Phylogenetic Lineages of Highly Virulent Non-O157 EHEC Serotypes
VerifiedAdded on 2022/08/17
|7
|7410
|13
Report
AI Summary
This report examines the phylogenetic lineages of highly virulent non-O157 enterohemorrhagic Escherichia coli (EHEC) serotypes, focusing on O26, O103, O111, and O145. The study employs multilocus sequence typing (MLST) to analyze 250 isolates from human and cattle sources, revealing that these serotypes cluster into distinct sequence type complexes (STCs). The findings suggest an ongoing microevolutionary scenario where the Shiga toxin gene (stx) is transferred between atypical enteropathogenic E. coli (aEPEC) and EHEC strains, highlighting the importance of aEPEC as potential pre- or post-EHEC isolates. The research emphasizes the role of virulence factors like Shiga toxin and the locus of enterocyte effacement (LEE) in EHEC pathogenesis, offering insights into the evolution and transmission of these bacteria and their implications for public health, particularly in relation to food-borne outbreaks and the development of hemolytic-uremic syndrome (HUS).

Highly Virulent Non-O157 Enterohemorrhagic Escherichia
(EHEC) Serotypes Reflect Similar Phylogenetic Lineages,Providing
New Insights into the Evolution of EHEC
Inga Eichhorn,a Katrin Heidemanns,a Torsten Semmler,a,b Bianca Kinnemann,a Alexander Mellmann,c Dag Harmsen,d
Muna F.Anjum,e Herbert Schmidt,f Angelika Fruth,b Peter Valentin-Weigand,g Jürgen Heesemann,h Sebastian Suerbaum,i
Helge Karch,c Lothar H.Wielera,b
Institute of Microbiology and Epizootics, Freie Universität Berlin, Centre for Infection Medicine, Berlin, Germanya; Robert Koch-Institut, Berlin, Germanyb; Institute of
Hygiene, University Hospital Muenster, Muenster, Germanyc; Department of Periodontology, University of Muenster, Muenster, Germanyd; Department of Bacteriology,
Animal and Plant Health Agency, Weybridge, Addlestone, Surrey, United Kingdome; Department of Food Microbiology and Hygiene, Institute of Food Science and
Biotechnology, University of Hohenheim, Stuttgart, Germanyf; Institute for Microbiology, University of Veterinary Medicine Hannover, Hannover, Germanyg; Max von
Pettenkofer-Institut, Ludwig-Maximilians-Universität, Munich, Germanyh; Institute for Medical Microbiology and Hospital Epidemiology, Hannover Medical Schoo
Hannover, Germanyi
Enterohemorrhagic Escherichia coli (EHEC) is the causative agent of bloody diarrhea and extraintestin
most importantly hemolytic-uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP).
phage-encoded Shiga toxin gene (stx), EHEC harbors the locus of enterocyte effacement (LEE), which
attaching and effacing lesions. Currently, the vast majority of EHEC infections are caused by strains b
groups (the “big five”), which, in addition to O157, the most important, comprise O26, O103, O111, a
that these four non-O157 EHEC serotypes differ in their phylogenies. To test this hypothesis, we used
ing (MLST) to analyze a large collection of 250 isolates of these four O serogroups, which were isolate
healthy humans and cattle between 1952 and 2009. The majority of the EHEC isolates of O serogroups
into one sequence type complex, STC29. Isolates of O103 clustered mainly in STC20, and most isolate
within STC32. In addition to these EHEC strains, STC29 also included stx-negative E. coli strains, term
genic E. coli (aEPEC), yet another intestinal pathogenic E. coli group. The finding that aEPEC and EHEC
serogroups share the same phylogeny suggests an ongoing microevolutionary scenario in which the p
gene stx is transferred between aEPEC and EHEC. As a consequence, aEPEC strains of STC29 can be re
EHEC isolates. Therefore, STC29 incorporates phylogenetic information useful for unraveling the evolu
Enterohemorrhagic Escherichia coli (EHEC) has emerged as a
serious public health concern, since it is responsible for a large
number of food-borne outbreaks of diarrheal diseases in humans
(1, 2). Bloody diarrhea, hemorrhagic colitis (HC), and the poten-
tially fatal hemolytic-uremic syndrome (HUS) belong to a wide
spectrum ofdisease presented by EHEC infections (2, 3). The
common virulence trait defining EHEC strains is the production
of Shiga toxin (Stx), which makes them a highly pathogenic sub-
group of Stx-producing E.coli (STEC).The Stx-encoding gene
(stx),uniquely defining the STEC pathotype,is encoded on a
lambdoid bacteriophage that is integrated into the bacterial chro-
mosome and functions as a mobile genetic element. Another im-
portant virulence factor in EHEC is a pathogenicity island desig-
nated the locus of enterocyte effacement (LEE),which encodes
proteins causing the formation of attaching and effacing (A/E)
lesions on intestinal epithelial cells through actin rearrangement
(2,3).While humans can develop serious illness when infected
with EHEC, its main reservoir, cattle, remains symptomless when
the gut is colonized with these bacteria (4).Other smallrumi-
nants, such as sheep or goats, and even wildlife ruminants, are also
known to carry and excrete EHEC (5, 6). EHEC is transmitted to
humans via the fecal-oral infection route, from animal feces to the
environment, water, and food of animal and organic origin. The
transmission from animals to humans, in addition to human-to-
human transmission, marks EHEC as a zoonotic agent (3, 7).
EHEC of serotype O157:H7 has caused severaldistinct out-
breaks in South America, Europe, Africa, North America, Japa
and China, and sporadic cases have been surveyed worldwid
12). Shortly after the first observation of an O157:H7 EHEC in
tion, other serotypes were also connected with diarrheal dise
Received 9 June 2015 Accepted 25 July 2015
Accepted manuscript posted online 31 July 2015
Citation Eichhorn I, Heidemanns K, Semmler T, Kinnemann B, Mellmann A,
Harmsen D, Anjum MF, Schmidt H, Fruth A, Valentin-Weigand P, Heesemann J,
Suerbaum S, Karch H, Wieler LH. 2015. Highly virulent non-O157
enterohemorrhagic Escherichia coli (EHEC) serotypes reflect similar phylogenet
lineages, providing new insights into the evolution of EHEC. Appl Environ
Microbiol 81:7041–7047.doi:10.1128/AEM.01921-15.
Editor: J. Björkroth
Address correspondence to Lothar H. Wieler, WielerLH@rki.de.
Supplemental material for this article may be found at http://dx.doi.org/10.1128
/AEM.01921-15.
Copyright © 2015, Eichhorn et al. This is an open-access article distributed und
the terms of the Creative Commons Attribution-Noncommercial-ShareAlike 3.0
Unported license, which permits unrestricted noncommercial use, distribution,
and reproduction in any medium, provided the original author and source are
credited.
doi:10.1128/AEM.01921-15
October 2015Volume 81Number 20 aem.asm.org7041Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
(EHEC) Serotypes Reflect Similar Phylogenetic Lineages,Providing
New Insights into the Evolution of EHEC
Inga Eichhorn,a Katrin Heidemanns,a Torsten Semmler,a,b Bianca Kinnemann,a Alexander Mellmann,c Dag Harmsen,d
Muna F.Anjum,e Herbert Schmidt,f Angelika Fruth,b Peter Valentin-Weigand,g Jürgen Heesemann,h Sebastian Suerbaum,i
Helge Karch,c Lothar H.Wielera,b
Institute of Microbiology and Epizootics, Freie Universität Berlin, Centre for Infection Medicine, Berlin, Germanya; Robert Koch-Institut, Berlin, Germanyb; Institute of
Hygiene, University Hospital Muenster, Muenster, Germanyc; Department of Periodontology, University of Muenster, Muenster, Germanyd; Department of Bacteriology,
Animal and Plant Health Agency, Weybridge, Addlestone, Surrey, United Kingdome; Department of Food Microbiology and Hygiene, Institute of Food Science and
Biotechnology, University of Hohenheim, Stuttgart, Germanyf; Institute for Microbiology, University of Veterinary Medicine Hannover, Hannover, Germanyg; Max von
Pettenkofer-Institut, Ludwig-Maximilians-Universität, Munich, Germanyh; Institute for Medical Microbiology and Hospital Epidemiology, Hannover Medical Schoo
Hannover, Germanyi
Enterohemorrhagic Escherichia coli (EHEC) is the causative agent of bloody diarrhea and extraintestin
most importantly hemolytic-uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP).
phage-encoded Shiga toxin gene (stx), EHEC harbors the locus of enterocyte effacement (LEE), which
attaching and effacing lesions. Currently, the vast majority of EHEC infections are caused by strains b
groups (the “big five”), which, in addition to O157, the most important, comprise O26, O103, O111, a
that these four non-O157 EHEC serotypes differ in their phylogenies. To test this hypothesis, we used
ing (MLST) to analyze a large collection of 250 isolates of these four O serogroups, which were isolate
healthy humans and cattle between 1952 and 2009. The majority of the EHEC isolates of O serogroups
into one sequence type complex, STC29. Isolates of O103 clustered mainly in STC20, and most isolate
within STC32. In addition to these EHEC strains, STC29 also included stx-negative E. coli strains, term
genic E. coli (aEPEC), yet another intestinal pathogenic E. coli group. The finding that aEPEC and EHEC
serogroups share the same phylogeny suggests an ongoing microevolutionary scenario in which the p
gene stx is transferred between aEPEC and EHEC. As a consequence, aEPEC strains of STC29 can be re
EHEC isolates. Therefore, STC29 incorporates phylogenetic information useful for unraveling the evolu
Enterohemorrhagic Escherichia coli (EHEC) has emerged as a
serious public health concern, since it is responsible for a large
number of food-borne outbreaks of diarrheal diseases in humans
(1, 2). Bloody diarrhea, hemorrhagic colitis (HC), and the poten-
tially fatal hemolytic-uremic syndrome (HUS) belong to a wide
spectrum ofdisease presented by EHEC infections (2, 3). The
common virulence trait defining EHEC strains is the production
of Shiga toxin (Stx), which makes them a highly pathogenic sub-
group of Stx-producing E.coli (STEC).The Stx-encoding gene
(stx),uniquely defining the STEC pathotype,is encoded on a
lambdoid bacteriophage that is integrated into the bacterial chro-
mosome and functions as a mobile genetic element. Another im-
portant virulence factor in EHEC is a pathogenicity island desig-
nated the locus of enterocyte effacement (LEE),which encodes
proteins causing the formation of attaching and effacing (A/E)
lesions on intestinal epithelial cells through actin rearrangement
(2,3).While humans can develop serious illness when infected
with EHEC, its main reservoir, cattle, remains symptomless when
the gut is colonized with these bacteria (4).Other smallrumi-
nants, such as sheep or goats, and even wildlife ruminants, are also
known to carry and excrete EHEC (5, 6). EHEC is transmitted to
humans via the fecal-oral infection route, from animal feces to the
environment, water, and food of animal and organic origin. The
transmission from animals to humans, in addition to human-to-
human transmission, marks EHEC as a zoonotic agent (3, 7).
