PVB 301 Assignment 2: Statistical Mechanics, Vibrations, Structure
VerifiedAdded on 2023/06/12
|10
|2204
|237
Homework Assignment
AI Summary
This document presents a student's solutions to PVB 301 Assignment 2, focusing on statistical mechanics, lattice vibrations, and electronic structure. It covers several key areas, including the partition function of a 2D gas, entropy and distinguishability (deriving the Sackur-Tetrode equation), Planck distribution from an ideal gas of photons, heat capacity of platinum, and the electronic properties of potassium. Detailed derivations and explanations are provided for each section, offering insights into the underlying principles and calculations. Desklib is a platform where students can find a wealth of similar solved assignments and study resources.

Running Head: PVB 301 1
PVB 301 Assignment 2
Student’s name
University affiliation
PVB 301 Assignment 2
Student’s name
University affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PVB 301 2
1. PARTITION FUNCTION OF A 2D GAS
The partition equation for a system of particles that obeys Boltzmann statistics has a
definition (Harris, 2012).
Z=∑
j
g j e−ε j/ kBT
………………………………… (1)
Where there are ∆G j energy states within the macro level then
Z=∑
j
∆ G j e−ε j/ kB T
………………………………….. (2) And ε j= h2 A−1
8 m n j
2
………………………………………………… (3) For single particle in a box using the
principle of quantum mechanics. For a gas confined in a 2D area;
Gj = 1
4 π n j
2
………………………………………………. (4)
∆ G j= 1
2 π nj ∆ n j substituting ∆ G j in the second equation;
Z= π
2 ∑
j
nj ∆ n j e−ε j /kB T
…………………………. (5)
Substituting ε jin equation (3) into equation (5);
Z= π
2 ∑
j
nj ∆ n j e−¿ h2 A−1
8 m k B T n j
2 ¿
Integrating from 0 to ∞; Z= π
2 ∫
0
∞
nj exp ( −h2 A−1
8 m k B T nj
2
) d n j;
Z=A ( 2 πm kB T
h2 )
2
2 = A
( h2
2 πm kB T )
= A
λth
2 Where λth= h
√ (2 πmk B T )
2. ENTROPY AND DISTINGUISHABILITY
1. PARTITION FUNCTION OF A 2D GAS
The partition equation for a system of particles that obeys Boltzmann statistics has a
definition (Harris, 2012).
Z=∑
j
g j e−ε j/ kBT
………………………………… (1)
Where there are ∆G j energy states within the macro level then
Z=∑
j
∆ G j e−ε j/ kB T
………………………………….. (2) And ε j= h2 A−1
8 m n j
2
………………………………………………… (3) For single particle in a box using the
principle of quantum mechanics. For a gas confined in a 2D area;
Gj = 1
4 π n j
2
………………………………………………. (4)
∆ G j= 1
2 π nj ∆ n j substituting ∆ G j in the second equation;
Z= π
2 ∑
j
nj ∆ n j e−ε j /kB T
…………………………. (5)
Substituting ε jin equation (3) into equation (5);
Z= π
2 ∑
j
nj ∆ n j e−¿ h2 A−1
8 m k B T n j
2 ¿
Integrating from 0 to ∞; Z= π
2 ∫
0
∞
nj exp ( −h2 A−1
8 m k B T nj
2
) d n j;
Z=A ( 2 πm kB T
h2 )
2
2 = A
( h2
2 πm kB T )
= A
λth
2 Where λth= h
√ (2 πmk B T )
2. ENTROPY AND DISTINGUISHABILITY

PVB 301 3
a) Probability density for distributing N1 particles in a sub volume V1, and N2 in
V2, is given by the binomial distribution (Santos, Dorini, & Cunha, 2008);
W = N !
