Report on Electrochemistry, Battery Systems, and EV Charging Costs
VerifiedAdded on 2022/10/11
|30
|5836
|24
Report
AI Summary
This report provides a comprehensive analysis of electric vehicle (EV) batteries, focusing on electrochemistry and battery systems. It begins with an executive summary covering battery characteristics, electrode types, open-circuit voltage, and the electrochemistry involved in charging and discharging. The report then compares the Tesla Model 3 (50 kWh) and the Hyundai Ioniq Electric (38 kWh), examining their styles, performance, efficiency, range, charging capabilities, passenger and cargo room, and safety features. The report delves into the principles of electrochemistry, including oxidation-reduction reactions and the components of batteries, particularly lithium-ion batteries. It discusses electrolytes, covering strong, weak, and non-electrolytes, as well as liquid and solid electrolytes. The report concludes with a discussion on charging methods and their impact on battery performance, offering insights into the costs associated with home charging for the Tesla Model 3. This assignment is a detailed exploration of EV battery technology and its implications for consumers.

Executive Summary
The report has extensively covered battery characteristics under electrochemistry, the battery systems such as electrodes types, open-circuit
voltage, electrolyte, and electrochemistry in batteries during charging and discharging. Finally concluding with a summary on Tesla Model 3
explaining the possibility of charging at home with a battery and the cost that can be incurred if that is practiced.
Comparison between Tesla Model 3 (50 kWh) and Hyundai Ioniq Electric (38kWh)
Tesla Model 3(50kWh)
The Tesla Model 3(50kWh) is a luxury vehicle with numerous advanced features. The Model 3 car sells for about $36000 because it is long-
range and has an impressive performance.
The report has extensively covered battery characteristics under electrochemistry, the battery systems such as electrodes types, open-circuit
voltage, electrolyte, and electrochemistry in batteries during charging and discharging. Finally concluding with a summary on Tesla Model 3
explaining the possibility of charging at home with a battery and the cost that can be incurred if that is practiced.
Comparison between Tesla Model 3 (50 kWh) and Hyundai Ioniq Electric (38kWh)
Tesla Model 3(50kWh)
The Tesla Model 3(50kWh) is a luxury vehicle with numerous advanced features. The Model 3 car sells for about $36000 because it is long-
range and has an impressive performance.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Style
The Tesla Model 3 is about the size of a BMW 3 series. It is a smart and well-proportioned car. The car keeps a fastback profile of it is owner
while replacing the lift gate using a conventional trunk cover. The lack of traditional grills used in other cars gives it a clean front facing
depiction. The tesla car also has unusual lush exterior handles. Its overage outlook and design represent the current modern trends being
absorbed, and therefore, it can appropriately attract considerable attention. (Crosbie, Jack (August 2, 2017).
Inside Tesla Model 3, they are curved glasses that extend from the windshield to the trunk. The glasses in tesla add up to the general sense of
space that makes the small electric vehicle look bigger than it is in the real sense. In the car, the most complex of its features is the console
configuration and the dashboard, which takes the minimalism of the vehicle to a different level compared to other cars. The electric vehicle does
The Tesla Model 3 is about the size of a BMW 3 series. It is a smart and well-proportioned car. The car keeps a fastback profile of it is owner
while replacing the lift gate using a conventional trunk cover. The lack of traditional grills used in other cars gives it a clean front facing
depiction. The tesla car also has unusual lush exterior handles. Its overage outlook and design represent the current modern trends being
absorbed, and therefore, it can appropriately attract considerable attention. (Crosbie, Jack (August 2, 2017).
