BTEC ND Applied Science: Electrical Energy Sources Report, Unit 14
VerifiedAdded on 2021/04/16
|6
|1371
|52
Report
AI Summary
This report, prepared for a BTEC ND Certificate in Applied Science at Westminster Kingsway College, analyzes electrical energy sources, focusing on primary and secondary cells. The report details the differences between these cell types, including their construction, operational principles, and applications. It describes the characteristics, merits, and limitations of each cell type in both home and industrial contexts, covering aspects such as capacity, behavior under load, and disposal hazards. The report also evaluates the use of primary and secondary cells in portable electronic applications, such as MP3 players and torches, and proposes an experimental plan for comparing the performance of devices using each cell type. The content is based on research from various sources, including Battery University and Wikipedia, providing a comprehensive overview of the subject matter.

College: Westminster Kingsway College Department: YAL-E: Science and Maths
Course title: BTEC ND Certificate in Applied Science
Assessor name (s): S Ikpe
Assignment title: Explaining the Properties of
Electrical Energy Sources
Unit title: Unit 14 – Energy Changes,
Sources and Applications
Date set: wb 09/12/2013 Date due: wb 06/01/2014
Task Brief / Scenario:
You work for an electrical supplier researching and producing a leaflet to inform the public
on up-to date developments in the manufacture of cells.
Work copied from other sources (e.g. books, websites, other students etc) and
presented as your own work will not be accepted – see Student Code &
Disciplinary Procedures
Grading criteria: Marks
P4
M4
D4
explain the difference between primary and secondary cells
describe the characteristics, merits and limitations of primary and
secondary cells related to their industrial applications.
evaluate the use of primary and secondary cells for portable applications.
Your assignment must be handed in by the deadline. Please sign the declaration below and
attach it to your work.
Declaration
Name: ...................................................................
This is my own work. Any sources I have used have been acknowledged appropriately.
I understand that work copied from other sources (e.g. books, websites, other students etc)
and presented as my own work will not be accepted.
Signed ……………………………………………….
Date ..........................................
I accept the grade I have been awarded and I am now submitting this piece of
work with the grade achieved
Name: ...................................................................
Signed ………………………………………………. Date ..........................................
Page 1 of 6
Course title: BTEC ND Certificate in Applied Science
Assessor name (s): S Ikpe
Assignment title: Explaining the Properties of
Electrical Energy Sources
Unit title: Unit 14 – Energy Changes,
Sources and Applications
Date set: wb 09/12/2013 Date due: wb 06/01/2014
Task Brief / Scenario:
You work for an electrical supplier researching and producing a leaflet to inform the public
on up-to date developments in the manufacture of cells.
Work copied from other sources (e.g. books, websites, other students etc) and
presented as your own work will not be accepted – see Student Code &
Disciplinary Procedures
Grading criteria: Marks
P4
M4
D4
explain the difference between primary and secondary cells
describe the characteristics, merits and limitations of primary and
secondary cells related to their industrial applications.
evaluate the use of primary and secondary cells for portable applications.
Your assignment must be handed in by the deadline. Please sign the declaration below and
attach it to your work.
Declaration
Name: ...................................................................
This is my own work. Any sources I have used have been acknowledged appropriately.
I understand that work copied from other sources (e.g. books, websites, other students etc)
and presented as my own work will not be accepted.
Signed ……………………………………………….
Date ..........................................
I accept the grade I have been awarded and I am now submitting this piece of
work with the grade achieved
Name: ...................................................................
Signed ………………………………………………. Date ..........................................
Page 1 of 6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
FEEDBACK: Unit 14 – Explaining the Properties of Electrical Energy Sources
Name: ____________________________________________________
Criter
ia
Teacher Feedback If you have failed the assignment or
just fallen short of a grade boundary
– then you may be given a re-
submission opportunity.
How to improve your grade
P4
M4
D4
Re-submission YES / NO Re-submission date (if set)
Internally moderated? Yes / No
Comments:
IM’s signature:
Page 2 of 6
Name: ____________________________________________________
Criter
ia
Teacher Feedback If you have failed the assignment or
just fallen short of a grade boundary
– then you may be given a re-
submission opportunity.