N1 ! N2 ! ( V 1
V )N1
( V 2
V )N2
¿ W =¿ Ωc ( V 1 N1 ) +¿ Ωc ( V 2 N2 ) −¿ Ωc ( V 1 N ) Ωc (V , N)=( V N
N ! )
Entire entropy;Ωp (U , N )=
( (2 πmU )
3 N
2
(3 N
2 )! )
Assuming N>>1 Ωp ( U , V , N ) =
( Ωc (V , N )Ωp (U , N )
h3 N )
Substituting : S=kInΩ ( U ,V , N )This gives the same Sackur-Tetrode equation for distinguishable
particles as for indistinguishable ones.
b) Helmholtz energy: F=U – TS
dA=−SdT − pdV =−SdT − pdV +∑
j
u j dN j
Where N j=no of particles
u j=corresponding chemical potentials
dA=−SdT −∑
i
ui dNi +∑
j
u j dN jCITATION Vid 08 ¿ 1033(Vidal ,2008)
Probability to find the system in some energy Eigen state r
Pr = e−βEr
Z Whereβ= 1
kT , Z=∑
r
e− βr
U = ⟨ E ⟩=∑
r
Pr Xr= 1
β
∂ logz
∂ x
a) Probability density for distributing N1 particles in a sub volume V1, and N2 in
V2, is given by the binomial distribution (Santos, Dorini, & Cunha, 2008);
W = N !
N1 ! N2 ! ( V 1
V )N1
( V 2
V )N2
¿ W =¿ Ωc ( V 1 N1 ) +¿ Ωc ( V 2 N2 ) −¿ Ωc ( V 1 N ) Ωc (V , N)=( V N
N ! )
Entire entropy;Ωp (U , N )=
( (2 πmU )
3 N
2
(3 N
2 )! )
Assuming N>>1 Ωp ( U , V , N ) =
( Ωc (V , N )Ωp (U , N )
h3 N )
Substituting : S=kInΩ ( U ,V , N )This gives the same Sackur-Tetrode equation for distinguishable
particles as for indistinguishable ones.
b) Helmholtz energy: F=U – TS
dA=−SdT − pdV =−SdT − pdV +∑
j
u j dN j