Inside Tesla Model 3, they are curved glasses that extend from the windshield to the trunk. The glasses in tesla add up to the general sense of
space that makes the small electric vehicle look bigger than it is in the real sense. In the car, the most complex of its features is the console
configuration and the dashboard, which takes the minimalism of the vehicle to a different level compared to other cars. The electric vehicle does
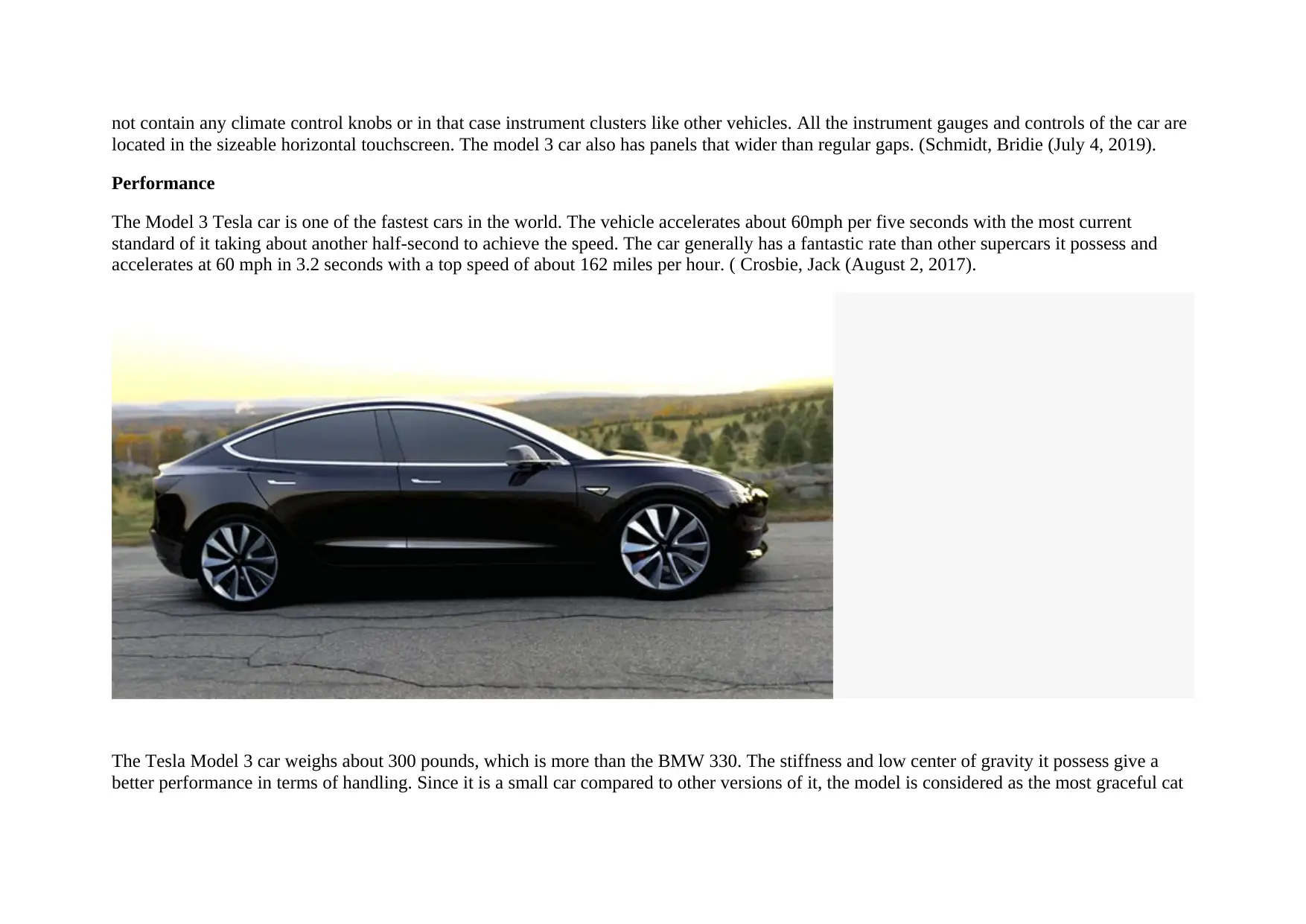
not contain any climate control knobs or in that case instrument clusters like other vehicles. All the instrument gauges and controls of the car are
located in the sizeable horizontal touchscreen. The model 3 car also has panels that wider than regular gaps. (Schmidt, Bridie (July 4, 2019).
Performance
The Model 3 Tesla car is one of the fastest cars in the world. The vehicle accelerates about 60mph per five seconds with the most current
standard of it taking about another half-second to achieve the speed. The car generally has a fantastic rate than other supercars it possess and
accelerates at 60 mph in 3.2 seconds with a top speed of about 162 miles per hour. ( Crosbie, Jack (August 2, 2017).
The Tesla Model 3 car weighs about 300 pounds, which is more than the BMW 330. The stiffness and low center of gravity it possess give a
better performance in terms of handling. Since it is a small car compared to other versions of it, the model is considered as the most graceful cat
located in the sizeable horizontal touchscreen. The model 3 car also has panels that wider than regular gaps. (Schmidt, Bridie (July 4, 2019).