EHEC of serotype O157:H7 has caused severaldistinct out-
breaks in South America, Europe, Africa, North America, Japa
and China, and sporadic cases have been surveyed worldwid
12). Shortly after the first observation of an O157:H7 EHEC in
tion, other serotypes were also connected with diarrheal dise
Received 9 June 2015 Accepted 25 July 2015
Accepted manuscript posted online 31 July 2015
Citation Eichhorn I, Heidemanns K, Semmler T, Kinnemann B, Mellmann A,
Harmsen D, Anjum MF, Schmidt H, Fruth A, Valentin-Weigand P, Heesemann J,
Suerbaum S, Karch H, Wieler LH. 2015. Highly virulent non-O157
enterohemorrhagic Escherichia coli (EHEC) serotypes reflect similar phylogenet
lineages, providing new insights into the evolution of EHEC. Appl Environ
Microbiol 81:7041–7047.doi:10.1128/AEM.01921-15.
Editor: J. Björkroth
Address correspondence to Lothar H. Wieler, WielerLH@rki.de.
Supplemental material for this article may be found at http://dx.doi.org/10.1128
/AEM.01921-15.
Copyright © 2015, Eichhorn et al. This is an open-access article distributed und
the terms of the Creative Commons Attribution-Noncommercial-ShareAlike 3.0
Unported license, which permits unrestricted noncommercial use, distribution,
and reproduction in any medium, provided the original author and source are
credited.
doi:10.1128/AEM.01921-15
October 2015Volume 81Number 20 aem.asm.org7041Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

in humans.Among the ⬎150 non-O157 EHEC O serogroups,
O26, O103, O111, and O145 occur most frequently in severe hu-
man diseases (3,13).It is a challenge for clinicians to identify
high-risk non-O157 serotypes, since the genetic basis that leads to
the diverse clinicaloutcomes ofdiseases caused by this EHEC
group remains unsolved (14). Comparison of genome sequences
of O157, O26, O103, and O111 revealed high similarities within
their whole gene repertoire and other factors specific for the
EHEC pathotype. Nevertheless, independent acquisition of mo-
bile genetic elements, such as pathogenicity islands (PAIs), bacte-
riophages,and plasmids,via horizontalgene transfer has been
identified as the primary driving force for a parallel evolution that
leads to different EHEC phylogenies (15). Phylogenetic analyses
from several studies suggest shared ancestors or common lineages
from which the different serotypes have evolved. For instance, a
group consisting of O103:H11 isolates was found to have the same
ancestor as EHEC O26:H11 when variants of two virulence genes
(fliC and eae) were analyzed (16). Also, since O26:H11 and O111:
H11 harbor highly similar PAI virulence profiles, it was suggested
that H11 isolates are closely related (17). Furthermore, complete
genome analyses of two O145 EHEC isolates demonstrated that
they had a common EPEC ancestor with O157:H7 before they
evolved further into another sublineage (18).
Another fact hinting at a more complex phylogeny than the
arrangementin serogroupsand evolutionary background of
EHEC strains is the frequent isolation of so-called atypical enter-
opathogenic E. coli (aEPEC) strains, yet another subgroup of in-
testinalpathogenic E.coli,which also belong to the same non-
O157 O serogroups as EHEC strains (19, 20). aEPEC strains do
not harbor the stx-converting bacteriophage but are similar to
EHEC strains capable of the A/E lesion formation caused by the
LEE. These strains are classified as “atypical” EPEC because they
lack the EAF plasmid,an adherence factor plasmid present in
EPEC strains (2). aEPEC is a common cause of nonbloody diar-
rhea in infants but is also frequently isolated from patients with
bloody diarrhea and HUS, as well as from ruminants (21). aEPEC
strains are thought to have developed during human infection via
loss ofthe stx gene and were therefore designated EHEC-LST
(EHEC that lost Stx) (22); they may convert back to EHEC upon
the acquisition of stx genes (23, 24).
In this study, the gold standard method multilocus sequence
typing (MLST) was used on randomly chosen O26, O103, O111,
and O145 E. coli strains in order to gain insight into their phylog-
eny. We tested the hypothesis that these four non-O157 O-sero-
groups are phylogenetically related.
Interestingly,our results showed that more than 40% of the
isolates examined were assigned to sequence types (STs) that form
the ST complex (STC) STC29. In addition, several aEPEC strains
from our strain collection also clustered within STC29, proving
their phylogenetic similarity.
MATERIALS AND METHODS
Bacterial isolates. A total of 250 Escherichia coli isolates were investigated.
Most of the bovine E. coli strains of O serogroups O26, O103, O111, and
O145 were chosen from strain collections of the Institute of Microbiology
and Epizootics (IMT), Freie Universität, Berlin, Berlin, Germany, as well
as the E. coli MLST database (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli)
(25).The human isolates of the relevant non-O157 O serogroups were
sampled within an exploratory clinical study of the “FBI-Zoo” research
consortium (www.fbi-zoo.de).In that study,stoolsamples of patients
with diarrhea were screened for STEC/EHEC and for Yersinia, Salmo-
nella,and Campylobacter spp.at the University HospitalMünster,the
Hannover MedicalSchool,and the Max von Pettenkofer-Institute for
Hygiene and MedicalMicrobiology (Ludwig-Maximilians-Universität
München).
In addition, 14 isolates of the HUS-associated EHEC (HUSEC) coll
tion (13) assigned to the non-O157 O serogroups O26, O103, O111,
O145 were included.Therefore,the greater part of the E.coliisolates
under investigation were sampled in Germany.Isolates obtained from
other E. coli studies, such as studies conducted in Australia, Canad
United States, and European countries such as Sweden, the United
dom,and Belgium,were also included in the study.The strains under
investigation had been sampled between the years 1952 and 2009The
strains were isolated mainly from cattle and humans,but three isolates
from sheep and one from a food source were also included in the a
After serotyping, 76 isolates were assigned to O serogroup O26 (NM
H19, K60), 96 isolates to O103 (NM, H2, H3, H11, H18, H21, H25, H
H43), 36 isolates to O111 (NM, H2, H8, H10), and 42 isolates to O14
(NM, H18, H25, H28, H34). Furthermore, 41 aEPEC isolates, of hum
(n ⫽ 10), bovine (n ⫽ 28), and ovine (n ⫽ 2) origin, as well as one iso
from a food source (n ⫽ 1), were included in our analysis. The isolat
belonged to the non-O157 O serogroups and clustered within STs o
STC29 (see Table S1 in the supplemental material).
Serotyping.Serotyping was performed using a microtiter method
with antisera against E. coli O antigens 1 to 182 and against H anti
56 according to the method of Prager et al. (26).
Pathotyping. Apart from the clinical data, the pathotypes of ind
vidual strains were determined on the basis of the presence or abs
of the virulence genes stx1, stx2, escV, and bfpB, which were identified
by PCR using published primer pairs and multiplex PCR protocols
(27). LEE-positive strains (aEPEC, EHEC) were identified by the de-
tection of escV, a LEE-located translocator gene. In addition, we als
screened for the intimin-encoding gene eae to confirm the presenc
the LEE (28). aEPEC strains do not harbor the EAF plasmid and ther
fore are bfpB negative.
The stx genes of strains positive for any stx gene were subtyped
ing to the work of Scheutz et al. (2012) by using the published prim
PCR protocols (29) (see also Fig. S2 in the supplemental material).
MLST. Multilocus sequence typing (MLST) was performed by ana
ing internal fragments of seven housekeeping genes (adk, fumC, g
mdh, purA, recA) (25). The alleles and sequence types (STs) were a
in accordance with the E. coli MLST website (http://mlst.warwick.ac
/mlst/dbs/Ecoli) using Ridom SeqSphere software (Ridom GmbH, Mü
ster, Germany) in order to generate a database. The genetic relatio
between different STs were determined on the basis of the allele pr
obtained using Bionumerics, version 7.1 (Applied Maths, Sint-Marte
Latem,Belgium).Bionumerics was also used to constructminimum
spanning trees (MSTs).
Statistical analysis. Host, O type, H type, and serotype inform
for isolates was summarized by sequence type complex and was st
cally analyzed for STC10, STC20, STC29, and STC32 with multinom
logistic regression using IBM SPSS Statistics software (version 20).
results are shown in Table S2 in the supplemental material, where
icant P values are highlighted.
RESULTS
Two-hundred fifty STEC strains belonging to O serogroups O2
O103,O111,and O145,of different origins,including human,
animal,and food sources,were screened for typicalvirulence-
associated genes using published primer pairs.
Next,we analyzed the non-O157 isolates using MLST.The
resulting phylogenetic relationships of the isolate population
displayed in a minimum spanning tree (MST) (Fig. 1). The va
majority of isolates (n ⫽ 234 [93.6%]) were assigned to four d
ferent ST complexes (STCs): STC10, STC20, STC29, and STC3
STC29 comprised 42.8% (n ⫽ 107) of the isolates and 15 STs
Eichhorn et al.
7042 aem.asm.org October 2015Volume 81 Number 20Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
O26, O103, O111, and O145 occur most frequently in severe hu-
man diseases (3,13).It is a challenge for clinicians to identify
high-risk non-O157 serotypes, since the genetic basis that leads to
the diverse clinicaloutcomes ofdiseases caused by this EHEC
group remains unsolved (14). Comparison of genome sequences
of O157, O26, O103, and O111 revealed high similarities within
their whole gene repertoire and other factors specific for the
EHEC pathotype. Nevertheless, independent acquisition of mo-
bile genetic elements, such as pathogenicity islands (PAIs), bacte-
riophages,and plasmids,via horizontalgene transfer has been
identified as the primary driving force for a parallel evolution that
leads to different EHEC phylogenies (15). Phylogenetic analyses
from several studies suggest shared ancestors or common lineages
from which the different serotypes have evolved. For instance, a
group consisting of O103:H11 isolates was found to have the same
ancestor as EHEC O26:H11 when variants of two virulence genes
(fliC and eae) were analyzed (16). Also, since O26:H11 and O111:
H11 harbor highly similar PAI virulence profiles, it was suggested
that H11 isolates are closely related (17). Furthermore, complete
genome analyses of two O145 EHEC isolates demonstrated that
they had a common EPEC ancestor with O157:H7 before they
evolved further into another sublineage (18).
Another fact hinting at a more complex phylogeny than the
arrangementin serogroupsand evolutionary background of
EHEC strains is the frequent isolation of so-called atypical enter-
opathogenic E. coli (aEPEC) strains, yet another subgroup of in-
testinalpathogenic E.coli,which also belong to the same non-
O157 O serogroups as EHEC strains (19, 20). aEPEC strains do
not harbor the stx-converting bacteriophage but are similar to
EHEC strains capable of the A/E lesion formation caused by the
LEE. These strains are classified as “atypical” EPEC because they
lack the EAF plasmid,an adherence factor plasmid present in
EPEC strains (2). aEPEC is a common cause of nonbloody diar-
rhea in infants but is also frequently isolated from patients with
bloody diarrhea and HUS, as well as from ruminants (21). aEPEC
strains are thought to have developed during human infection via
loss ofthe stx gene and were therefore designated EHEC-LST
(EHEC that lost Stx) (22); they may convert back to EHEC upon
the acquisition of stx genes (23, 24).