Where N j=no of particles
u j=corresponding chemical potentials
dA=−SdT −∑
i
ui dNi +∑
j
u j dN jCITATION Vid 08 ¿ 1033(Vidal ,2008)
Probability to find the system in some energy Eigen state r
Pr = e−βEr
Z Whereβ= 1
kT , Z=∑
r
e− βr
U = ⟨ E ⟩=∑
r
Pr Xr= 1
β
∂ logz
∂ x
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
PVB 301 4
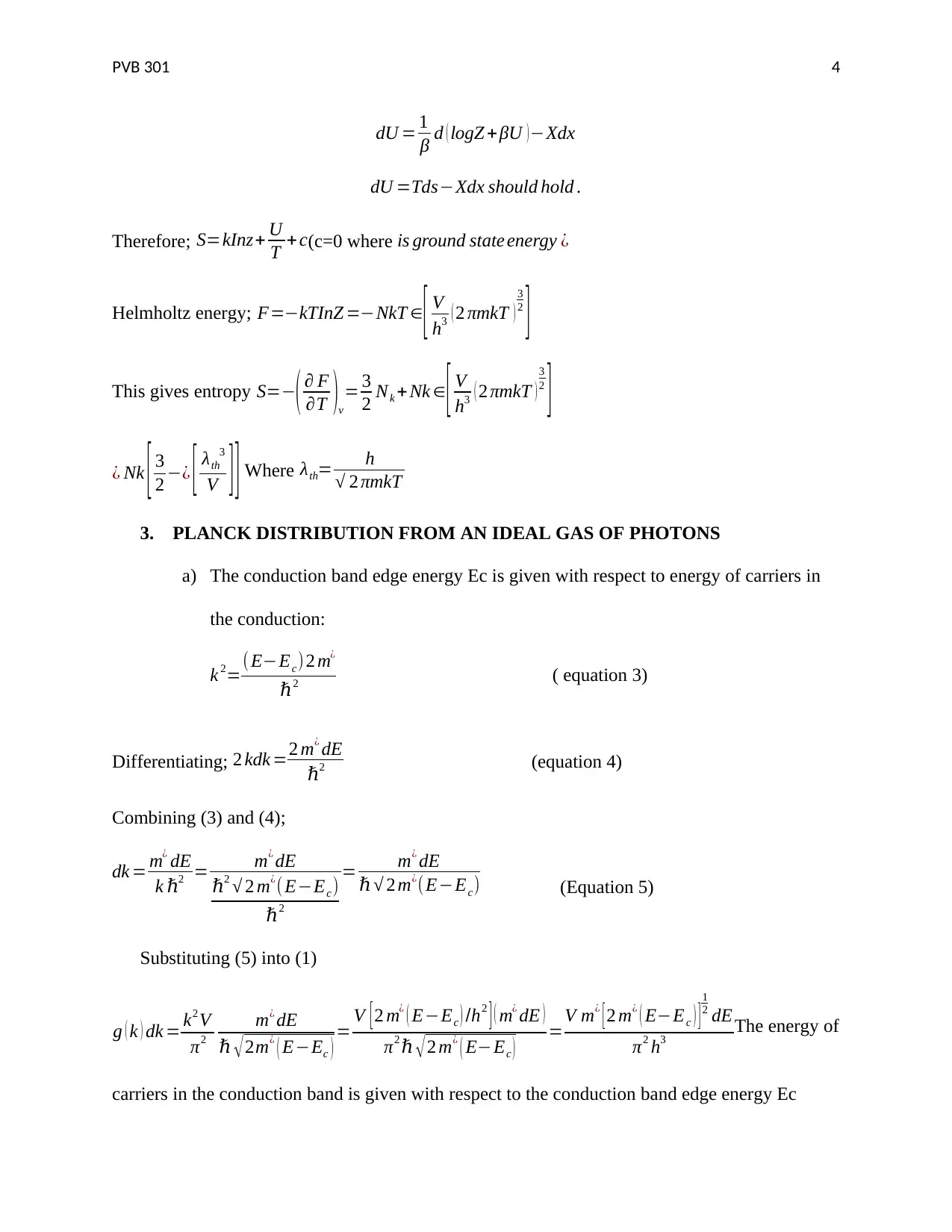
dU = 1
β d ( logZ + βU )−Xdx
dU =Tds−Xdx should hold .
Therefore; S=kInz+ U
T +c(c=0 where is ground state energy ¿
Helmholtz energy; F=−kTInZ =−NkT ∈[ V
h3 ( 2 πmkT )
3
2
]
This gives entropy S=−( ∂ F
∂T )v
= 3
2 Nk + Nk ∈
[ V
h3 ( 2 πmkT )
3
2
]
¿ Nk [ 3
2 −¿ [ λth
3
V ] ] Where λth= h
√ 2 πmkT
3. PLANCK DISTRIBUTION FROM AN IDEAL GAS OF PHOTONS
a) The conduction band edge energy Ec is given with respect to energy of carriers in
the conduction:
k 2=( E−Ec) 2 m¿
ℏ2 ( equation 3)
Differentiating; 2 kdk =2 m¿ dE
ℏ2 (equation 4)
Combining (3) and (4);
dk = m¿ dE
k ℏ2 = m¿ dE
ℏ2 √ 2 m¿(E−Ec)
ℏ2
= m¿ dE
ℏ √ 2 m¿(E−Ec) (Equation 5)
Substituting (5) into (1)
g ( k ) dk = k2 V
π2
m¿ dE
ℏ √2m¿
( E−Ec ) = V [2 m¿ ( E−Ec ) /h2
] ( m¿ dE )
π2 ℏ √2 m¿
( E−Ec ) =V m¿
[ 2 m¿ ( E−Ec ) ] 1
2 dE
π2 h3
The energy of
carriers in the conduction band is given with respect to the conduction band edge energy Ec
dU = 1
β d ( logZ + βU )−Xdx
dU =Tds−Xdx should hold .