How to improve your grade
P4
M4
D4
Re-submission YES / NO Re-submission date (if set)
Internally moderated? Yes / No
Comments:
IM’s signature:
Page 2 of 6
P4 tasks
1. Name two ways in which electricity can be produced. Identify what type of electricity is produced in
each
a) Magnetic induction (Spinning a magnet inside a coil) can produce DC or AC current
depending on the spin
b) Photoelectric effect: produces a DC current
2. What is a battery? Explain with the aid of large labelled diagrams, the difference between primary
and secondary cells. Give three examples of the uses of each type.
Battery is a collection of more than one cells where the chemical reactions create a
flow of electrons in a circuit
Differences:
Primary cells: Primary cells is only used once and then discarded. That is, it is
impossible to reused or recharged.. examples of primary cells are Mercury cell and
Dry cell. Primary cell is essentially a chemical cell and produces electrical current by
an irreversible chemical reaction. Once the reaction has taken place, it cannot be
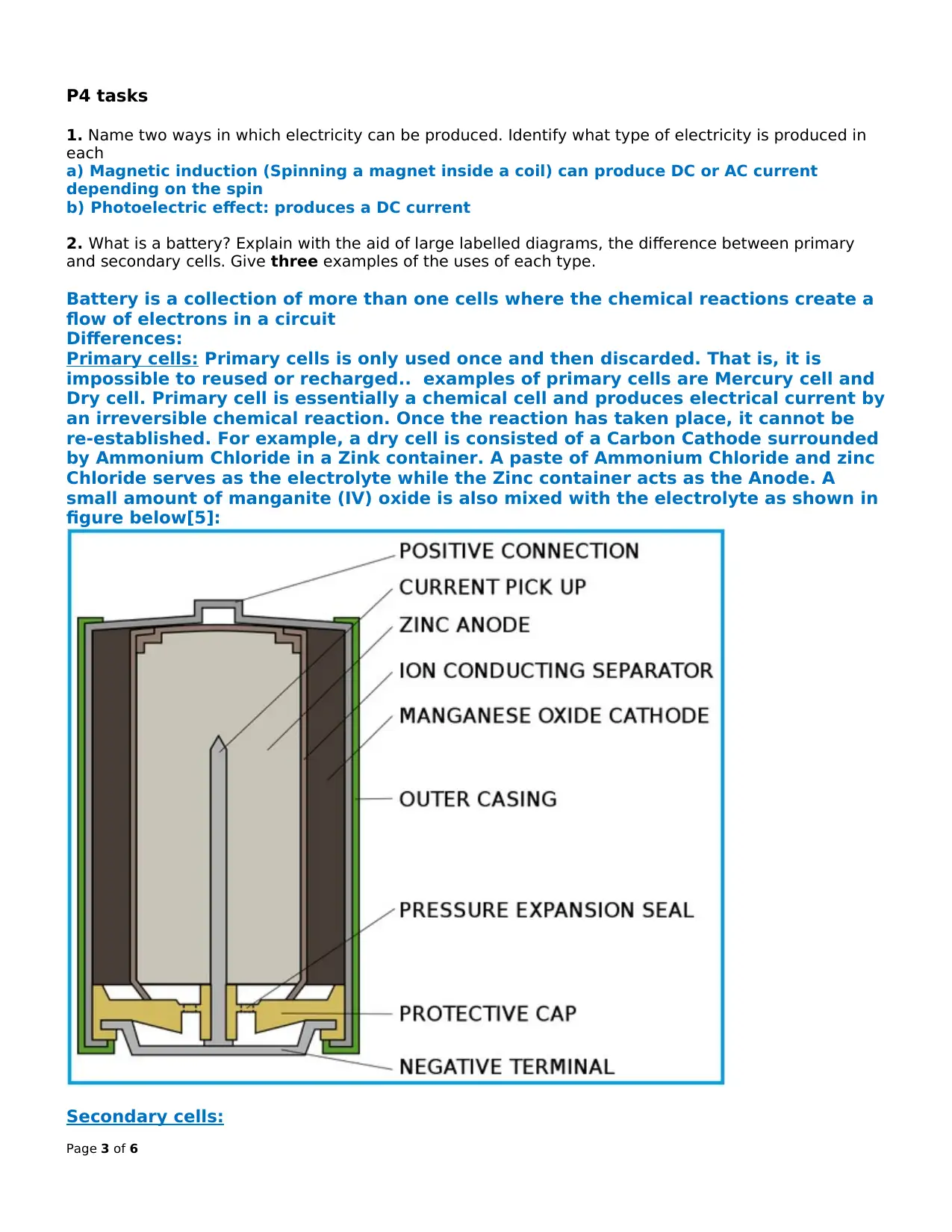
re-established. For example, a dry cell is consisted of a Carbon Cathode surrounded
by Ammonium Chloride in a Zink container. A paste of Ammonium Chloride and zinc
Chloride serves as the electrolyte while the Zinc container acts as the Anode. A
small amount of manganite (IV) oxide is also mixed with the electrolyte as shown in
figure below[5]:
Secondary cells:
Page 3 of 6
1. Name two ways in which electricity can be produced. Identify what type of electricity is produced in
each
a) Magnetic induction (Spinning a magnet inside a coil) can produce DC or AC current
depending on the spin
b) Photoelectric effect: produces a DC current
2. What is a battery? Explain with the aid of large labelled diagrams, the difference between primary
and secondary cells. Give three examples of the uses of each type.