In this study, the gold standard method multilocus sequence
typing (MLST) was used on randomly chosen O26, O103, O111,
and O145 E. coli strains in order to gain insight into their phylog-
eny. We tested the hypothesis that these four non-O157 O-sero-
groups are phylogenetically related.
Interestingly,our results showed that more than 40% of the
isolates examined were assigned to sequence types (STs) that form
the ST complex (STC) STC29. In addition, several aEPEC strains
from our strain collection also clustered within STC29, proving
their phylogenetic similarity.
MATERIALS AND METHODS
Bacterial isolates. A total of 250 Escherichia coli isolates were investigated.
Most of the bovine E. coli strains of O serogroups O26, O103, O111, and
O145 were chosen from strain collections of the Institute of Microbiology
and Epizootics (IMT), Freie Universität, Berlin, Berlin, Germany, as well
as the E. coli MLST database (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli)
(25).The human isolates of the relevant non-O157 O serogroups were
sampled within an exploratory clinical study of the “FBI-Zoo” research
consortium (www.fbi-zoo.de).In that study,stoolsamples of patients
with diarrhea were screened for STEC/EHEC and for Yersinia, Salmo-
nella,and Campylobacter spp.at the University HospitalMünster,the
Hannover MedicalSchool,and the Max von Pettenkofer-Institute for
Hygiene and MedicalMicrobiology (Ludwig-Maximilians-Universität
München).
In addition, 14 isolates of the HUS-associated EHEC (HUSEC) coll
tion (13) assigned to the non-O157 O serogroups O26, O103, O111,
O145 were included.Therefore,the greater part of the E.coliisolates
under investigation were sampled in Germany.Isolates obtained from
other E. coli studies, such as studies conducted in Australia, Canad
United States, and European countries such as Sweden, the United
dom,and Belgium,were also included in the study.The strains under
investigation had been sampled between the years 1952 and 2009The
strains were isolated mainly from cattle and humans,but three isolates
from sheep and one from a food source were also included in the a
After serotyping, 76 isolates were assigned to O serogroup O26 (NM
H19, K60), 96 isolates to O103 (NM, H2, H3, H11, H18, H21, H25, H
H43), 36 isolates to O111 (NM, H2, H8, H10), and 42 isolates to O14
(NM, H18, H25, H28, H34). Furthermore, 41 aEPEC isolates, of hum
(n ⫽ 10), bovine (n ⫽ 28), and ovine (n ⫽ 2) origin, as well as one iso
from a food source (n ⫽ 1), were included in our analysis. The isolat
belonged to the non-O157 O serogroups and clustered within STs o
STC29 (see Table S1 in the supplemental material).
Serotyping.Serotyping was performed using a microtiter method
with antisera against E. coli O antigens 1 to 182 and against H anti
56 according to the method of Prager et al. (26).
Pathotyping. Apart from the clinical data, the pathotypes of ind
vidual strains were determined on the basis of the presence or abs
of the virulence genes stx1, stx2, escV, and bfpB, which were identified
by PCR using published primer pairs and multiplex PCR protocols
(27). LEE-positive strains (aEPEC, EHEC) were identified by the de-
tection of escV, a LEE-located translocator gene. In addition, we als
screened for the intimin-encoding gene eae to confirm the presenc
the LEE (28). aEPEC strains do not harbor the EAF plasmid and ther
fore are bfpB negative.
The stx genes of strains positive for any stx gene were subtyped
ing to the work of Scheutz et al. (2012) by using the published prim
PCR protocols (29) (see also Fig. S2 in the supplemental material).
MLST. Multilocus sequence typing (MLST) was performed by ana
ing internal fragments of seven housekeeping genes (adk, fumC, g
mdh, purA, recA) (25). The alleles and sequence types (STs) were a
in accordance with the E. coli MLST website (http://mlst.warwick.ac
/mlst/dbs/Ecoli) using Ridom SeqSphere software (Ridom GmbH, Mü
ster, Germany) in order to generate a database. The genetic relatio
between different STs were determined on the basis of the allele pr
obtained using Bionumerics, version 7.1 (Applied Maths, Sint-Marte
Latem,Belgium).Bionumerics was also used to constructminimum
spanning trees (MSTs).
Statistical analysis. Host, O type, H type, and serotype inform
for isolates was summarized by sequence type complex and was st
cally analyzed for STC10, STC20, STC29, and STC32 with multinom
logistic regression using IBM SPSS Statistics software (version 20).
results are shown in Table S2 in the supplemental material, where
icant P values are highlighted.
RESULTS
Two-hundred fifty STEC strains belonging to O serogroups O2
O103,O111,and O145,of different origins,including human,
animal,and food sources,were screened for typicalvirulence-
associated genes using published primer pairs.
Next,we analyzed the non-O157 isolates using MLST.The
resulting phylogenetic relationships of the isolate population
displayed in a minimum spanning tree (MST) (Fig. 1). The va
majority of isolates (n ⫽ 234 [93.6%]) were assigned to four d
ferent ST complexes (STCs): STC10, STC20, STC29, and STC3
STC29 comprised 42.8% (n ⫽ 107) of the isolates and 15 STs
Eichhorn et al.
7042 aem.asm.org October 2015Volume 81 Number 20Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
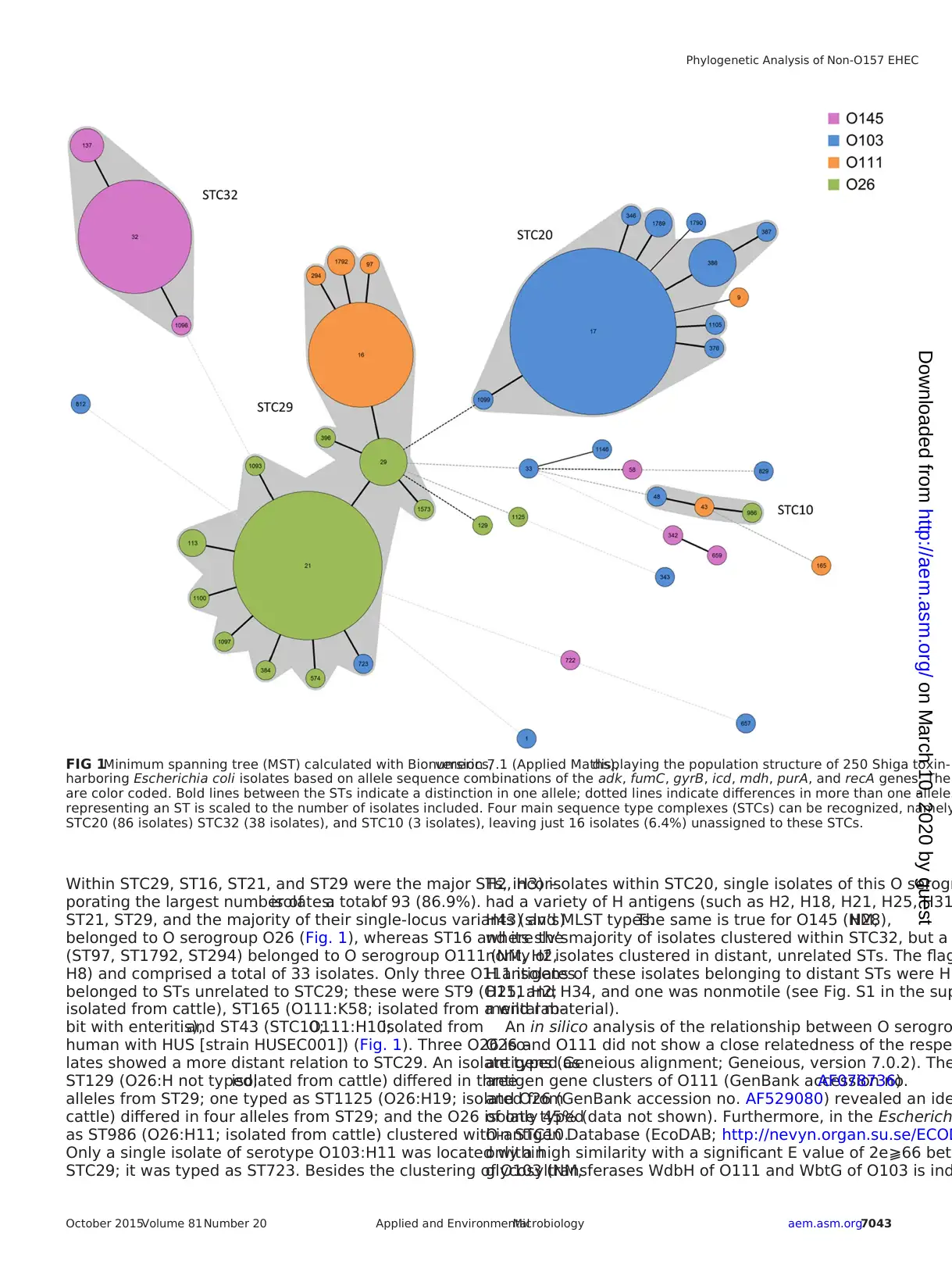
Within STC29, ST16, ST21, and ST29 were the major STs, incor-
porating the largest number ofisolates:a totalof 93 (86.9%).
ST21, ST29, and the majority of their single-locus variants (slv’s)
belonged to O serogroup O26 (Fig. 1), whereas ST16 and its slv’s
(ST97, ST1792, ST294) belonged to O serogroup O111 (NM, H2,
H8) and comprised a total of 33 isolates. Only three O111 isolates
belonged to STs unrelated to STC29; these were ST9 (O111:H2;
isolated from cattle), ST165 (O111:K58; isolated from a wild rab-
bit with enteritis),and ST43 (STC10;O111:H10;isolated from
human with HUS [strain HUSEC001]) (Fig. 1). Three O26 iso-
lates showed a more distant relation to STC29. An isolate typed as
ST129 (O26:H not typed;isolated from cattle) differed in three
alleles from ST29; one typed as ST1125 (O26:H19; isolated from
cattle) differed in four alleles from ST29; and the O26 isolate typed
as ST986 (O26:H11; isolated from cattle) clustered within STC10.
Only a single isolate of serotype O103:H11 was located within
STC29; it was typed as ST723. Besides the clustering of O103 (NM,
H2, H3) isolates within STC20, single isolates of this O serogr
had a variety of H antigens (such as H2, H18, H21, H25, H31
H43) and MLST types.The same is true for O145 (NM,H28),
where the majority of isolates clustered within STC32, but a
nority of isolates clustered in distant, unrelated STs. The flag
H antigens of these isolates belonging to distant STs were H1
H25, and H34, and one was nonmotile (see Fig. S1 in the sup
mental material).