Therefore; S=kInz+ U
T +c(c=0 where is ground state energy ¿
Helmholtz energy; F=−kTInZ =−NkT ∈[ V
h3 ( 2 πmkT )
3
2
]
This gives entropy S=−( ∂ F
∂T )v
= 3
2 Nk + Nk ∈
[ V
h3 ( 2 πmkT )
3
2
]
¿ Nk [ 3
2 −¿ [ λth
3
V ] ] Where λth= h
√ 2 πmkT
3. PLANCK DISTRIBUTION FROM AN IDEAL GAS OF PHOTONS
a) The conduction band edge energy Ec is given with respect to energy of carriers in
the conduction:
k 2=( E−Ec) 2 m¿
ℏ2 ( equation 3)
Differentiating; 2 kdk =2 m¿ dE
ℏ2 (equation 4)
Combining (3) and (4);
dk = m¿ dE
k ℏ2 = m¿ dE
ℏ2 √ 2 m¿(E−Ec)
ℏ2
= m¿ dE
ℏ √ 2 m¿(E−Ec) (Equation 5)
Substituting (5) into (1)
g ( k ) dk = k2 V
π2
m¿ dE
ℏ √2m¿
( E−Ec ) = V [2 m¿ ( E−Ec ) /h2
] ( m¿ dE )
π2 ℏ √2 m¿
( E−Ec ) =V m¿
[ 2 m¿ ( E−Ec ) ] 1
2 dE
π2 h3
The energy of
carriers in the conduction band is given with respect to the conduction band edge energy Ec
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PVB 301 5
(Piroth, 2008):
The number of electron states in the conduction band per unit volume over an energy range dE is
g ( E ) d ( E )= m¿
[ 2 m¿ ( E−Ec ) ]1
2
π 2 h3 dE
We consider an energy range dE and calculate the value of electron states per unit volume in
the conduction band.
g ( E ) d ( E )= m¿
[ 2 m¿ ( E−Ec ) ]1
2
π 2 h3 dE
b) Define N(E) as density of occupied states;
N(E) = 2f(E) ρ(E)
Number of states ; N ( E ) =
( 1
8 × 4
3 π n3)
1 = 1
6 π n3
Since E=ℏ2 π2 n2
2 ma2 therefore n=
( 2 m a2 E
ℏ2 π 2 ) 1
2
Replacing;N ( E )= 1
6 π (2 m a2 E
ℏ2 π2 )3
2 E
3
2
ρ ( E ) = dN ( E )
dE = d
DE [ 1
6 π ( 2 m a2 E
ℏ2 π2 ) 3
2 E
3
2
]
ρ ( E ) = 1
4 π ( 2 m a2
ℏ2 π2 ) 3
2 E
1
2
c) Using partition function Z;
F=−kTlogZ
(Piroth, 2008):
The number of electron states in the conduction band per unit volume over an energy range dE is
g ( E ) d ( E )= m¿
[ 2 m¿ ( E−Ec ) ]1
2
π 2 h3 dE
We consider an energy range dE and calculate the value of electron states per unit volume in
the conduction band.
