of validity is referred
to as ‘Bias’ or ‘systematic error’.
Thus, to make the study better, an average can be taken or a range of the measurement can be
taken, for instance –
SUBJECT WEIGHT RANGE
1 150 – 162
2 175 – 185
3 120 – 130
4 110 – 120
5 140 – 150
Q7. In a cohort study, an ideal comparison group would be a group which would be similar to an
exposed group through they would remain unexposed. It is described as “counterfactual ideal”
since it is difficult for the similar individual to be both exposed and unexposed at the same time.
A randomized trial supports by ensuring that selection of individuals for the control group is not
impacted by any kind of bias. Randomized controlled trials (RCTs) are the guidelines for linking
the factual with the counterfactual ideals.
Q8. A placebo is an object which does not have any kind of medical effects. For instance
solution of the saline, sterile water or might be a sugar pill. In order for the placebo to work, it is
necessary for the person to expect something. The more the person expects the placebo to
function more the placebo will generate a response. It is generally a perception of the brain. The
placebo just creates a response but does not cure it.
The average will of the two scales (scale 1 + scale 2) (Sophia, 2020). Lack of validity is referred
to as ‘Bias’ or ‘systematic error’.
Thus, to make the study better, an average can be taken or a range of the measurement can be
taken, for instance –
SUBJECT WEIGHT RANGE
1 150 – 162
2 175 – 185
3 120 – 130
4 110 – 120
5 140 – 150
Q7. In a cohort study, an ideal comparison group would be a group which would be similar to an
exposed group through they would remain unexposed. It is described as “counterfactual ideal”
since it is difficult for the similar individual to be both exposed and unexposed at the same time.
A randomized trial supports by ensuring that selection of individuals for the control group is not
impacted by any kind of bias. Randomized controlled trials (RCTs) are the guidelines for linking
the factual with the counterfactual ideals.
Q8. A placebo is an object which does not have any kind of medical effects. For instance
solution of the saline, sterile water or might be a sugar pill. In order for the placebo to work, it is
necessary for the person to expect something. The more the person expects the placebo to
function more the placebo will generate a response. It is generally a perception of the brain. The
placebo just creates a response but does not cure it.

5QUESTION & ANSWER
For instance, two groups of women A & B were selected who were having menstruation. Group
A was given warm water to drink whereas the group B was given normal water. It was observed
that women who drank warm water felt better as it helped in relaxing the cramped muscles;
whereas women had normal water did not improve much.
Q9. Randomization is the process by which the study participants are randomly allocated to a
treatment group. This process helps in decreasing the variation as it equally allocates individuals
with specific traits among all the trial arms. The three major objectives in a randomized clinical
trial are –
To estimate essential quantities
To examine a possible relationship among different treatment, and
To decrease sources of bias while examining the efficacy of new treatments
Q10. An unethical RCT scenario could be –
Conducting a human experimentation wherein selecting 100 people from a community and
conducting a study by using an unknown drug on the people to check its effectiveness. There is
no information regarding the drug, that is, the side effects or the positive effects are unknown
and without conducting a mice experimentation, directly using it on humans (Weyzig &
Schipper, 2006).
Q11. A Randomized Controlled Trial (RCT) is a research experiment which is directed in such a
way that all the sources of bias are terminated from the procedure. Thus, RCT is referred to as
the golden standard.
For instance, two groups of women A & B were selected who were having menstruation. Group
A was given warm water to drink whereas the group B was given normal water. It was observed
that women who drank warm water felt better as it helped in relaxing the cramped muscles;
whereas women had normal water did not improve much.
Q9. Randomization is the process by which the study participants are randomly allocated to a
treatment group. This process helps in decreasing the variation as it equally allocates individuals
with specific traits among all the trial arms. The three major objectives in a randomized clinical
trial are –
To estimate essential quantities
To examine a possible relationship among different treatment, and
To decrease sources of bias while examining the efficacy of new treatments
Q10. An unethical RCT scenario could be –
Conducting a human experimentation wherein selecting 100 people from a community and
conducting a study by using an unknown drug on the people to check its effectiveness. There is
no information regarding the drug, that is, the side effects or the positive effects are unknown
and without conducting a mice experimentation, directly using it on humans (Weyzig &
Schipper, 2006).
Q11. A Randomized Controlled Trial (RCT) is a research experiment which is directed in such a
way that all the sources of bias are terminated from the procedure. Thus, RCT is referred to as
the golden standard.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6QUESTION & ANSWER
Q12. Examples 1 – large difference – Two groups of women A and B were selected going
through periods. Group A was given warm water and group B was given normal water. It was
observed that women who drank warm water felt well compared to women who had normal
water. There was a large difference in feeling of muscle cramps in the two group as group A was
far more relaxed compared to group B.
Examples 2 – small difference – Two groups of women C and D were selected going through
periods. Group C was given cold water and group D was given normal water. It was observed
that women who drank normal water felt normal compared to women who had cold water as it
did not improve the health condition though made the feeling worse. Thus, there was hardly any
difference in the sensation of pain.
Q12. Examples 1 – large difference – Two groups of women A and B were selected going
through periods. Group A was given warm water and group B was given normal water. It was
observed that women who drank warm water felt well compared to women who had normal
water. There was a large difference in feeling of muscle cramps in the two group as group A was
far more relaxed compared to group B.
Examples 2 – small difference – Two groups of women C and D were selected going through
periods. Group C was given cold water and group D was given normal water. It was observed
that women who drank normal water felt normal compared to women who had cold water as it
did not improve the health condition though made the feeling worse. Thus, there was hardly any
difference in the sensation of pain.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7QUESTION & ANSWER
References
Weyzig, F., & Schipper, I. (2006). SOMO briefing paper on ethics in clinical trials. Centre for
research on multinational corporations, dutch ministry of foreign affairs, 16.
Sophia. (2020). Accuracy and Precision. Retrieved 17 January 2020, from
https://www.sophia.org/tutorials/accuracy-and-precision--3
References
Weyzig, F., & Schipper, I. (2006). SOMO briefing paper on ethics in clinical trials. Centre for
research on multinational corporations, dutch ministry of foreign affairs, 16.
Sophia. (2020). Accuracy and Precision. Retrieved 17 January 2020, from
https://www.sophia.org/tutorials/accuracy-and-precision--3
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