An in silico analysis of the relationship between O serogro
O26 and O111 did not show a close relatedness of the respe
antigens (Geneious alignment; Geneious, version 7.0.2). The
antigen gene clusters of O111 (GenBank accession no.AF078736)
and O26 (GenBank accession no. AF529080) revealed an ide
of only 45% (data not shown). Furthermore, in the Escherich
O-antigen Database (EcoDAB; http://nevyn.organ.su.se/ECOD
only a high similarity with a significant E value of 2e⫺66 betw
glycosyltransferases WdbH of O111 and WbtG of O103 is ind
FIG 1Minimum spanning tree (MST) calculated with Bionumerics,version 7.1 (Applied Maths),displaying the population structure of 250 Shiga toxin-
harboring Escherichia coli isolates based on allele sequence combinations of the adk, fumC, gyrB, icd, mdh, purA, and recA genes. The
are color coded. Bold lines between the STs indicate a distinction in one allele; dotted lines indicate differences in more than one allele.
representing an ST is scaled to the number of isolates included. Four main sequence type complexes (STCs) can be recognized, namely
STC20 (86 isolates) STC32 (38 isolates), and STC10 (3 isolates), leaving just 16 isolates (6.4%) unassigned to these STCs.
Phylogenetic Analysis of Non-O157 EHEC
October 2015Volume 81Number 20 aem.asm.org7043Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
porating the largest number ofisolates:a totalof 93 (86.9%).
ST21, ST29, and the majority of their single-locus variants (slv’s)
belonged to O serogroup O26 (Fig. 1), whereas ST16 and its slv’s
(ST97, ST1792, ST294) belonged to O serogroup O111 (NM, H2,
H8) and comprised a total of 33 isolates. Only three O111 isolates
belonged to STs unrelated to STC29; these were ST9 (O111:H2;
isolated from cattle), ST165 (O111:K58; isolated from a wild rab-
bit with enteritis),and ST43 (STC10;O111:H10;isolated from
human with HUS [strain HUSEC001]) (Fig. 1). Three O26 iso-
lates showed a more distant relation to STC29. An isolate typed as
ST129 (O26:H not typed;isolated from cattle) differed in three
alleles from ST29; one typed as ST1125 (O26:H19; isolated from
cattle) differed in four alleles from ST29; and the O26 isolate typed
as ST986 (O26:H11; isolated from cattle) clustered within STC10.
Only a single isolate of serotype O103:H11 was located within
STC29; it was typed as ST723. Besides the clustering of O103 (NM,
H2, H3) isolates within STC20, single isolates of this O serogr
had a variety of H antigens (such as H2, H18, H21, H25, H31
H43) and MLST types.The same is true for O145 (NM,H28),
where the majority of isolates clustered within STC32, but a
nority of isolates clustered in distant, unrelated STs. The flag
H antigens of these isolates belonging to distant STs were H1
H25, and H34, and one was nonmotile (see Fig. S1 in the sup
mental material).
An in silico analysis of the relationship between O serogro
O26 and O111 did not show a close relatedness of the respe
antigens (Geneious alignment; Geneious, version 7.0.2). The
antigen gene clusters of O111 (GenBank accession no.AF078736)
and O26 (GenBank accession no. AF529080) revealed an ide
of only 45% (data not shown). Furthermore, in the Escherich
O-antigen Database (EcoDAB; http://nevyn.organ.su.se/ECOD
only a high similarity with a significant E value of 2e⫺66 betw
glycosyltransferases WdbH of O111 and WbtG of O103 is ind
FIG 1Minimum spanning tree (MST) calculated with Bionumerics,version 7.1 (Applied Maths),displaying the population structure of 250 Shiga toxin-
harboring Escherichia coli isolates based on allele sequence combinations of the adk, fumC, gyrB, icd, mdh, purA, and recA genes. The
are color coded. Bold lines between the STs indicate a distinction in one allele; dotted lines indicate differences in more than one allele.
representing an ST is scaled to the number of isolates included. Four main sequence type complexes (STCs) can be recognized, namely
STC20 (86 isolates) STC32 (38 isolates), and STC10 (3 isolates), leaving just 16 isolates (6.4%) unassigned to these STCs.
Phylogenetic Analysis of Non-O157 EHEC
October 2015Volume 81Number 20 aem.asm.org7043Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

cated, whereas for glycosyltransferases of O111 and O26, no sim-
ilarity at all is mentioned.
The unexpected clustering of mainly EHEC isolates from O
serogroups O26 and O111 in STC29 prompted us to analyze atyp-
icalEPEC strains from these serotypes as well.Of a totalof 41
aEPEC strains included in our analysis, we identified human (n ⫽
10), bovine (n ⫽ 28), ovine (n ⫽ 2), and food (n ⫽ 1) isolates that
belonged to the non-O157 O serogroups investigated and clus-
tered into STC29. The majority (37 isolates [90.2%]) shared the
STs with the EHEC/STEC isolates;only four additional STs oc-
curred as slv’s ofST21 or ST29:ST389,ST575,ST1107,and
ST1108 (Fig. 2).
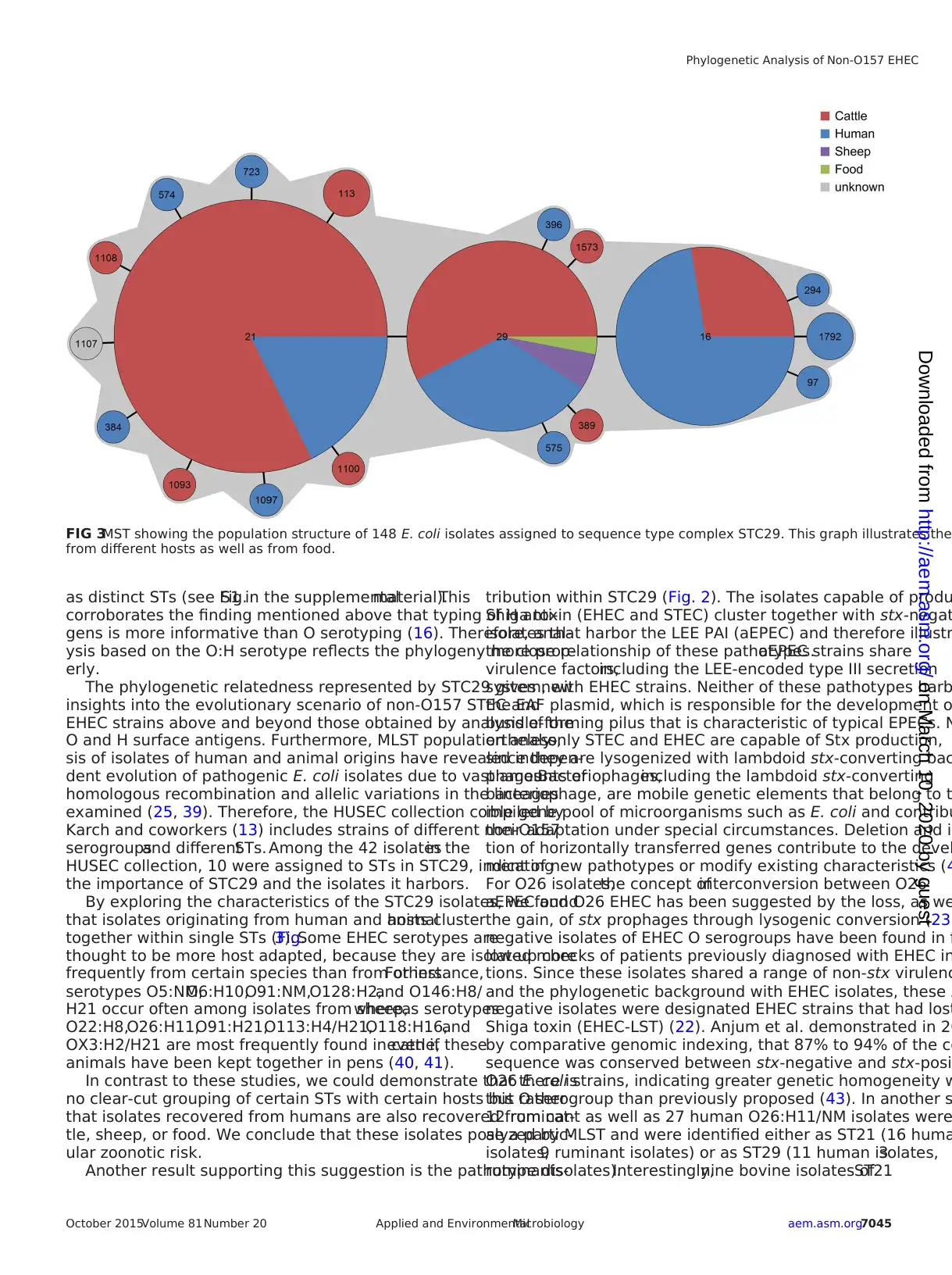
Analysis of the origins of the 148 isolates of STC29 demon-
strated no clear association between origin and ST, since the three
most common STs (ST16, ST21, ST29) were found in humans as
well as in cattle. Because isolates from food or sheep were quite
rare, no valid conclusion could be drawn here (Fig. 3).
DISCUSSION
Several non-O157 O serogroups are important causes of diarrheal
diseases producing clinical symptoms of similar severity to those
for serotype O157:H7.The most frequent non-O157 causative
agents belong to O serogroups O26, O103, O111, and O145 (30),
which are capable of causing significant diarrheal outbreaks (31–
34). Since they were first associated with human disease, the de-
tection rate ofnon-O157 strains in diarrhealdiseases has in-
creased.This could be due to better detection techniques and
increased awareness of the non-O157 serogroup-associated risk of
infection, as well as to a higher frequency of these pathogens oc-
curring in human and animal infections (11, 35, 36). In this study,
we analyzed a collection of 250 non-O157 STEC and EHEC iso-
lates by use of MLST. The MLST results revealed a close relation-
ship of the two most prevalent non-O157 EHEC O serogroups
O26 and O111, since they clustered together within a single
STC29. This was also published for three EHEC isolates of O2
H11 that clustered together with three EPEC isolates of serot
O111:H8 in distinct STs of STC29 (25).
The common classification of E. coli isolates into different
serogroups is thought to indicate a distant relationship of the
isolates. Thus, an O serogroup would also suggest a detache
logeny of this lineage with accompanying differences in its b
ical characteristics. The divergence in the O-antigen genes o
and O26 could be due to lateral gene transfer of the rfb-like
which caused the evolution of O157:H7 isolates from a comm
O55:H7-like ancestor, where this region is not present (37). Com-
parable recombination events of the O-antigen coding region
could be responsible for the evolution of EHEC and aEPEC str
of O serogroups O26 and O111 from a common ancestor, ex
ing the differences in the somatic antigen. The usage of O se
groups as descriptive indicators for pathogenicity results in d
tinct phylogenetic lineages,although the isolates investigated
might be polyphyletic.
In contrast,phylogenetic analyses of the flagellar H antigen
described them as monophyletic and revealed a close relate
of O111:H11 and O26:H11 strains in a parsimony phylogenet
tree based on genome-wide single nucleotide polymorphism
(SNPs) (38).This led to the hypothesis that O26:H11 evolved
from an ancestralO111:H11 strain by an antigenic shift from
O111 to O26 and generally that STEC strains with the same H
antigens might share an ancestor (16).In our study,the genes
encoding flagellar H antigens showed a similar result in the M
as the O antigens. Those isolates that were distributed outsid
major STCs were assigned to various H types (H10,H19,H11,
H18, H21, H25, H31, H34, and H43), which fits their designat
FIG 2MST displaying the population structure of 148 E. coli isolates belonging to pathotypes STEC, EHEC, and aEPEC and assigned to se
STC29. The distribution of the pathotypes of isolates is illustrated by different colors.