g ( E ) d ( E )= m¿
[ 2 m¿ ( E−Ec ) ]1
2
π 2 h3 dE
b) Define N(E) as density of occupied states;
N(E) = 2f(E) ρ(E)
Number of states ; N ( E ) =
( 1
8 × 4
3 π n3)
1 = 1
6 π n3
Since E=ℏ2 π2 n2
2 ma2 therefore n=
( 2 m a2 E
ℏ2 π 2 ) 1
2
Replacing;N ( E )= 1
6 π (2 m a2 E
ℏ2 π2 )3
2 E
3
2
ρ ( E ) = dN ( E )
dE = d
DE [ 1
6 π ( 2 m a2 E
ℏ2 π2 ) 3
2 E
3
2
]
ρ ( E ) = 1
4 π ( 2 m a2
ℏ2 π2 ) 3
2 E
1
2
c) Using partition function Z;
F=−kTlogZ

PVB 301 6
F=−kTlog ∏
s=1
r
(1−e− β ∈s )−1
¿−kT ∑
s=1
r
log ( 1−e− β ∈s )−1
¿−kT 2∫ dΓ
h3 log ( 1−e− β ∈s
)−1
AnddΓ =dV 4 π p2 dp , ∈= pc , x=βϵ =βpc
F=−1
3 {−8 π k4
( hc)3 ∫
0
∞
d ( x)3 log ( 1−e− x) }V T 4
I =∫
0
∞
d (x )3 log (1−e−x)− ( x3 ) log ¿|∞0+∫
0
∞
x3 d [ log (1−e−x) ]
(a ¿ lim
x→ ∞
( x)3 log (1−e−x )≈ lim
x→ ∞
x3 e−x →0
(b) lim
x→ 0
(x)3 log (1−e−x )≈ lim
x →0
x3 logx → 0
And I =∫
0
∞
dx x3
ex−1 = π 4
15
F= −1
3 aV T 4
4. HEAT CAPACITY OF PLATINUM
a. Consider the wave vector k =k x , k y , k zand define
lx = k x
|k | ly= k y
|k | lz= k z
|k|
lx
2 +ly
2 +lz
2=1 (1)
Solutions to the wave equation;
F=−kTlog ∏
s=1
r
(1−e− β ∈s )−1
¿−kT ∑
s=1
r
log ( 1−e− β ∈s )−1
¿−kT 2∫ dΓ
h3 log ( 1−e− β ∈s
)−1
AnddΓ =dV 4 π p2 dp , ∈= pc , x=βϵ =βpc
F=−1
3 {−8 π k4
( hc)3 ∫
0
∞
d ( x)3 log ( 1−e− x) }V T 4
I =∫
0
∞
d (x )3 log (1−e−x)− ( x3 ) log ¿|∞0+∫
0
∞
x3 d [ log (1−e−x) ]
(a ¿ lim
x→ ∞
( x)3 log (1−e−x )≈ lim
x→ ∞
x3 e−x →0
(b) lim
x→ 0
(x)3 log (1−e−x )≈ lim
x →0
x3 logx → 0
And I =∫
0
∞
dx x3
ex−1 = π 4
15
F= −1
3 aV T 4
4. HEAT CAPACITY OF PLATINUM
a. Consider the wave vector k =k x , k y , k zand define
lx = k x
|k | ly= k y
|k | lz= k z
|k|
lx
2 +ly
2 +lz
2=1 (1)
Solutions to the wave equation;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PVB 301 7
u ( x , y , z , t )=sin ( 2 πvt ) sin ( 2 π lx x
λ )sin (2 π l y y
λ )sin( 2 π lz z
λ ¿
boundary conditions u = 0 at x , y , z=0 , x=¿ Lx , y=Ly , z =Lz
nx=( 2 lx Lx
λ ), ny= (2 ly Ly
λ ),nz =( 2lz Lz
λ ) (2)
Substituting (2) in (1) and using the relation c= λv;
nx
2
¿ ¿ ¿ ¿
The number of nodes N(v) with a frequency range of 0,v is given by
N ( v ) =¿ ¿ where V =Lx Ly Lz
The waves can be differentiated either once or twice to the longitudinal and then to the transverse
direction respectively (Avison , 2014).