Performance
The Model 3 Tesla car is one of the fastest cars in the world. The vehicle accelerates about 60mph per five seconds with the most current
standard of it taking about another half-second to achieve the speed. The car generally has a fantastic rate than other supercars it possess and
accelerates at 60 mph in 3.2 seconds with a top speed of about 162 miles per hour. ( Crosbie, Jack (August 2, 2017).
The Tesla Model 3 car weighs about 300 pounds, which is more than the BMW 330. The stiffness and low center of gravity it possess give a
better performance in terms of handling. Since it is a small car compared to other versions of it, the model is considered as the most graceful cat
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

that can be found on the roads. Model 3 tesla does not have one-pedal driving experience; therefore, an individual is always advised to hit the
brake to make a stop when necessary. The unique performance features of the car include a $5000 self-driving characteristics which require only
a single tap on the gear selector and another that activates the best lane-keeping system in the world. ( "Tesla, Mercedes, and Škoda Score a
Touchdown in Euro NCAP's Latest Safety Tests,2019)
.
Efficiency/Range
They are usually four choices available in Model 3 Tesla, and they are outlined below.
325-mile Long Range Rear-Wheel Drive
240-mile Standard Range Plus
310-mile Long Range Dual-Motor and Dual-Motor Performance
220-mile Standard
The most standard version of model 3 always uses a 50kWh battery. At 325 miles, model 3 tesla provides more speed than all electric vehicles
available in the world. The model beats out the Nissan leaf, Chevy bolt, and BMW i3 on its efficiency because it has an efficiency rate of almost
130 miles per gallon.
brake to make a stop when necessary. The unique performance features of the car include a $5000 self-driving characteristics which require only
a single tap on the gear selector and another that activates the best lane-keeping system in the world. ( "Tesla, Mercedes, and Škoda Score a
Touchdown in Euro NCAP's Latest Safety Tests,2019)
.
Efficiency/Range
They are usually four choices available in Model 3 Tesla, and they are outlined below.
325-mile Long Range Rear-Wheel Drive
240-mile Standard Range Plus
310-mile Long Range Dual-Motor and Dual-Motor Performance
220-mile Standard
The most standard version of model 3 always uses a 50kWh battery. At 325 miles, model 3 tesla provides more speed than all electric vehicles
available in the world. The model beats out the Nissan leaf, Chevy bolt, and BMW i3 on its efficiency because it has an efficiency rate of almost
130 miles per gallon.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Charging
The cars 16.5kW always add an approximate of 50 miles for every hour the charger is plugged in which beats the average 25 miles that most
electric vehicles require from a standard charger. The fastest charging chargers have the ability to bringing the battery of a model 3 tesla to
approximately 80 percent within 40 minutes. Tesla as a brand does not offer to charge services for free and hence to charge your car at home
may be about one-third of the total cost in a tesla charging station. The most important of electric vehicles is that they charge fast when empty.
( Schmidt, Bridie (July 4, 2019).
The cars 16.5kW always add an approximate of 50 miles for every hour the charger is plugged in which beats the average 25 miles that most
electric vehicles require from a standard charger. The fastest charging chargers have the ability to bringing the battery of a model 3 tesla to
approximately 80 percent within 40 minutes. Tesla as a brand does not offer to charge services for free and hence to charge your car at home
may be about one-third of the total cost in a tesla charging station. The most important of electric vehicles is that they charge fast when empty.
( Schmidt, Bridie (July 4, 2019).

Passenger/Cargo Room
Model 3 tesla is a five-seat vehicle making it the most spacious among electric cars; this is possible because the cars lack an engine. Its rear
trunk is quite roomy, and they increase when the car’s rear seats are folded in a downward manner. In Tesla, they are no use of keys to enter the
vehicle, and owners use credit card shaped devices or a paired phone in the event of wanting to open the door to start it up. ( Grant, Alex
(November 7, 2017). The electric vehicle comprises of two control buttons located on the steering; two column-mounted stalks used to turn
signals, electronic door release buttons, and power window switches different from standard cars that possess buttons. ( Schmidt, Bridie (July 4,
2019).