Eichhorn et al.
7044 aem.asm.org October 2015Volume 81 Number 20Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
ilarity at all is mentioned.
The unexpected clustering of mainly EHEC isolates from O
serogroups O26 and O111 in STC29 prompted us to analyze atyp-
icalEPEC strains from these serotypes as well.Of a totalof 41
aEPEC strains included in our analysis, we identified human (n ⫽
10), bovine (n ⫽ 28), ovine (n ⫽ 2), and food (n ⫽ 1) isolates that
belonged to the non-O157 O serogroups investigated and clus-
tered into STC29. The majority (37 isolates [90.2%]) shared the
STs with the EHEC/STEC isolates;only four additional STs oc-
curred as slv’s ofST21 or ST29:ST389,ST575,ST1107,and
ST1108 (Fig. 2).
Analysis of the origins of the 148 isolates of STC29 demon-
strated no clear association between origin and ST, since the three
most common STs (ST16, ST21, ST29) were found in humans as
well as in cattle. Because isolates from food or sheep were quite
rare, no valid conclusion could be drawn here (Fig. 3).
DISCUSSION
Several non-O157 O serogroups are important causes of diarrheal
diseases producing clinical symptoms of similar severity to those
for serotype O157:H7.The most frequent non-O157 causative
agents belong to O serogroups O26, O103, O111, and O145 (30),
which are capable of causing significant diarrheal outbreaks (31–
34). Since they were first associated with human disease, the de-
tection rate ofnon-O157 strains in diarrhealdiseases has in-
creased.This could be due to better detection techniques and
increased awareness of the non-O157 serogroup-associated risk of
infection, as well as to a higher frequency of these pathogens oc-
curring in human and animal infections (11, 35, 36). In this study,
we analyzed a collection of 250 non-O157 STEC and EHEC iso-
lates by use of MLST. The MLST results revealed a close relation-
ship of the two most prevalent non-O157 EHEC O serogroups
O26 and O111, since they clustered together within a single
STC29. This was also published for three EHEC isolates of O2
H11 that clustered together with three EPEC isolates of serot
O111:H8 in distinct STs of STC29 (25).
The common classification of E. coli isolates into different
serogroups is thought to indicate a distant relationship of the
isolates. Thus, an O serogroup would also suggest a detache
logeny of this lineage with accompanying differences in its b
ical characteristics. The divergence in the O-antigen genes o
and O26 could be due to lateral gene transfer of the rfb-like
which caused the evolution of O157:H7 isolates from a comm
O55:H7-like ancestor, where this region is not present (37). Com-
parable recombination events of the O-antigen coding region
could be responsible for the evolution of EHEC and aEPEC str
of O serogroups O26 and O111 from a common ancestor, ex
ing the differences in the somatic antigen. The usage of O se
groups as descriptive indicators for pathogenicity results in d
tinct phylogenetic lineages,although the isolates investigated
might be polyphyletic.
In contrast,phylogenetic analyses of the flagellar H antigen
described them as monophyletic and revealed a close relate
of O111:H11 and O26:H11 strains in a parsimony phylogenet
tree based on genome-wide single nucleotide polymorphism
(SNPs) (38).This led to the hypothesis that O26:H11 evolved
from an ancestralO111:H11 strain by an antigenic shift from
O111 to O26 and generally that STEC strains with the same H
antigens might share an ancestor (16).In our study,the genes
encoding flagellar H antigens showed a similar result in the M
as the O antigens. Those isolates that were distributed outsid
major STCs were assigned to various H types (H10,H19,H11,
H18, H21, H25, H31, H34, and H43), which fits their designat
FIG 2MST displaying the population structure of 148 E. coli isolates belonging to pathotypes STEC, EHEC, and aEPEC and assigned to se
STC29. The distribution of the pathotypes of isolates is illustrated by different colors.
Eichhorn et al.
7044 aem.asm.org October 2015Volume 81 Number 20Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
as distinct STs (see Fig.S1 in the supplementalmaterial).This
corroborates the finding mentioned above that typing of H anti-
gens is more informative than O serotyping (16). Therefore, anal-
ysis based on the O:H serotype reflects the phylogeny more prop-
erly.
The phylogenetic relatedness represented by STC29 gives new
insights into the evolutionary scenario of non-O157 STEC and
EHEC strains above and beyond those obtained by analysis of the
O and H surface antigens. Furthermore, MLST population analy-
sis of isolates of human and animal origins have revealed indepen-
dent evolution of pathogenic E. coli isolates due to vast amounts of
homologous recombination and allelic variations in the lineages
examined (25, 39). Therefore, the HUSEC collection compiled by
Karch and coworkers (13) includes strains of different non-O157
serogroupsand differentSTs. Among the 42 isolatesin the
HUSEC collection, 10 were assigned to STs in STC29, indicating
the importance of STC29 and the isolates it harbors.
By exploring the characteristics of the STC29 isolates, we found
that isolates originating from human and animalhosts cluster
together within single STs (Fig.3). Some EHEC serotypes are
thought to be more host adapted, because they are isolated more
frequently from certain species than from others.For instance,
serotypes O5:NM,O6:H10,O91:NM,O128:H2,and O146:H8/
H21 occur often among isolates from sheep,whereas serotypes
O22:H8,O26:H11,O91:H21,O113:H4/H21,O118:H16,and
OX3:H2/H21 are most frequently found in cattle,even if these
animals have been kept together in pens (40, 41).
In contrast to these studies, we could demonstrate that there is
no clear-cut grouping of certain STs with certain hosts but rather
that isolates recovered from humans are also recovered from cat-
tle, sheep, or food. We conclude that these isolates pose a partic-
ular zoonotic risk.
Another result supporting this suggestion is the pathotype dis-
tribution within STC29 (Fig. 2). The isolates capable of produ
Shiga toxin (EHEC and STEC) cluster together with stx-negat
isolates that harbor the LEE PAI (aEPEC) and therefore illustr
the close relationship of these pathotypes.aEPEC strains share
virulence factors,including the LEE-encoded type III secretion
system, with EHEC strains. Neither of these pathotypes harb
the EAF plasmid, which is responsible for the development o
bundle-forming pilus that is characteristic of typical EPECs. N
ertheless,only STEC and EHEC are capable of Stx production,
since they are lysogenized with lambdoid stx-converting bac
phages.Bacteriophages,including the lambdoid stx-converting
bacteriophage, are mobile genetic elements that belong to t
ible gene pool of microorganisms such as E. coli and contribu
their adaptation under special circumstances. Deletion and in
tion of horizontally transferred genes contribute to the devel
ment of new pathotypes or modify existing characteristics (4
For O26 isolates,the concept ofinterconversion between O26
aEPEC and O26 EHEC has been suggested by the loss, as we
the gain, of stx prophages through lysogenic conversion (23)
negative isolates of EHEC O serogroups have been found in f
low-up checks of patients previously diagnosed with EHEC in
tions. Since these isolates shared a range of non-stx virulenc
and the phylogenetic background with EHEC isolates, these s
negative isolates were designated EHEC strains that had lost
Shiga toxin (EHEC-LST) (22). Anjum et al. demonstrated in 20
by comparative genomic indexing, that 87% to 94% of the co
sequence was conserved between stx-negative and stx-posi
O26 E. coli strains, indicating greater genetic homogeneity w
this O serogroup than previously proposed (43). In another s
12 ruminant as well as 27 human O26:H11/NM isolates were
alyzed by MLST and were identified either as ST21 (16 huma
isolates,9 ruminant isolates) or as ST29 (11 human isolates,3
ruminantisolates).Interestingly,nine bovine isolates ofST21
FIG 3MST showing the population structure of 148 E. coli isolates assigned to sequence type complex STC29. This graph illustrates the
from different hosts as well as from food.
Phylogenetic Analysis of Non-O157 EHEC
October 2015Volume 81Number 20 aem.asm.org7045Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
corroborates the finding mentioned above that typing of H anti-
gens is more informative than O serotyping (16). Therefore, anal-
ysis based on the O:H serotype reflects the phylogeny more prop-
erly.
The phylogenetic relatedness represented by STC29 gives new
insights into the evolutionary scenario of non-O157 STEC and
EHEC strains above and beyond those obtained by analysis of the
O and H surface antigens. Furthermore, MLST population analy-
sis of isolates of human and animal origins have revealed indepen-
dent evolution of pathogenic E. coli isolates due to vast amounts of
homologous recombination and allelic variations in the lineages
examined (25, 39). Therefore, the HUSEC collection compiled by
Karch and coworkers (13) includes strains of different non-O157
serogroupsand differentSTs. Among the 42 isolatesin the
HUSEC collection, 10 were assigned to STs in STC29, indicating
the importance of STC29 and the isolates it harbors.
By exploring the characteristics of the STC29 isolates, we found
that isolates originating from human and animalhosts cluster
together within single STs (Fig.3). Some EHEC serotypes are
thought to be more host adapted, because they are isolated more
frequently from certain species than from others.For instance,
serotypes O5:NM,O6:H10,O91:NM,O128:H2,and O146:H8/
H21 occur often among isolates from sheep,whereas serotypes
O22:H8,O26:H11,O91:H21,O113:H4/H21,O118:H16,and
OX3:H2/H21 are most frequently found in cattle,even if these
animals have been kept together in pens (40, 41).
In contrast to these studies, we could demonstrate that there is
no clear-cut grouping of certain STs with certain hosts but rather
that isolates recovered from humans are also recovered from cat-
tle, sheep, or food. We conclude that these isolates pose a partic-
ular zoonotic risk.
Another result supporting this suggestion is the pathotype dis-
tribution within STC29 (Fig. 2). The isolates capable of produ
Shiga toxin (EHEC and STEC) cluster together with stx-negat
isolates that harbor the LEE PAI (aEPEC) and therefore illustr
the close relationship of these pathotypes.aEPEC strains share
virulence factors,including the LEE-encoded type III secretion
system, with EHEC strains. Neither of these pathotypes harb
the EAF plasmid, which is responsible for the development o
bundle-forming pilus that is characteristic of typical EPECs. N
ertheless,only STEC and EHEC are capable of Stx production,
since they are lysogenized with lambdoid stx-converting bac
phages.Bacteriophages,including the lambdoid stx-converting
bacteriophage, are mobile genetic elements that belong to t
ible gene pool of microorganisms such as E. coli and contribu
their adaptation under special circumstances. Deletion and in
tion of horizontally transferred genes contribute to the devel
ment of new pathotypes or modify existing characteristics (4
For O26 isolates,the concept ofinterconversion between O26
aEPEC and O26 EHEC has been suggested by the loss, as we
the gain, of stx prophages through lysogenic conversion (23)
negative isolates of EHEC O serogroups have been found in f
low-up checks of patients previously diagnosed with EHEC in
tions. Since these isolates shared a range of non-stx virulenc
and the phylogenetic background with EHEC isolates, these s
negative isolates were designated EHEC strains that had lost
Shiga toxin (EHEC-LST) (22). Anjum et al. demonstrated in 20
by comparative genomic indexing, that 87% to 94% of the co
sequence was conserved between stx-negative and stx-posi
O26 E. coli strains, indicating greater genetic homogeneity w
this O serogroup than previously proposed (43). In another s
12 ruminant as well as 27 human O26:H11/NM isolates were
alyzed by MLST and were identified either as ST21 (16 huma
isolates,9 ruminant isolates) or as ST29 (11 human isolates,3
ruminantisolates).Interestingly,nine bovine isolates ofST21
FIG 3MST showing the population structure of 148 E. coli isolates assigned to sequence type complex STC29. This graph illustrates the
from different hosts as well as from food.