3
c3 = 1
c3
l
+ 2
c3
t
3 N=N vD
=(4 π v D
3 V )
3 c3
Defining vD = k T D
h substituting into N(v) we get Nv =3 N h3 v3
k3 T D
3
Number of particles with energy Ei is ni= 1
A e− Ei/(kT ¿)¿= 1
A e−¿ ¿¿ where Ei=¿
Energy contribution for oscillators with frequency v;
dU ( v )=∑
i=0
∞
Ei
1
A e− Ei/(kT ¿)¿ ¿
dN ( v )= 1
A e
− 1
2 hv/(kT ¿)∑i=0
∞
e−ihv /(kT ¿)= 1
A e−1/2hv
kT
1
1−e
−hv
kT
¿¿
Substituting in the energy equation;
u ( x , y , z , t )=sin ( 2 πvt ) sin ( 2 π lx x
λ )sin (2 π l y y
λ )sin( 2 π lz z
λ ¿
boundary conditions u = 0 at x , y , z=0 , x=¿ Lx , y=Ly , z =Lz
nx=( 2 lx Lx
λ ), ny= (2 ly Ly
λ ),nz =( 2lz Lz
λ ) (2)
Substituting (2) in (1) and using the relation c= λv;
nx
2
¿ ¿ ¿ ¿
The number of nodes N(v) with a frequency range of 0,v is given by
N ( v ) =¿ ¿ where V =Lx Ly Lz
The waves can be differentiated either once or twice to the longitudinal and then to the transverse
direction respectively (Avison , 2014).
3
c3 = 1
c3
l
+ 2
c3
t
3 N=N vD
=(4 π v D
3 V )
3 c3
Defining vD = k T D
h substituting into N(v) we get Nv =3 N h3 v3
k3 T D
3
Number of particles with energy Ei is ni= 1