Safety
In the years 2018, the National Highway Traffic Safety administration gave tesla a five-star rating on each category it was grouped. The electric
vehicle has a wide range of safety features that include lane-keeping commands, active cruise controls, and automatic brake controls. ( Grant,
Alex (November 7, 2017).
Hyundai Ioniq Electric (38kWh
The Hyundai Ioniq Electric battery has a range of 183 miles with a powerful motor of about 134 horsepower. The vehicle poses a 7.2Kw charger
that’s is better than the initial version that used 6.6kW. Charging the car using a 100kW charger will take approximately 50 minutes to ensure
that it is full.
Model 3 tesla is a five-seat vehicle making it the most spacious among electric cars; this is possible because the cars lack an engine. Its rear
trunk is quite roomy, and they increase when the car’s rear seats are folded in a downward manner. In Tesla, they are no use of keys to enter the
vehicle, and owners use credit card shaped devices or a paired phone in the event of wanting to open the door to start it up. ( Grant, Alex
(November 7, 2017). The electric vehicle comprises of two control buttons located on the steering; two column-mounted stalks used to turn
signals, electronic door release buttons, and power window switches different from standard cars that possess buttons. ( Schmidt, Bridie (July 4,
2019).
Safety
In the years 2018, the National Highway Traffic Safety administration gave tesla a five-star rating on each category it was grouped. The electric
vehicle has a wide range of safety features that include lane-keeping commands, active cruise controls, and automatic brake controls. ( Grant,
Alex (November 7, 2017).
Hyundai Ioniq Electric (38kWh
The Hyundai Ioniq Electric battery has a range of 183 miles with a powerful motor of about 134 horsepower. The vehicle poses a 7.2Kw charger
that’s is better than the initial version that used 6.6kW. Charging the car using a 100kW charger will take approximately 50 minutes to ensure
that it is full.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The Hyundai electric vehicle is rated at 150MPGe in the city and about 122MPGe while on the highway. The car is available and can be
purchased in California. The blue link app in the Hyundai Ioniq electric vehicle will allow drivers to monitor battery levels remotely with
accessing them physically, and the drivers will be able to control the Ac of the car. ( Schmidt, Bridie (July 4, 2019).
.Hyundai Ioniq possess smart sense that whose function is to ensure safety and also be driver assistant. With the Hyundai, Ioniq owners will be
able to get about five years free of Hyundai line services whose function is satellite navigation on weather, traffic, parking and charging stations.
The ionic electric vehicle poses one pedal that gives the drivers the ability to avoid using the food brake available in the cars. The cars are
affordable and better priced for customers.
Electrochemistry.
This is a branch of physical chemistry that involves studying the relationship between electricity and an identified chemical change in which
electricity is considered as the overall outcome of the chemical change. The movement of electrons called electricity is generated by the move
from one type of element through oxidation-reduction. A redox reaction is one that Include a difference within the oxidation situation of the
elements. Fox example
2HF→ H2+¿ F2 ¿
It can be written, as shown below:
Oxidation reaction
2 e−¿+H 2+ ¿2 H +¿ ¿ ¿¿
Reduction reaction
purchased in California. The blue link app in the Hyundai Ioniq electric vehicle will allow drivers to monitor battery levels remotely with
accessing them physically, and the drivers will be able to control the Ac of the car. ( Schmidt, Bridie (July 4, 2019).
.Hyundai Ioniq possess smart sense that whose function is to ensure safety and also be driver assistant. With the Hyundai, Ioniq owners will be
able to get about five years free of Hyundai line services whose function is satellite navigation on weather, traffic, parking and charging stations.
The ionic electric vehicle poses one pedal that gives the drivers the ability to avoid using the food brake available in the cars. The cars are
affordable and better priced for customers.
Electrochemistry.
This is a branch of physical chemistry that involves studying the relationship between electricity and an identified chemical change in which
electricity is considered as the overall outcome of the chemical change. The movement of electrons called electricity is generated by the move
from one type of element through oxidation-reduction. A redox reaction is one that Include a difference within the oxidation situation of the
elements. Fox example
2HF→ H2+¿ F2 ¿
It can be written, as shown below:
Oxidation reaction
2 e−¿+H 2+ ¿2 H +¿ ¿ ¿¿
Reduction reaction

2 F−¿ → F2+ ¿2 e−¿ ¿ ¿ ¿
Overall reaction
2 H+¿+2 F−¿→ H2+¿ F 2¿ ¿
¿
Oxidation is a process that involves the misplacement of electrons, while the reduction process requires the addition of new electrons, which is
shown in the above element equation. The element being reduced in a chemical reaction is called the oxidizing agent, while the element that is
always being added to is called the reducing agent. From the above reactions H2is the reducing agent while F2 is the oxidizing agent. ( Grant,
Alex (November 7, 2017).