Phylogenetic Analysis of Non-O157 EHEC
October 2015Volume 81Number 20 aem.asm.org7045Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from

were identified as aEPEC, and one stx2a-positive bovine isolate of
ST29 showed virulence characteristics of the emerging human-
pathogenic clone, hinting at interconversion between stx-negative
O26 and STEC strains and the emergence of new STEC clones
with human-pathogenic potential in cattle (44).
Whole-genome sequencing of aEPEC and EHEC isolates of
STC29 will help verify the homogeneity of these pathotypes and
further determine the bacteriophage integration sites.Further-
more, whole-genome data of a considerable number of non-O157
isolates sampled over a definite time period will allow long-term
studies that might unravel whether aEPEC strains have lost the
stx phage (EHEC-LST) or have been transformed to EHEC
strains via lysogenic conversion.
Applying the concept of bidirectional conversion (the possible
loss and gain ofmobile genetic elements) (24) to the isolates
within STC29, we can hypothesize that aEPEC strains might func-
tion as pre-EHEC strains that again integrate the stx prophage into
their genomes.On the other hand,aEPEC strains may also be
EHEC-LST strains,which have lost the stx bacteriophage and,
simultaneously, the ability to secrete Stx. This has to be proven in
further lysogenic conversion experiments,lysogenizing aEPEC
strains of STC29 with stx-converting bacteriophages.
In conclusion, STC29 incorporates highly virulent non-O157
EHEC and aEPEC O serogroups. Thus, STC29 is a highly interest-
ing group with which to analyze the microevolution of EHEC
pathogens.Future genomic as wellas functionalstudies will
providegreaterinsightinto the evolutionarymechanisms
shaping isolates ofthis phylogenetic lineage into important
human pathogens.
ACKNOWLEDGMENTS
This study was supported by the BMBF network “FBI-Zoo” (Food-
Borne Zoonotic Infections of Humans, grant 01KI1012A) and by grant
GRK1673/1 from the German Research Foundation (DFG).
REFERENCES
1. Levine MM.1987.Escherichia coli that cause diarrhea:enterotoxigenic,
enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadher-
ent. J Infect Dis 155:377–389. http://dx.doi.org/10.1093/infdis/155.3.377.
2. Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol
Rev 11:142–201.
3. Karch H, Mellmann A, Bielaszewska M. 2009. Epidemiology and patho-
genesis ofenterohaemorrhagic Escherichia coli.BerlMunch Tierarztl
Wochenschr 122:417– 424.
4. Lowe RM, Baines D, Selinger LB, Thomas JE, McAllister TA, Sharma R.
2009.Escherichia coli O157:H7 strain origin,lineage,and Shiga toxin 2
expression affect colonization of cattle. Appl Environ Microbiol 75:5074 –
5081. http://dx.doi.org/10.1128/AEM.00391-09.
5. Hofer E,Cernela N,Stephan R.2012.Shiga toxin subtypes associated
with Shiga toxin-producing Escherichia coli strains isolated from red deer,
roe deer, chamois, and ibex. Foodborne Pathog Dis 9:792–795. http://dx
.doi.org/10.1089/fpd.2012.1156.
6. Zweifel C,Blanco JE,Blanco M,Blanco J,Stephan R.2004.Serotypes
and virulence genes of ovine non-O157 Shiga toxin-producing Escherichia
coli in Switzerland. Int J Food Microbiol 95:19 –27. http://dx.doi.org/10
.1016/j.ijfoodmicro.2004.01.015.
7. Beutin L. 2006. Emerging enterohaemorrhagic Escherichia coli, causes and
effects of the rise of a human pathogen. J Vet Med B Infect Dis Vet Public
Health 53:299 –305. http://dx.doi.org/10.1111/j.1439-0450.2006.00968.x.
8. Akashi S, Joh K, Mori T, Tsuji A, Ito H, Hoshi H, Hayakawa T, Ihara
J, Abe T, Hatori M, Nakamura T, Akashi S. 1994. A severe outbreak of
haemorrhagic colitis and haemolytic uraemic syndrome associated with
Escherichia coli O157:H7 in Japan. Eur J Pediatr 153:650 – 655. http://dx
.doi.org/10.1007/BF02190685.
9. Cole D, Griffin PM, Fullerton KE, Ayers T, Smith K, Ingram LA, Kissler
B, Hoekstra RM.2014.Attributing sporadic and outbreak-associated
infections to sources:blending epidemiologicaldata.EpidemiolInfect
142:295–302. http://dx.doi.org/10.1017/S0950268813000915.
10.Effler E, Isaacson M, Arntzen L, Heenan R, Canter P, Barrett T
Mambo C, Levine W, Zaidi A, Griffin PM. 2001. Factors contribu
the emergence of Escherichia coli O157 in Africa. Emerg Infect Dis 7
819.http://dx.doi.org/10.3201/eid0705.017507.
11.Gormley FJ,Little CL,RawalN, Gillespie IA,Lebaigue S,Adak GK.
2011. A 17-year review of foodborne outbreaks: describing the cont
decline in England and Wales (1992–2008).Epidemiol Infect 139:688 –
699.http://dx.doi.org/10.1017/S0950268810001858.
12.Xiong Y, Wang P, Lan R, Ye C, Wang H, Ren J, Jing H, Wang Y
Z, Bai X, Cui Z, Luo X, Zhao A, Zhang S, Sun H, Wang L, Xu J.
A novel Escherichia coli O157:H7 clone causing a major hemolytic u
syndrome outbreak in China.PLoS One 7:e36144.http://dx.doi.org/10
.1371/journal.pone.0036144.
13.Mellmann A,Bielaszewska M,Kock R,Friedrich AW,Fruth A,Mid-
dendorfB, Harmsen D,SchmidtMA, Karch H.2008.Analysisof
collection of hemolytic uremic syndrome-associated enterohemorrha
Escherichia coli. Emerg Infect Dis 14:1287–1290.http://dx.doi.org/10.3201
/eid1408.071082.
14.Coombes BK,Gilmour MW,Goodman CD.2011.The evolution of
virulence in non-O157 Shiga toxin-producing Escherichia coli. Front
crobiol 2:90.http://dx.doi.org/10.3389/fmicb.2011.00090.
15.Ogura Y,Ooka T,IguchiA, Toh H, AsadulghaniM, Oshima K,
Kodama T,Abe H,Nakayama K,Kurokawa K,Tobe T,HattoriM,
HayashiT. 2009.Comparative genomics revealthe mechanism of the
parallel evolution of O157 and non-O157 enterohemorrhagic Escher
coli. Proc Natl Acad Sci U S A 106:17939 –17944.http://dx.doi.org/10.1073
/pnas.0903585106.
16.Iguchi A, Iyoda S, Ohnishi M. 2012. Molecular characterization re
three distinct clonal groups among clinical Shiga toxin-producing Es
richia coli strains of serogroup O103. J Clin Microbiol 50:2894 –2900http:
//dx.doi.org/10.1128/JCM.00789-12.
17.Ju W, Rump L,Toro M,Shen J,Cao G,Zhao S,Meng J.2014.
Pathogenicity islands in Shiga toxin-producing Escherichia coliO26,
O103, and O111 isolates from humans and animals. Foodborne Path
Dis 11:342–345.http://dx.doi.org/10.1089/fpd.2013.1696.
18.Cooper KK,Mandrell RE,Louie JW,Korlach J,Clark TA,Parker CT,
Huynh S, Chain PS, Ahmed S, Carter MQ. 2014. Comparative ge
of enterohemorrhagic Escherichia coli O145:H28 demonstrates a co
evolutionary lineage with Escherichia coli O157:H7. BMC Genomics
http://dx.doi.org/10.1186/1471-2164-15-17.
19.Bokete TN,Whittam TS,Wilson RA,Clausen CR,O’Callahan CM,
Moseley SL, Fritsche TR, Tarr PI. 1997. Genetic and phenotypic
of Escherichia coli with enteropathogenic characteristics isolated fro
attle children.J Infect Dis 175:1382–1389.http://dx.doi.org/10.1086
/516470.
20.Eklund M,Bielaszewska M,NakariUM,Karch H,Siitonen A.2006.
Molecular and phenotypic profiling of sorbitol-fermenting Escherichi
O157:H⫺ human isolates from Finland.Clin MicrobiolInfect 12:634 –
641. http://dx.doi.org/10.1111/j.1469-0691.2006.01478.x.
21.Wieler LH, Sobjinski G, Schlapp T, Failing K, Weiss R, Menge
G. 2007. Longitudinal prevalence study of diarrheagenic Escherichia
dairy calves. Berl Munch Tierarztl Wochenschr 120:296 –306.
22.Bielaszewska M,Kock R,Friedrich AW,von Eiff C,Zimmerhackl LB,
Karch H,Mellmann A.2007.Shiga toxin-mediated hemolytic uremic
syndrome: time to change the diagnostic paradigm? PLoS One 2:e1
http://dx.doi.org/10.1371/journal.pone.0001024.
23.Bielaszewska M, Prager R, Kock R, Mellmann A, Zhang W, Ts
Tarr PI, Karch H. 2007. Shiga toxin gene loss and transfer in vitro
vivo during enterohemorrhagic Escherichia coli O26 infection in hum
ApplEnviron Microbiol73:3144 –3150.http://dx.doi.org/10.1128/AEM
.02937-06.
24.Mellmann A,Lu S,Karch H,Xu JG,Harmsen D,Schmidt MA,Bie-
laszewska M.2008.Recycling ofShiga toxin 2 genesin sorbitol-
fermenting enterohemorrhagic Escherichia coli O157:NM. Appl Envir
Microbiol 74:67–72. http://dx.doi.org/10.1128/AEM.01906-07.
25.Wirth T,Falush D,Lan R,Colles F,Mensa P,Wieler LH,Karch H,
Reeves PR,Maiden MC,Ochman H,Achtman M.2006.Sex and viru-
lence in Escherichia coli: an evolutionary perspective. Mol Microbiol
1136 –1151. http://dx.doi.org/10.1111/j.1365-2958.2006.05172.x.
26.Prager R, Strutz U, Fruth A, Tschape H. 2003. Subtyping of path
Eichhorn et al.