A e− Ei/(kT ¿)¿= 1
A e−¿ ¿¿ where Ei=¿
Energy contribution for oscillators with frequency v;
dU ( v )=∑
i=0
∞
Ei
1
A e− Ei/(kT ¿)¿ ¿
dN ( v )= 1
A e
− 1
2 hv/(kT ¿)∑i=0
∞
e−ihv /(kT ¿)= 1
A e−1/2hv
kT
1
1−e
−hv
kT
¿¿
Substituting in the energy equation;
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
PVB 301 8
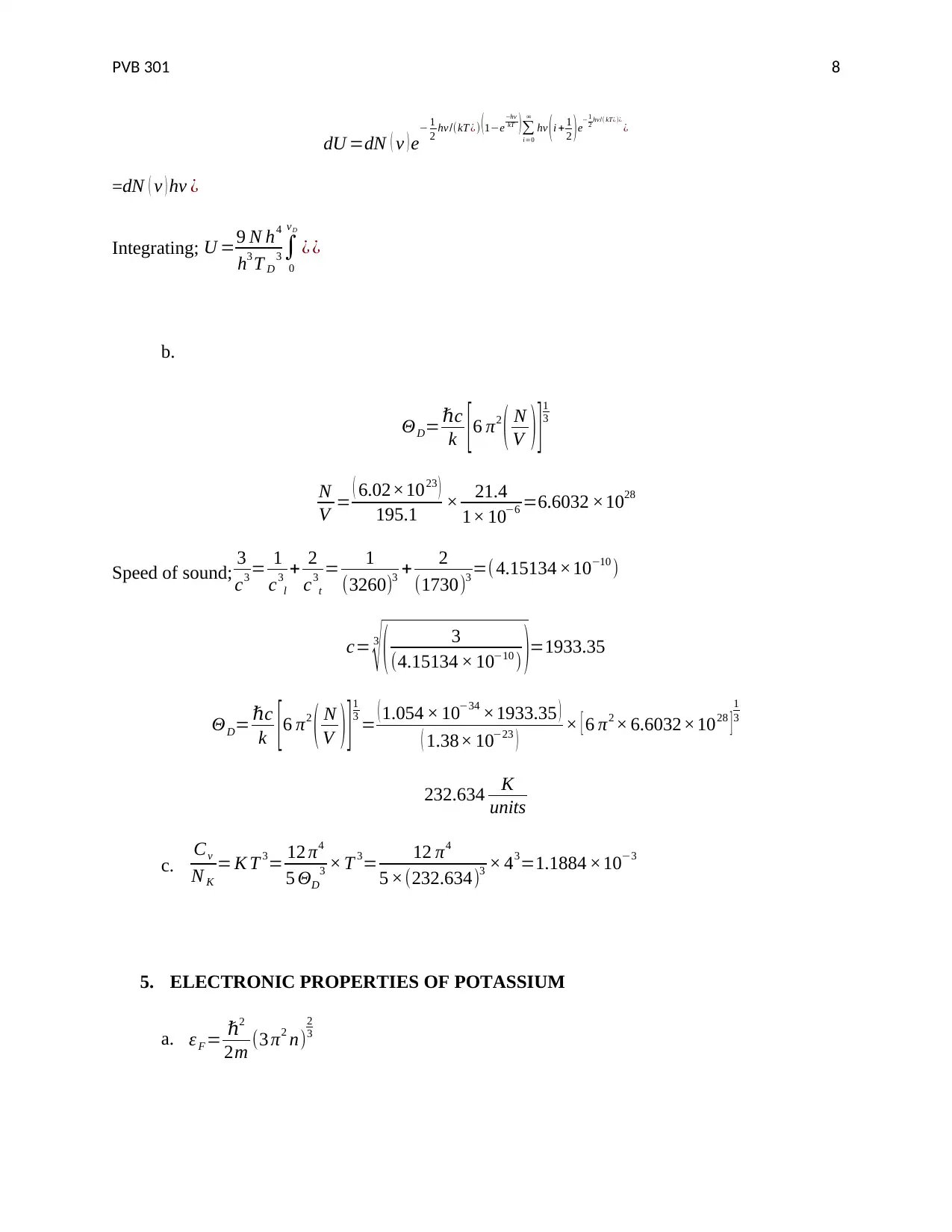
dU =dN ( v ) e− 1
2 hv/(kT ¿) (1−e
−hv
kT )∑
i=0
∞
hv (i + 1
2 )e−1
2 hv/( kT¿)¿
¿
=dN ( v ) hv ¿
Integrating; U =9 N h4
h3 T D
3 ∫
0
vD
¿ ¿
b.
ΘD= cℏ
k [6 π2
( N
V ) ]1
3
N
V = ( 6.02×1023 )
195.1 × 21.4
1× 10−6 =6.6032 ×1028
Speed of sound; 3
c3 = 1
c3
l
+ 2
c3
t
= 1
(3260)3 + 2
(1730)3 =( 4.15134 ×10−10 )
c= 3
√ ( 3
(4.15134 × 10−10 ) )=1933.35
ΘD= cℏ
k [ 6 π2
( N
V ) ] 1
3 = ( 1.054 × 10−34 ×1933.35 )
( 1.38× 10−23 ) × [ 6 π2 × 6.6032×1028 ] 1
3
232.634 K
units
c. Cv
N K
= K T 3= 12 π4
5 ΘD
3 × T 3= 12 π4
5 ×(232.634)3 × 43=1.1884 ×10−3
5. ELECTRONIC PROPERTIES OF POTASSIUM
a. ε F = ℏ2
2m (3 π2 n)
2
3
dU =dN ( v ) e− 1
2 hv/(kT ¿) (1−e
−hv
kT )∑
i=0
∞
hv (i + 1
2 )e−1
2 hv/( kT¿)¿
¿
=dN ( v ) hv ¿
Integrating; U =9 N h4
h3 T D
3 ∫
0
vD
¿ ¿
b.