Batteries consist of electrochemical cells that can store chemical energy which can be converted later into electrical energy.
In this report, lithium batteries were recommended on the following basis.
Both the cathode and anode allow the lithium ions to move freely through a process called extraction. As the atoms move freely through the
Anode and cathode, the batteries involved are known as swing batteries. The positive ions in the lithium move from the anode to the cathode
during discharging through an electrolyte while the electrons move freely through the external circuit towards the same direction. When charging
the reserve occurs, the negative ions move to the anode with higher energy. The following chemical reactions summarise the whole process.( .
( Grant, Alex (November 7, 2017).
Cathode reaction with the lithium doped cobalt oxide substrate.
CoO2+Li+¿¿+e−¿ ⇌¿ LiCo O2
Overall reaction
2 H+¿+2 F−¿→ H2+¿ F 2¿ ¿
¿
Oxidation is a process that involves the misplacement of electrons, while the reduction process requires the addition of new electrons, which is
shown in the above element equation. The element being reduced in a chemical reaction is called the oxidizing agent, while the element that is
always being added to is called the reducing agent. From the above reactions H2is the reducing agent while F2 is the oxidizing agent. ( Grant,
Alex (November 7, 2017).
Batteries consist of electrochemical cells that can store chemical energy which can be converted later into electrical energy.
In this report, lithium batteries were recommended on the following basis.
Both the cathode and anode allow the lithium ions to move freely through a process called extraction. As the atoms move freely through the
Anode and cathode, the batteries involved are known as swing batteries. The positive ions in the lithium move from the anode to the cathode
during discharging through an electrolyte while the electrons move freely through the external circuit towards the same direction. When charging
the reserve occurs, the negative ions move to the anode with higher energy. The following chemical reactions summarise the whole process.( .
( Grant, Alex (November 7, 2017).
Cathode reaction with the lithium doped cobalt oxide substrate.
CoO2+Li+¿¿+e−¿ ⇌¿ LiCo O2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Anode reaction with the graphite.
LiC6 ⇌ C6+Li+¿¿+e−¿¿
A full reaction whereby the left to right discharging and vice versa
LiC6+Co O2 ⇌C6+ LiCo O2
Overall reaction. Overcharging saturates lithium cobalt oxide, therefore, leading to the production of lithium oxide shown in the following
chemical irreversible reactions.
Li+¿¿+ei+ LiCoO2 ⟶ L i2O + CoO
Overcharging by up to 5.2 volts, can lead to the synthesis of cobalt (IV) oxide, as evidenced by the extraction method.
LiCo O2 ⟶ Li+¿+¿¿ CoO2 +e−¿¿
In Lithium-ion battery the ions are transported from the electrode or anode by an oxidizing the transitional metal, Cobalt (Co), in L i1−x CoO2
from C O3+¿ ¿to C O4 +¿¿ during the charging process and reducing from CO4 +¿¿ to CO3+¿ ¿ during discharging.
Electrolytes.
This is a substance that contains both negative and positive ions when electrically passed through a solution such as water.
1. Strong electrolyte: completely dissociates into ions in solution, highly conducting. e.g., Strong acids, bases and soluble ionic salts (NaCl)
2. Weak electrolyte: partially dissociate into ions in solution, weakly
Conducting. E.g., weak acids and bases acetic acid (HC2H3O2)
3. Non-electrolytes: molecule does not dissociate or ionize in solution. Non-conducting.e.g. methanol (CH3OH)
LiC6 ⇌ C6+Li+¿¿+e−¿¿
A full reaction whereby the left to right discharging and vice versa
LiC6+Co O2 ⇌C6+ LiCo O2
Overall reaction. Overcharging saturates lithium cobalt oxide, therefore, leading to the production of lithium oxide shown in the following
chemical irreversible reactions.