7046 aem.asm.org October 2015Volume 81 Number 20Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
ST29 showed virulence characteristics of the emerging human-
pathogenic clone, hinting at interconversion between stx-negative
O26 and STEC strains and the emergence of new STEC clones
with human-pathogenic potential in cattle (44).
Whole-genome sequencing of aEPEC and EHEC isolates of
STC29 will help verify the homogeneity of these pathotypes and
further determine the bacteriophage integration sites.Further-
more, whole-genome data of a considerable number of non-O157
isolates sampled over a definite time period will allow long-term
studies that might unravel whether aEPEC strains have lost the
stx phage (EHEC-LST) or have been transformed to EHEC
strains via lysogenic conversion.
Applying the concept of bidirectional conversion (the possible
loss and gain ofmobile genetic elements) (24) to the isolates
within STC29, we can hypothesize that aEPEC strains might func-
tion as pre-EHEC strains that again integrate the stx prophage into
their genomes.On the other hand,aEPEC strains may also be
EHEC-LST strains,which have lost the stx bacteriophage and,
simultaneously, the ability to secrete Stx. This has to be proven in
further lysogenic conversion experiments,lysogenizing aEPEC
strains of STC29 with stx-converting bacteriophages.
In conclusion, STC29 incorporates highly virulent non-O157
EHEC and aEPEC O serogroups. Thus, STC29 is a highly interest-
ing group with which to analyze the microevolution of EHEC
pathogens.Future genomic as wellas functionalstudies will
providegreaterinsightinto the evolutionarymechanisms
shaping isolates ofthis phylogenetic lineage into important
human pathogens.
ACKNOWLEDGMENTS
This study was supported by the BMBF network “FBI-Zoo” (Food-
Borne Zoonotic Infections of Humans, grant 01KI1012A) and by grant
GRK1673/1 from the German Research Foundation (DFG).
REFERENCES
1. Levine MM.1987.Escherichia coli that cause diarrhea:enterotoxigenic,
enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadher-
ent. J Infect Dis 155:377–389. http://dx.doi.org/10.1093/infdis/155.3.377.
2. Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol
Rev 11:142–201.
3. Karch H, Mellmann A, Bielaszewska M. 2009. Epidemiology and patho-
genesis ofenterohaemorrhagic Escherichia coli.BerlMunch Tierarztl
Wochenschr 122:417– 424.
4. Lowe RM, Baines D, Selinger LB, Thomas JE, McAllister TA, Sharma R.
2009.Escherichia coli O157:H7 strain origin,lineage,and Shiga toxin 2
expression affect colonization of cattle. Appl Environ Microbiol 75:5074 –
5081. http://dx.doi.org/10.1128/AEM.00391-09.
5. Hofer E,Cernela N,Stephan R.2012.Shiga toxin subtypes associated
with Shiga toxin-producing Escherichia coli strains isolated from red deer,
roe deer, chamois, and ibex. Foodborne Pathog Dis 9:792–795. http://dx
.doi.org/10.1089/fpd.2012.1156.
6. Zweifel C,Blanco JE,Blanco M,Blanco J,Stephan R.2004.Serotypes
and virulence genes of ovine non-O157 Shiga toxin-producing Escherichia
coli in Switzerland. Int J Food Microbiol 95:19 –27. http://dx.doi.org/10
.1016/j.ijfoodmicro.2004.01.015.
7. Beutin L. 2006. Emerging enterohaemorrhagic Escherichia coli, causes and
effects of the rise of a human pathogen. J Vet Med B Infect Dis Vet Public
Health 53:299 –305. http://dx.doi.org/10.1111/j.1439-0450.2006.00968.x.
8. Akashi S, Joh K, Mori T, Tsuji A, Ito H, Hoshi H, Hayakawa T, Ihara
J, Abe T, Hatori M, Nakamura T, Akashi S. 1994. A severe outbreak of
haemorrhagic colitis and haemolytic uraemic syndrome associated with
Escherichia coli O157:H7 in Japan. Eur J Pediatr 153:650 – 655. http://dx
.doi.org/10.1007/BF02190685.
9. Cole D, Griffin PM, Fullerton KE, Ayers T, Smith K, Ingram LA, Kissler
B, Hoekstra RM.2014.Attributing sporadic and outbreak-associated
infections to sources:blending epidemiologicaldata.EpidemiolInfect
142:295–302. http://dx.doi.org/10.1017/S0950268813000915.
10.Effler E, Isaacson M, Arntzen L, Heenan R, Canter P, Barrett T
Mambo C, Levine W, Zaidi A, Griffin PM. 2001. Factors contribu
the emergence of Escherichia coli O157 in Africa. Emerg Infect Dis 7
819.http://dx.doi.org/10.3201/eid0705.017507.
11.Gormley FJ,Little CL,RawalN, Gillespie IA,Lebaigue S,Adak GK.
2011. A 17-year review of foodborne outbreaks: describing the cont
decline in England and Wales (1992–2008).Epidemiol Infect 139:688 –
699.http://dx.doi.org/10.1017/S0950268810001858.
12.Xiong Y, Wang P, Lan R, Ye C, Wang H, Ren J, Jing H, Wang Y
Z, Bai X, Cui Z, Luo X, Zhao A, Zhang S, Sun H, Wang L, Xu J.
A novel Escherichia coli O157:H7 clone causing a major hemolytic u
syndrome outbreak in China.PLoS One 7:e36144.http://dx.doi.org/10
.1371/journal.pone.0036144.
13.Mellmann A,Bielaszewska M,Kock R,Friedrich AW,Fruth A,Mid-
dendorfB, Harmsen D,SchmidtMA, Karch H.2008.Analysisof
collection of hemolytic uremic syndrome-associated enterohemorrha
Escherichia coli. Emerg Infect Dis 14:1287–1290.http://dx.doi.org/10.3201
/eid1408.071082.
14.Coombes BK,Gilmour MW,Goodman CD.2011.The evolution of
virulence in non-O157 Shiga toxin-producing Escherichia coli. Front
crobiol 2:90.http://dx.doi.org/10.3389/fmicb.2011.00090.
15.Ogura Y,Ooka T,IguchiA, Toh H, AsadulghaniM, Oshima K,
Kodama T,Abe H,Nakayama K,Kurokawa K,Tobe T,HattoriM,
HayashiT. 2009.Comparative genomics revealthe mechanism of the
parallel evolution of O157 and non-O157 enterohemorrhagic Escher
coli. Proc Natl Acad Sci U S A 106:17939 –17944.http://dx.doi.org/10.1073
/pnas.0903585106.
16.Iguchi A, Iyoda S, Ohnishi M. 2012. Molecular characterization re
three distinct clonal groups among clinical Shiga toxin-producing Es
richia coli strains of serogroup O103. J Clin Microbiol 50:2894 –2900http:
//dx.doi.org/10.1128/JCM.00789-12.
17.Ju W, Rump L,Toro M,Shen J,Cao G,Zhao S,Meng J.2014.
Pathogenicity islands in Shiga toxin-producing Escherichia coliO26,
O103, and O111 isolates from humans and animals. Foodborne Path
Dis 11:342–345.http://dx.doi.org/10.1089/fpd.2013.1696.
18.Cooper KK,Mandrell RE,Louie JW,Korlach J,Clark TA,Parker CT,
Huynh S, Chain PS, Ahmed S, Carter MQ. 2014. Comparative ge
of enterohemorrhagic Escherichia coli O145:H28 demonstrates a co
evolutionary lineage with Escherichia coli O157:H7. BMC Genomics
http://dx.doi.org/10.1186/1471-2164-15-17.
19.Bokete TN,Whittam TS,Wilson RA,Clausen CR,O’Callahan CM,
Moseley SL, Fritsche TR, Tarr PI. 1997. Genetic and phenotypic
of Escherichia coli with enteropathogenic characteristics isolated fro
attle children.J Infect Dis 175:1382–1389.http://dx.doi.org/10.1086
/516470.
20.Eklund M,Bielaszewska M,NakariUM,Karch H,Siitonen A.2006.
Molecular and phenotypic profiling of sorbitol-fermenting Escherichi
O157:H⫺ human isolates from Finland.Clin MicrobiolInfect 12:634 –
641. http://dx.doi.org/10.1111/j.1469-0691.2006.01478.x.
21.Wieler LH, Sobjinski G, Schlapp T, Failing K, Weiss R, Menge
G. 2007. Longitudinal prevalence study of diarrheagenic Escherichia
dairy calves. Berl Munch Tierarztl Wochenschr 120:296 –306.
22.Bielaszewska M,Kock R,Friedrich AW,von Eiff C,Zimmerhackl LB,
Karch H,Mellmann A.2007.Shiga toxin-mediated hemolytic uremic
syndrome: time to change the diagnostic paradigm? PLoS One 2:e1
http://dx.doi.org/10.1371/journal.pone.0001024.
23.Bielaszewska M, Prager R, Kock R, Mellmann A, Zhang W, Ts
Tarr PI, Karch H. 2007. Shiga toxin gene loss and transfer in vitro
vivo during enterohemorrhagic Escherichia coli O26 infection in hum
ApplEnviron Microbiol73:3144 –3150.http://dx.doi.org/10.1128/AEM
.02937-06.
24.Mellmann A,Lu S,Karch H,Xu JG,Harmsen D,Schmidt MA,Bie-
laszewska M.2008.Recycling ofShiga toxin 2 genesin sorbitol-
fermenting enterohemorrhagic Escherichia coli O157:NM. Appl Envir
Microbiol 74:67–72. http://dx.doi.org/10.1128/AEM.01906-07.
25.Wirth T,Falush D,Lan R,Colles F,Mensa P,Wieler LH,Karch H,
Reeves PR,Maiden MC,Ochman H,Achtman M.2006.Sex and viru-
lence in Escherichia coli: an evolutionary perspective. Mol Microbiol
1136 –1151. http://dx.doi.org/10.1111/j.1365-2958.2006.05172.x.
26.Prager R, Strutz U, Fruth A, Tschape H. 2003. Subtyping of path
Eichhorn et al.
7046 aem.asm.org October 2015Volume 81 Number 20Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Escherichia coli strains using flagellar (H)-antigens: serotyping versus fliC
polymorphisms. Int J Med Microbiol 292:477– 486. http://dx.doi.org/10
.1078/1438-4221-00226.
27.Müller D, Greune L, Heusipp G, Karch H, Fruth A, Tschape H, Schmidt
MA. 2007. Identification of unconventional intestinal pathogenic Esche-
richia coli isolates expressing intermediate virulence factor profiles by us-
ing a novel single-step multiplex PCR. Appl Environ Microbiol 73:3380 –
3390. http://dx.doi.org/10.1128/AEM.02855-06.
28.Friedrich AW, Borell J, Bielaszewska M, Fruth A, Tschape H, Karch
H. 2003.Shiga toxin 1c-producing Escherichia coli strains:phenotypic
and genetic characterization and association with human disease. J Clin
Microbiol 41:2448 –2453. http://dx.doi.org/10.1128/JCM.41.6.2448-2453
.2003.
