EAEC: Virulence Factors and Clinical Manifestations of Infection
VerifiedAdded on 2022/08/17
|32
|17557
|18
Report
AI Summary
This document provides a comprehensive overview of Enteroaggregative Escherichia coli (EAEC), a significant food-borne pathogen responsible for causing acute and persistent diarrhea, particularly in children and immunocompromised individuals. The report delves into the definition of EAEC, characterized by its aggregative adherence pattern on epithelial cells, and its emergence as a global health concern, especially in developing countries. It explores the general concepts, including the evolution of the term 'aggregative adherence' and the classification of EAEC strains. The document also examines the general epidemiology of EAEC, highlighting its association with both acute and persistent diarrhea, traveler's diarrhea, and outbreaks worldwide, including those linked to contaminated food. Clinical features, such as watery diarrhea, abdominal pain, and fever, are discussed, along with the heterogeneity of symptoms and the role of virulence factors. Furthermore, the document explores histopathological studies, illustrating EAEC's interactions with intestinal cells and its impact on the intestinal mucosa. The heterogeneity of EAEC strains, in terms of serotypes, genetic determinants, and virulence factors, is also addressed, emphasizing the ongoing research to understand the pathogenesis of this complex pathogen.
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/309172847
Enteroaggregative Escherichia coli (EAEC)
Chapter · October 2016
DOI: 10.1007/978-3-319-45092-6_2
CITATIONS
6
READS
977
2 authors, including:
Some of the authors of this publication are also working on these related projects:
Inflammation induced by the different diarrheagenic E. coli pathotypesView project
Effect of Bacterial proteases on biological substratesView project
Fernando Navarro-Garcia
Center for Research and Advanced Studies of the National Polytechnic Institute
105PUBLICATIONS3,924CITATIONS
SEE PROFILE
Enteroaggregative Escherichia coli (EAEC)
Chapter · October 2016
DOI: 10.1007/978-3-319-45092-6_2
CITATIONS
6
READS
977
2 authors, including:
Some of the authors of this publication are also working on these related projects:
Inflammation induced by the different diarrheagenic E. coli pathotypesView project
Effect of Bacterial proteases on biological substratesView project
Fernando Navarro-Garcia
Center for Research and Advanced Studies of the National Polytechnic Institute
105PUBLICATIONS3,924CITATIONS
SEE PROFILE
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

27© Springer International Publishing Switzerland 2016
A.G. Torres (ed.), Escherichia coli in the Americas,
DOI 10.1007/978-3-319-45092-6_2
Chapter 2
EnteroaggregativeEscherichia coli (EAEC)
Waldir P. Elias and Fernando Navarro-Garcia
Summary EnteroaggregativeEscherichia coli (EAEC) is defi ned by the production
of the characteristic aggregative adherence pattern on cultured epithelial cel
pathotype is a food-borne emerging enteropathogen , responsible for case
and persistent diarrhea in children and immunocompromised patients in deve
countries, as well as in travelers returning from endemic areas. Growth and c
impairment are linked to EAEC infections in children living in developing coun
The pathogenesis of EAEC is characterized by abundant adherence to the in
mucosa,elaborationof enterotoxins/cytotoxins,and inductionof mucosal
infl ammation. Several putative virulence factors associated with these three
have been identifi ed and characterized, but none of them is present in all str
The virulence gene(s) that defi ne virulent strains within this complex heter
is yet to be determined. An increasing attention to this pathotype emerged si
massive outbreak caused by a hybrid hypervirulent Shiga toxin-expressing EA
strain with severe clinical consequences.