Battery is a collection of more than one cells where the chemical reactions create a
flow of electrons in a circuit
Differences:
Primary cells: Primary cells is only used once and then discarded. That is, it is
impossible to reused or recharged.. examples of primary cells are Mercury cell and
Dry cell. Primary cell is essentially a chemical cell and produces electrical current by
an irreversible chemical reaction. Once the reaction has taken place, it cannot be
re-established. For example, a dry cell is consisted of a Carbon Cathode surrounded
by Ammonium Chloride in a Zink container. A paste of Ammonium Chloride and zinc
Chloride serves as the electrolyte while the Zinc container acts as the Anode. A
small amount of manganite (IV) oxide is also mixed with the electrolyte as shown in
figure below[5]:
Secondary cells:
Page 3 of 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
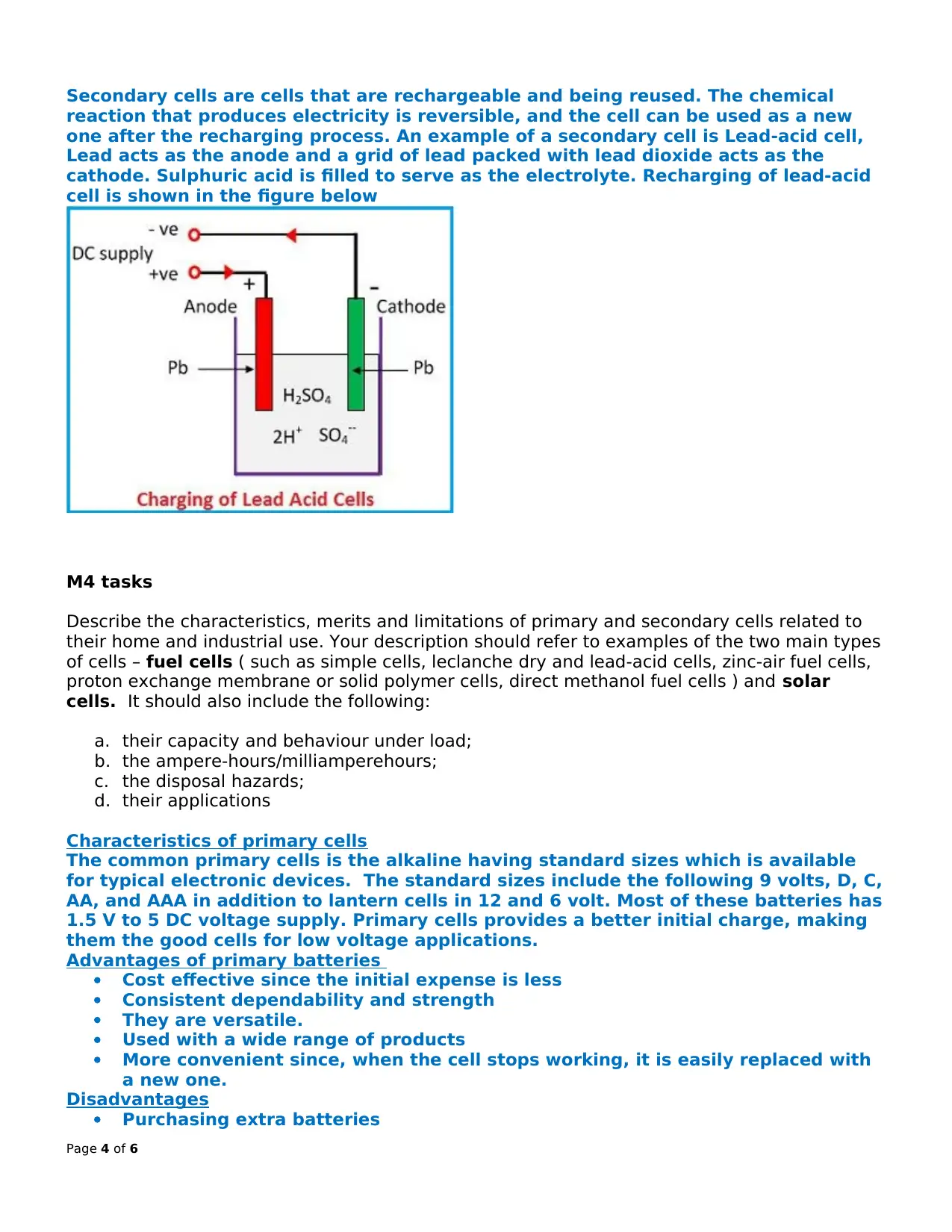
Secondary cells are cells that are rechargeable and being reused. The chemical
reaction that produces electricity is reversible, and the cell can be used as a new
one after the recharging process. An example of a secondary cell is Lead-acid cell,
Lead acts as the anode and a grid of lead packed with lead dioxide acts as the
cathode. Sulphuric acid is filled to serve as the electrolyte. Recharging of lead-acid
cell is shown in the figure below
M4 tasks
Describe the characteristics, merits and limitations of primary and secondary cells related to
their home and industrial use. Your description should refer to examples of the two main types
of cells – fuel cells ( such as simple cells, leclanche dry and lead-acid cells, zinc-air fuel cells,
proton exchange membrane or solid polymer cells, direct methanol fuel cells ) and solar
cells. It should also include the following:
a. their capacity and behaviour under load;
b. the ampere-hours/milliamperehours;
c. the disposal hazards;
d. their applications
Characteristics of primary cells
The common primary cells is the alkaline having standard sizes which is available
for typical electronic devices. The standard sizes include the following 9 volts, D, C,