29.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann
A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR,
Sanchez M,Persson S,O’Brien AD.2012.Multicenter evaluation of a
sequence-based protocol for subtyping Shiga toxins and standardizing Stx
nomenclature. J Clin Microbiol 50:2951–2963. http://dx.doi.org/10.1128
/JCM.00860-12.
30.Bielaszewska M,Karch H.2000.Non-O157:H7 Shiga toxin (verocyto-
toxin)-producing Escherichia coli strains: epidemiological significance and
microbiologicaldiagnosis.World J MicrobiolBiotechnol16:711–718.
http://dx.doi.org/10.1023/A:1008972605514.
31.Bielaszewska M,Zhang W,Mellmann A,Karch H.2007.Enterohaem-
orrhagic Escherichia coli O26:H11/H⫺: a human pathogen in emergence.
Berl Munch Tierarztl Wochenschr 120:279 –287.
32.Brooks JT, Sowers EG, Wells JG, Greene KD, Griffin PM, Hoekstra RM,
Strockbine NA. 2005. Non-O157 Shiga toxin-producing Escherichia coli
infections in the United States,1983–2002.J Infect Dis 192:1422–1429.
http://dx.doi.org/10.1086/466536.
33.Mariani-Kurkdjian P,Denamur E,Milon A,Picard B,Cave H,Lam-
bert-Zechovsky N,LoiratC, GoulletP, SansonettiPJ, Elion J.1993.
Identification of a clone of Escherichia coli O103:H2 as a potential agent of
hemolytic-uremic syndrome in France. J Clin Microbiol 31:296 –301.
34.Taylor EV,Nguyen TA,Machesky KD,Koch E,Sotir MJ,Bohm SR,
Folster JP, Bokanyi R, Kupper A, Bidol SA, Emanuel A, Arends KD,
Johnson SA,Dunn J,Stroika S,PatelMK,Williams I.2013.Multi-
state outbreak of Escherichia coli O145 infections associated with romaine
lettuce consumption, 2010. J Food Prot 76:939 –944. http://dx.doi.org/10
.4315/0362-028X.JFP-12-503.
35.Bettelheim KA. 2003. Non-O157 verotoxin-producing Escherichia c
problem,paradox,and paradigm.Exp BiolMed (Maywood) 228:333–
344.
36.Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garma
Lathrop S,Medus C,Spina NL,Webb TH,White PL,Wymore K,
Gierke RE, Mahon BE, Griffin PM. 2013. Increased recognition of
O157 Shiga toxin-producing Escherichia coliinfections in the United
States during 2000 –2010: epidemiologic features and comparison w
coli O157 infections. Foodborne Pathog Dis 10:453– 460.http://dx.doi.org
/10.1089/fpd.2012.1401.
37.Feng P,Lampel KA,Karch H,Whittam TS.1998.Genotypic and phe-
notypic changes in the emergence of Escherichia coli O157:H7. J Inf
177:1750 –1753. http://dx.doi.org/10.1086/517438.
38.Ju W,Cao G,Rump L,Strain E,Luo Y,Timme R,Allard M,Zhao S,
Brown E, Meng J. 2012. Phylogenetic analysis of non-O157 Shiga t
producing Escherichia colistrains by whole-genome sequencing.J Clin
Microbiol 50:4123– 4127.http://dx.doi.org/10.1128/JCM.02262-12.
39.Reid SD, Herbelin CJ, Bumbaugh AC, Selander RK, Whittam T
Parallel evolution of virulence in pathogenic Escherichia coli. Nature
64 – 67. http://dx.doi.org/10.1038/35017546.
40.UrdahlAM,Beutin L,Skjerve E,Zimmermann S,Wasteson Y.2003.
Animal host associated differences in Shiga toxin-producing Escheri
coli isolated from sheep and cattle on the same farm.J Appl Microbiol
95:92–101.http://dx.doi.org/10.1046/j.1365-2672.2003.01964.x.
41.Wieler LH, Busse B, Steinruck H, Beutin L, Weber A, Karch H,
G. 2000. Enterohemorrhagic Escherichia coli (EHEC) strains of serog
O118 display three distinctive clonal groups of EHEC pathogens. J Cl
Microbiol 38:2162–2169.
42.Mellmann A, Bielaszewska M, Karch H. 2009. Intrahost genome
tions in enterohemorrhagic Escherichia coli. Gastroenterology 136:1
1938.http://dx.doi.org/10.1053/j.gastro.2008.12.072.
43.Anjum MF, Lucchini S, Thompson A, Hinton JC, Woodward MJ
Comparative genomic indexing reveals the phylogenomics of Esche
colipathogens.Infect Immun 71:4674 – 4683.http://dx.doi.org/10.1128
/IAI.71.8.4674-4683.2003.
44.Zweifel C, Cernela N, Stephan R. 2013. Detection of the emergin
toxin-producing Escherichia coli O26:H11/H⫺ sequence type 29 (ST29)
clone in human patients and healthy cattle in Switzerland. Appl Envi
Microbiol 79:5411–5413. http://dx.doi.org/10.1128/AEM.01728-13.
Phylogenetic Analysis of Non-O157 EHEC
October 2015Volume 81Number 20 aem.asm.org7047Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
polymorphisms. Int J Med Microbiol 292:477– 486. http://dx.doi.org/10
.1078/1438-4221-00226.
27.Müller D, Greune L, Heusipp G, Karch H, Fruth A, Tschape H, Schmidt
MA. 2007. Identification of unconventional intestinal pathogenic Esche-
richia coli isolates expressing intermediate virulence factor profiles by us-
ing a novel single-step multiplex PCR. Appl Environ Microbiol 73:3380 –
3390. http://dx.doi.org/10.1128/AEM.02855-06.
28.Friedrich AW, Borell J, Bielaszewska M, Fruth A, Tschape H, Karch
H. 2003.Shiga toxin 1c-producing Escherichia coli strains:phenotypic
and genetic characterization and association with human disease. J Clin
Microbiol 41:2448 –2453. http://dx.doi.org/10.1128/JCM.41.6.2448-2453
.2003.
29.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann
A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR,
Sanchez M,Persson S,O’Brien AD.2012.Multicenter evaluation of a
sequence-based protocol for subtyping Shiga toxins and standardizing Stx
nomenclature. J Clin Microbiol 50:2951–2963. http://dx.doi.org/10.1128
/JCM.00860-12.
30.Bielaszewska M,Karch H.2000.Non-O157:H7 Shiga toxin (verocyto-
toxin)-producing Escherichia coli strains: epidemiological significance and
microbiologicaldiagnosis.World J MicrobiolBiotechnol16:711–718.
http://dx.doi.org/10.1023/A:1008972605514.
31.Bielaszewska M,Zhang W,Mellmann A,Karch H.2007.Enterohaem-
orrhagic Escherichia coli O26:H11/H⫺: a human pathogen in emergence.
Berl Munch Tierarztl Wochenschr 120:279 –287.
32.Brooks JT, Sowers EG, Wells JG, Greene KD, Griffin PM, Hoekstra RM,
Strockbine NA. 2005. Non-O157 Shiga toxin-producing Escherichia coli
infections in the United States,1983–2002.J Infect Dis 192:1422–1429.
http://dx.doi.org/10.1086/466536.
33.Mariani-Kurkdjian P,Denamur E,Milon A,Picard B,Cave H,Lam-
bert-Zechovsky N,LoiratC, GoulletP, SansonettiPJ, Elion J.1993.
Identification of a clone of Escherichia coli O103:H2 as a potential agent of
hemolytic-uremic syndrome in France. J Clin Microbiol 31:296 –301.
34.Taylor EV,Nguyen TA,Machesky KD,Koch E,Sotir MJ,Bohm SR,
Folster JP, Bokanyi R, Kupper A, Bidol SA, Emanuel A, Arends KD,
Johnson SA,Dunn J,Stroika S,PatelMK,Williams I.2013.Multi-
state outbreak of Escherichia coli O145 infections associated with romaine
lettuce consumption, 2010. J Food Prot 76:939 –944. http://dx.doi.org/10
.4315/0362-028X.JFP-12-503.
35.Bettelheim KA. 2003. Non-O157 verotoxin-producing Escherichia c
problem,paradox,and paradigm.Exp BiolMed (Maywood) 228:333–
344.
36.Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garma
Lathrop S,Medus C,Spina NL,Webb TH,White PL,Wymore K,
Gierke RE, Mahon BE, Griffin PM. 2013. Increased recognition of
O157 Shiga toxin-producing Escherichia coliinfections in the United
States during 2000 –2010: epidemiologic features and comparison w
coli O157 infections. Foodborne Pathog Dis 10:453– 460.http://dx.doi.org
/10.1089/fpd.2012.1401.
37.Feng P,Lampel KA,Karch H,Whittam TS.1998.Genotypic and phe-
notypic changes in the emergence of Escherichia coli O157:H7. J Inf
177:1750 –1753. http://dx.doi.org/10.1086/517438.
38.Ju W,Cao G,Rump L,Strain E,Luo Y,Timme R,Allard M,Zhao S,
Brown E, Meng J. 2012. Phylogenetic analysis of non-O157 Shiga t
producing Escherichia colistrains by whole-genome sequencing.J Clin
Microbiol 50:4123– 4127.http://dx.doi.org/10.1128/JCM.02262-12.
39.Reid SD, Herbelin CJ, Bumbaugh AC, Selander RK, Whittam T
Parallel evolution of virulence in pathogenic Escherichia coli. Nature
64 – 67. http://dx.doi.org/10.1038/35017546.
40.UrdahlAM,Beutin L,Skjerve E,Zimmermann S,Wasteson Y.2003.
Animal host associated differences in Shiga toxin-producing Escheri
coli isolated from sheep and cattle on the same farm.J Appl Microbiol
95:92–101.http://dx.doi.org/10.1046/j.1365-2672.2003.01964.x.
41.Wieler LH, Busse B, Steinruck H, Beutin L, Weber A, Karch H,
G. 2000. Enterohemorrhagic Escherichia coli (EHEC) strains of serog
O118 display three distinctive clonal groups of EHEC pathogens. J Cl
Microbiol 38:2162–2169.
42.Mellmann A, Bielaszewska M, Karch H. 2009. Intrahost genome
tions in enterohemorrhagic Escherichia coli. Gastroenterology 136:1
1938.http://dx.doi.org/10.1053/j.gastro.2008.12.072.
43.Anjum MF, Lucchini S, Thompson A, Hinton JC, Woodward MJ
Comparative genomic indexing reveals the phylogenomics of Esche
colipathogens.Infect Immun 71:4674 – 4683.http://dx.doi.org/10.1128
/IAI.71.8.4674-4683.2003.
44.Zweifel C, Cernela N, Stephan R. 2013. Detection of the emergin
toxin-producing Escherichia coli O26:H11/H⫺ sequence type 29 (ST29)
clone in human patients and healthy cattle in Switzerland. Appl Envi
Microbiol 79:5411–5413. http://dx.doi.org/10.1128/AEM.01728-13.
Phylogenetic Analysis of Non-O157 EHEC
October 2015Volume 81Number 20 aem.asm.org7047Applied and EnvironmentalMicrobiology
on March 10, 2020 by guesthttp://aem.asm.org/Downloaded from
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