Li+¿¿+ei+ LiCoO2 ⟶ L i2O + CoO
Overcharging by up to 5.2 volts, can lead to the synthesis of cobalt (IV) oxide, as evidenced by the extraction method.
LiCo O2 ⟶ Li+¿+¿¿ CoO2 +e−¿¿
In Lithium-ion battery the ions are transported from the electrode or anode by an oxidizing the transitional metal, Cobalt (Co), in L i1−x CoO2
from C O3+¿ ¿to C O4 +¿¿ during the charging process and reducing from CO4 +¿¿ to CO3+¿ ¿ during discharging.
Electrolytes.
This is a substance that contains both negative and positive ions when electrically passed through a solution such as water.
1. Strong electrolyte: completely dissociates into ions in solution, highly conducting. e.g., Strong acids, bases and soluble ionic salts (NaCl)
2. Weak electrolyte: partially dissociate into ions in solution, weakly
Conducting. E.g., weak acids and bases acetic acid (HC2H3O2)
3. Non-electrolytes: molecule does not dissociate or ionize in solution. Non-conducting.e.g. methanol (CH3OH)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Liquid electrolytes.
Most lithium-ion batteries consist of salts such as LIB F4, LIB F6 Insolvents such as diethyl carbonate, dimethyl carbonate, and ethylene
carbonate. Liquid electrolytes always act as pathways to allow movement of ions from the Anode to the Cathode during discharge. The rate of
conduction for a liquid electrolyte at average temperature is 20 degrees Celsius which also at times increases by 30 to 40% at 40 degrees and
also sometimes decreases slightly at 0 degrees Celsius.
Combination of ethylene carbonate and dimethyl carbonate results in high conduction and also the formation of a solid electrolyte. The solvents
used can decompose quickly on the negatively charged electron during the charging process. When the required solutions are used as the
electrolytes, the solvent decomposes on the first charging, and they form layers that are called solid electrolyte interphase which is electrically
insulated to provide ionic conductivity. The electrolyte interphase produced reduces the decomposition levels on the electrolyte during and after
the second charge. An example that can relate to the above is ethylene carbonate decomposes at a very high voltage of 0.7, which leads to the
formation of a stable and dense interface. (Lambert, Fred (September 20, 2018)
Solid electrolytes.
Most researches in the recent present have adopted the use of solid as an electrolyte material. Solid ceramic electrolytes mainly are lithium metal
oxides that allow lithium-ion movement through the solid more easily because of natural lithium. Ceramic electrolytes can be grouped down into
two: glassy and ceramic itself. The solid electrolyte always contains components with crystal structures that contain mostly ion movement
channels. The commonly known and used ceramic electrolytes are perovskites and super ion conductors. The other type of ceramic glassy
electrolytes is always amorphous atomic structures that are usually made of the same elements to the ceramic solid electrolytes, although they
typically have high conductivity levels than the ceramic solid electrolyte. (Lambert, Fred September 20, 2018)
Ceramic and glassy electrolytes conductivity level of ions can be accelerated by using sulfur instead of oxygen. Sulfur has a larger radius that
gives it the ability to be polarised hence allowing for higher conductivity of the lithium metal.
Most lithium-ion batteries consist of salts such as LIB F4, LIB F6 Insolvents such as diethyl carbonate, dimethyl carbonate, and ethylene
carbonate. Liquid electrolytes always act as pathways to allow movement of ions from the Anode to the Cathode during discharge. The rate of
conduction for a liquid electrolyte at average temperature is 20 degrees Celsius which also at times increases by 30 to 40% at 40 degrees and
also sometimes decreases slightly at 0 degrees Celsius.
Combination of ethylene carbonate and dimethyl carbonate results in high conduction and also the formation of a solid electrolyte. The solvents
used can decompose quickly on the negatively charged electron during the charging process. When the required solutions are used as the
electrolytes, the solvent decomposes on the first charging, and they form layers that are called solid electrolyte interphase which is electrically
insulated to provide ionic conductivity. The electrolyte interphase produced reduces the decomposition levels on the electrolyte during and after
the second charge. An example that can relate to the above is ethylene carbonate decomposes at a very high voltage of 0.7, which leads to the
formation of a stable and dense interface. (Lambert, Fred (September 20, 2018)
Solid electrolytes.