W. P.Elias
Laboratório de Bacteriologia, Instituto Butantan, São Paulo, Brazil
e-mail: waldir.elias@butantan.gov.br
F.Navarro-Garcia(* )
Department of Cell Biology, Centro de Investigación y de Estudios Avanzados del IPN
(CINVESTAV-IPN), México, Distrito Federal, Mexico
e-mail: fnavarro@cell.cinvestav.mx
A.G. Torres (ed.), Escherichia coli in the Americas,
DOI 10.1007/978-3-319-45092-6_2
Chapter 2
EnteroaggregativeEscherichia coli (EAEC)
Waldir P. Elias and Fernando Navarro-Garcia
Summary EnteroaggregativeEscherichia coli (EAEC) is defi ned by the production
of the characteristic aggregative adherence pattern on cultured epithelial cel
pathotype is a food-borne emerging enteropathogen , responsible for case
and persistent diarrhea in children and immunocompromised patients in deve
countries, as well as in travelers returning from endemic areas. Growth and c
impairment are linked to EAEC infections in children living in developing coun
The pathogenesis of EAEC is characterized by abundant adherence to the in
mucosa,elaborationof enterotoxins/cytotoxins,and inductionof mucosal
infl ammation. Several putative virulence factors associated with these three
have been identifi ed and characterized, but none of them is present in all str
The virulence gene(s) that defi ne virulent strains within this complex heter
is yet to be determined. An increasing attention to this pathotype emerged si
massive outbreak caused by a hybrid hypervirulent Shiga toxin-expressing EA
strain with severe clinical consequences.
W. P.Elias
Laboratório de Bacteriologia, Instituto Butantan, São Paulo, Brazil
e-mail: waldir.elias@butantan.gov.br
F.Navarro-Garcia(* )
Department of Cell Biology, Centro de Investigación y de Estudios Avanzados del IPN
(CINVESTAV-IPN), México, Distrito Federal, Mexico
e-mail: fnavarro@cell.cinvestav.mx
28
1 General Concepts
1.1 Defi ning EAEC
The term aggregative adherence was coined by Nataro and colleagues
examining the adherence properties ofE. coli strains isolated in an epidemiological
study of childhood diarrhea in the city of Santiago, Chile (Nataro et al.1987 ). The
isolates were tested for interaction with HEp-2 cells, and three adherence pat
were described. In addition to the previously described localized adherence (L
pattern, the term “diffuse adherence” was distinguished into the truly diffuse
sion (DA) and the aggregative adherence (AA). Standard AA is defi ned as ba
adhering to each other, on the surface of epithelial cells as well as on the sur
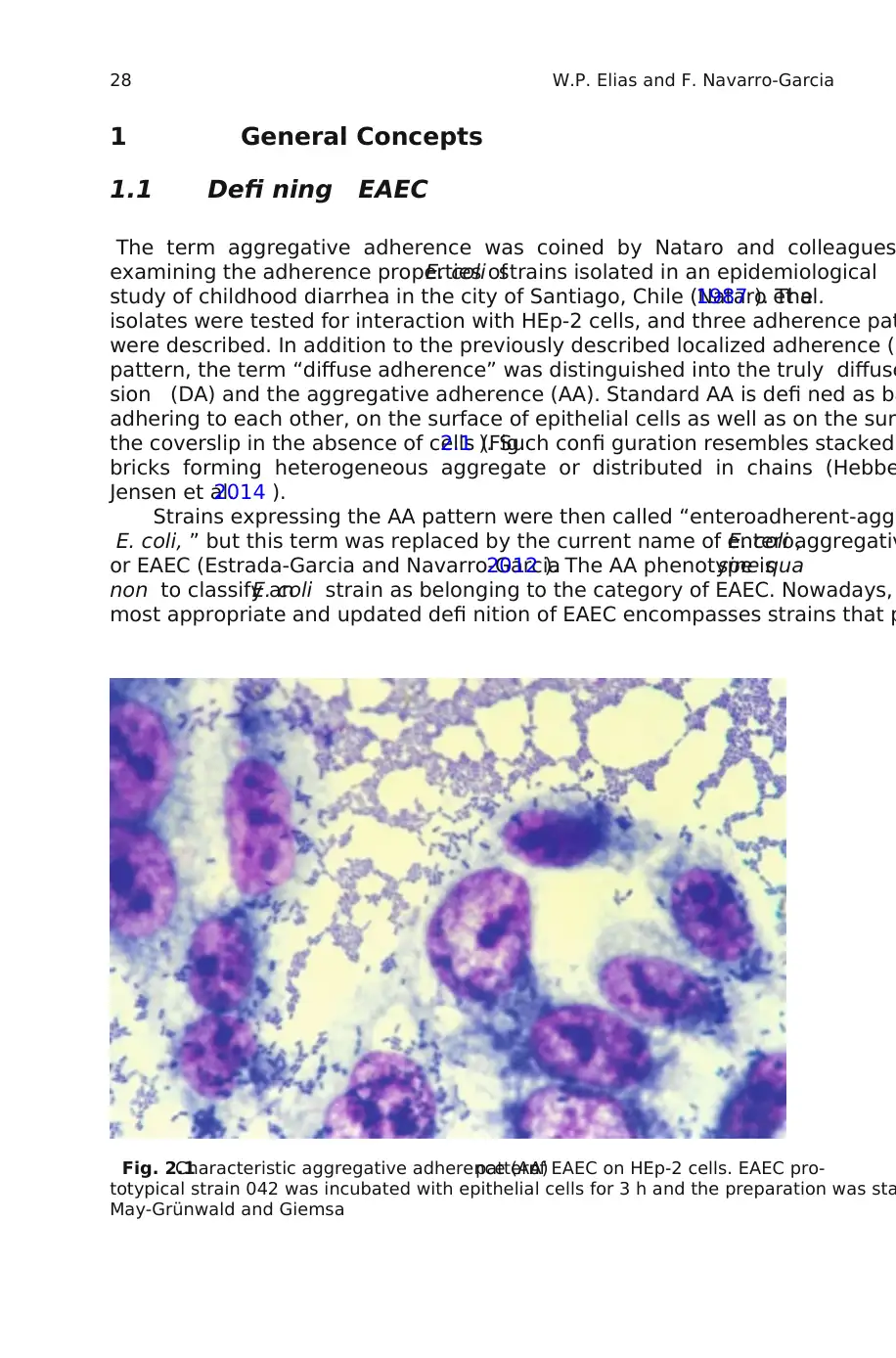