ΘD= cℏ
k [6 π2
( N
V ) ]1
3
N
V = ( 6.02×1023 )
195.1 × 21.4
1× 10−6 =6.6032 ×1028
Speed of sound; 3
c3 = 1
c3
l
+ 2
c3
t
= 1
(3260)3 + 2
(1730)3 =( 4.15134 ×10−10 )
c= 3
√ ( 3
(4.15134 × 10−10 ) )=1933.35
ΘD= cℏ
k [ 6 π2
( N
V ) ] 1
3 = ( 1.054 × 10−34 ×1933.35 )
( 1.38× 10−23 ) × [ 6 π2 × 6.6032×1028 ] 1
3
232.634 K
units
c. Cv
N K
= K T 3= 12 π4
5 ΘD
3 × T 3= 12 π4
5 ×(232.634)3 × 43=1.1884 ×10−3
5. ELECTRONIC PROPERTIES OF POTASSIUM
a. ε F = ℏ2
2m (3 π2 n)
2
3

PVB 301 9
ℏ=1.055× 10−34 J . s
m=9.20× 10−31 kg
1eV =1.602× 10−19 J
n=(6.02 ×1023)
39.0983 × 0.890
(1 ×10−6 ) ≅ (1.37 ×1028 )/m3
ε F = ( 1.055 ×10−34 ) 2
2× ( 9.20 ×10−31 ) × [ 3 π 2 × ( 1.37× 1028 ) ] 2
3
=3.3147 ×10−19 J
b. eV = 3.3147× 10−19
1.602× 10−19 ≅ 2.069 eV
c. ε F =1
2 m v2
V = √ 2 ε F
m = √ 2 ×3.3147 ×10−19
9.20× 10−31 =8.48873× 105 m/s
d. It is assumed that the valence electrons can be treated as free, or at least moving in a
region of constant potential and non- interacting (Arora, 2013).
ℏ=1.055× 10−34 J . s
m=9.20× 10−31 kg
1eV =1.602× 10−19 J
n=(6.02 ×1023)
39.0983 × 0.890
(1 ×10−6 ) ≅ (1.37 ×1028 )/m3
ε F = ( 1.055 ×10−34 ) 2
2× ( 9.20 ×10−31 ) × [ 3 π 2 × ( 1.37× 1028 ) ] 2
3
=3.3147 ×10−19 J
b. eV = 3.3147× 10−19
1.602× 10−19 ≅ 2.069 eV
c. ε F =1
2 m v2
V = √ 2 ε F
m = √ 2 ×3.3147 ×10−19
9.20× 10−31 =8.48873× 105 m/s
d. It is assumed that the valence electrons can be treated as free, or at least moving in a
region of constant potential and non- interacting (Arora, 2013).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PVB 301 10
References
Arora, C. L. (2013). S.Chand'S Success Guide R/C B.Sc Physics Vol -3. New Delhi: S. Chand Publishing.
Avison , J. (2014). The World of Physics. Cape Town: Nelson Thornes.
Harris, S. (2012). An Introduction to the Theory of the Boltzmann Equation. Chelmsford: Courier
Corporation.
Piroth, A. (2008). Fundamentals of the Physics of Solids: Volume II: Electronic Properties. Berlin: Springer
Science & Business Media.
Santos, L. T., Dorini, F. A., & Cunha, M. C. (2008). The probability density function to the random linear
transport equation. Sao Paulo: Unicamp.
Vidal, J. (2008). Thermodynamics. France: Editions OPHRYS.
References
Arora, C. L. (2013). S.Chand'S Success Guide R/C B.Sc Physics Vol -3. New Delhi: S. Chand Publishing.
Avison , J. (2014). The World of Physics. Cape Town: Nelson Thornes.
Harris, S. (2012). An Introduction to the Theory of the Boltzmann Equation. Chelmsford: Courier
Corporation.
Piroth, A. (2008). Fundamentals of the Physics of Solids: Volume II: Electronic Properties. Berlin: Springer
Science & Business Media.
Santos, L. T., Dorini, F. A., & Cunha, M. C. (2008). The probability density function to the random linear
transport equation. Sao Paulo: Unicamp.
Vidal, J. (2008). Thermodynamics. France: Editions OPHRYS.
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