AA, and AAA in addition to lantern cells in 12 and 6 volt. Most of these batteries has
1.5 V to 5 DC voltage supply. Primary cells provides a better initial charge, making
them the good cells for low voltage applications.
Advantages of primary batteries
Cost effective since the initial expense is less
Consistent dependability and strength
They are versatile.
Used with a wide range of products
More convenient since, when the cell stops working, it is easily replaced with
a new one.
Disadvantages
Purchasing extra batteries
Page 4 of 6
reaction that produces electricity is reversible, and the cell can be used as a new
one after the recharging process. An example of a secondary cell is Lead-acid cell,
Lead acts as the anode and a grid of lead packed with lead dioxide acts as the
cathode. Sulphuric acid is filled to serve as the electrolyte. Recharging of lead-acid
cell is shown in the figure below
M4 tasks
Describe the characteristics, merits and limitations of primary and secondary cells related to
their home and industrial use. Your description should refer to examples of the two main types
of cells – fuel cells ( such as simple cells, leclanche dry and lead-acid cells, zinc-air fuel cells,
proton exchange membrane or solid polymer cells, direct methanol fuel cells ) and solar
cells. It should also include the following:
a. their capacity and behaviour under load;
b. the ampere-hours/milliamperehours;
c. the disposal hazards;
d. their applications
Characteristics of primary cells
The common primary cells is the alkaline having standard sizes which is available
for typical electronic devices. The standard sizes include the following 9 volts, D, C,
AA, and AAA in addition to lantern cells in 12 and 6 volt. Most of these batteries has
1.5 V to 5 DC voltage supply. Primary cells provides a better initial charge, making
them the good cells for low voltage applications.
Advantages of primary batteries
Cost effective since the initial expense is less
Consistent dependability and strength
They are versatile.
Used with a wide range of products
More convenient since, when the cell stops working, it is easily replaced with
a new one.