Most researches in the recent present have adopted the use of solid as an electrolyte material. Solid ceramic electrolytes mainly are lithium metal
oxides that allow lithium-ion movement through the solid more easily because of natural lithium. Ceramic electrolytes can be grouped down into
two: glassy and ceramic itself. The solid electrolyte always contains components with crystal structures that contain mostly ion movement
channels. The commonly known and used ceramic electrolytes are perovskites and super ion conductors. The other type of ceramic glassy
electrolytes is always amorphous atomic structures that are usually made of the same elements to the ceramic solid electrolytes, although they
typically have high conductivity levels than the ceramic solid electrolyte. (Lambert, Fred September 20, 2018)
Ceramic and glassy electrolytes conductivity level of ions can be accelerated by using sulfur instead of oxygen. Sulfur has a larger radius that
gives it the ability to be polarised hence allowing for higher conductivity of the lithium metal.
Charging.
Lithium-ion batteries always offer the best performance and to maintain that they must be charged appropriately and in the correct manner
required. Charging of the battery if it’s not done appropriately, it might slow down the performance of the battery or even lead to the destruction
of the cell. Proper charging leads to the best performance and the longest life to be obtained from this batteries, therefore; as a result, lithium-ion
battery charging is done with the conjunction of a battery management system that enables the discharge and charge levels. Charging lithium-ion
batteries is always very unique and different from charging the Nickel-metal hydride battery or Nickel-cadmium battery diagram. Lithium-ion
batteries charging is always voltage-sensitive and more different from charging the regular lead-acid batteries. The differences always come in
that lithium-ion batteries do contain high voltage per cell; therefore, they require more voltage tolerance to be able to detect full charge of the
batteries, and once full they always do not allow to be float charged. Lithium-ion batteries do not tolerate the events of being overcharged;
therefore, it’s still important to be able to detect the occasion of the cells is fully charged. Many consumers used lithium-ion batteries always
charge to a voltage of 4.2 volts per cell, and this has enabled a tolerance of about ± 50 mV per cell. Charging lithium-ion batteries beyond this
causes high stress to the cell, and this results in oxidation and therefore reduces service capacity, safety, and life. ( Grant, Alex (November 7,
2017).
A summary table of lithium batteries
Mistry
Lithiu
m
Cobalt
Oxide
Lithium
Mangan
ese
Oxide
Lithium Nickel
Manganese
Oxide
Lithiu
m Iron
Phosph
ate
Lithium
Nickel
Cobalt Alumi
num Oxide
Lithium
Titanate
Oxide
Short
form
Li-
cobalt
Li-
mangane
NMC Li-
phosph
Li-aluminum Li-titanate
Lithium-ion batteries always offer the best performance and to maintain that they must be charged appropriately and in the correct manner
required. Charging of the battery if it’s not done appropriately, it might slow down the performance of the battery or even lead to the destruction
of the cell. Proper charging leads to the best performance and the longest life to be obtained from this batteries, therefore; as a result, lithium-ion
battery charging is done with the conjunction of a battery management system that enables the discharge and charge levels. Charging lithium-ion
batteries is always very unique and different from charging the Nickel-metal hydride battery or Nickel-cadmium battery diagram. Lithium-ion
batteries charging is always voltage-sensitive and more different from charging the regular lead-acid batteries. The differences always come in
that lithium-ion batteries do contain high voltage per cell; therefore, they require more voltage tolerance to be able to detect full charge of the
batteries, and once full they always do not allow to be float charged. Lithium-ion batteries do not tolerate the events of being overcharged;
therefore, it’s still important to be able to detect the occasion of the cells is fully charged. Many consumers used lithium-ion batteries always
charge to a voltage of 4.2 volts per cell, and this has enabled a tolerance of about ± 50 mV per cell. Charging lithium-ion batteries beyond this
causes high stress to the cell, and this results in oxidation and therefore reduces service capacity, safety, and life. ( Grant, Alex (November 7,
2017).
A summary table of lithium batteries
Mistry
Lithiu
m
Cobalt
Oxide
Lithium
Mangan
ese
Oxide
Lithium Nickel
Manganese
Oxide
Lithiu
m Iron
Phosph
ate
Lithium
Nickel
Cobalt Alumi
num Oxide
Lithium
Titanate
Oxide
Short
form
Li-
cobalt
Li-
mangane
NMC Li-
phosph
Li-aluminum Li-titanate
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 30
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