Disadvantages
Purchasing extra batteries
Page 4 of 6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Contains hazardous materials and must be handled with care
They have higher impact on water pollution, air acidification, ozone pollution
and global warming as compared to secondary cells
Characteristics of secondary cells.
Secondary cells includes sealed lead acid, lithium, nickel metal hydride and nickel
cadmium. These batteries comes in a variety of voltages and sizes. Secondary cells
normally are used for devices demanding higher power and regular battery
replacements. The batteries assurance an extended battery life, making them good
batteries for high-drain frequent-use applications.
Advantages secondary cell
Provide better cost savings particularly with appliances which are frequently
used and provide thousands of hours of use, and last for several years
They have less impact on water pollution, air acidification, ozone pollution
and global warming.
Disadvantages:
Higher initial cost, the secondary cell are more expensive that primary
batteries. Als expensive while recharging.
With time, secondary cells loses the ability of holding charge, thus loss of
dependability and strength
They lack versatility
They are rarely interchangeable hence less products can be used
Less convenient, since, when recharging the battery, one waits for the battery
to charge fully before they are reused
They are more prone to exploding or starting fires than disposable batteries
when exposed to condition such as high temperature and connection which
are faulty
D4 tasks
For D4 you are required to evaluate the use of primary and secondary cells for portable
electronic applications such as in an mp3 player, a torch light, a TV remote controller, a toy, a
personal alarm, etc.
1. Plan a short experiment to compare two portable devices which use primary and
secondary cells, eg MP3 players, torches.
2. Discuss your plan with me and/or the technicians. Arrange with the technicians to have the
required materials made available for your experiment.
3. Carry out the experiment and present a report of your findings following standard
procedures.
Page 5 of 6
They have higher impact on water pollution, air acidification, ozone pollution
and global warming as compared to secondary cells
Characteristics of secondary cells.
Secondary cells includes sealed lead acid, lithium, nickel metal hydride and nickel
cadmium. These batteries comes in a variety of voltages and sizes. Secondary cells
normally are used for devices demanding higher power and regular battery
replacements. The batteries assurance an extended battery life, making them good
batteries for high-drain frequent-use applications.
Advantages secondary cell
Provide better cost savings particularly with appliances which are frequently
used and provide thousands of hours of use, and last for several years
They have less impact on water pollution, air acidification, ozone pollution
and global warming.
Disadvantages:
Higher initial cost, the secondary cell are more expensive that primary
batteries. Als expensive while recharging.
With time, secondary cells loses the ability of holding charge, thus loss of
dependability and strength
They lack versatility
They are rarely interchangeable hence less products can be used
Less convenient, since, when recharging the battery, one waits for the battery
to charge fully before they are reused
They are more prone to exploding or starting fires than disposable batteries
when exposed to condition such as high temperature and connection which
are faulty
D4 tasks
For D4 you are required to evaluate the use of primary and secondary cells for portable
electronic applications such as in an mp3 player, a torch light, a TV remote controller, a toy, a
personal alarm, etc.
1. Plan a short experiment to compare two portable devices which use primary and
secondary cells, eg MP3 players, torches.
2. Discuss your plan with me and/or the technicians. Arrange with the technicians to have the
required materials made available for your experiment.
3. Carry out the experiment and present a report of your findings following standard
procedures.
Page 5 of 6

Resources:
1. Battery University: http://batteryuniversity.com/
2. Battery (electricity): http://en.wikipedia.org/wiki/Battery_(electricity)
3. Galvanic cell: http://en.wikipedia.org/wiki/Galvanic_cell
4. Fuel cell: http://en.wikipedia.org/wiki/Fuel_cell
5. PRIMARY CELL: http://www.differencebetween.com/difference-between-primary-and-vs-
secondary-cells/
Page 6 of 6
1. Battery University: http://batteryuniversity.com/
2. Battery (electricity): http://en.wikipedia.org/wiki/Battery_(electricity)
3. Galvanic cell: http://en.wikipedia.org/wiki/Galvanic_cell
4. Fuel cell: http://en.wikipedia.org/wiki/Fuel_cell
5. PRIMARY CELL: http://www.differencebetween.com/difference-between-primary-and-vs-
secondary-cells/
Page 6 of 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