the coverslip in the absence of cells (Fig.2.1 ). Such confi guration resembles stacked
bricks forming heterogeneous aggregate or distributed in chains (Hebbe
Jensen et al.2014 ).
Strains expressing the AA pattern were then called “enteroadherent-aggr
E. coli, ” but this term was replaced by the current name of enteroaggregativE. coli ,
or EAEC (Estrada-Garcia and Navarro-Garcia2012 ). The AA phenotype issine qua
non to classify anE. coli strain as belonging to the category of EAEC. Nowadays,
most appropriate and updated defi nition of EAEC encompasses strains that p
Fig. 2.1Characteristic aggregative adherence (AA)patternof EAEC on HEp-2 cells. EAEC pro-
totypical strain 042 was incubated with epithelial cells for 3 h and the preparation was sta
May-Grünwald and Giemsa
W.P. Elias and F. Navarro-Garcia
1 General Concepts
1.1 Defi ning EAEC
The term aggregative adherence was coined by Nataro and colleagues
examining the adherence properties ofE. coli strains isolated in an epidemiological
study of childhood diarrhea in the city of Santiago, Chile (Nataro et al.1987 ). The
isolates were tested for interaction with HEp-2 cells, and three adherence pat
were described. In addition to the previously described localized adherence (L
pattern, the term “diffuse adherence” was distinguished into the truly diffuse
sion (DA) and the aggregative adherence (AA). Standard AA is defi ned as ba
adhering to each other, on the surface of epithelial cells as well as on the sur
the coverslip in the absence of cells (Fig.2.1 ). Such confi guration resembles stacked
bricks forming heterogeneous aggregate or distributed in chains (Hebbe
Jensen et al.2014 ).
Strains expressing the AA pattern were then called “enteroadherent-aggr
E. coli, ” but this term was replaced by the current name of enteroaggregativE. coli ,
or EAEC (Estrada-Garcia and Navarro-Garcia2012 ). The AA phenotype issine qua
non to classify anE. coli strain as belonging to the category of EAEC. Nowadays,
most appropriate and updated defi nition of EAEC encompasses strains that p
Fig. 2.1Characteristic aggregative adherence (AA)patternof EAEC on HEp-2 cells. EAEC pro-
totypical strain 042 was incubated with epithelial cells for 3 h and the preparation was sta
May-Grünwald and Giemsa
W.P. Elias and F. Navarro-Garcia
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
29
the AA pattern on HeLa or HEp-2 cells (3 or 6 h adhesion assay) and are devo
virulence markers that defi ne other types of diarrheagenicE. coli. An exception is for
strains presenting the AA pattern and other EAEC-specifi c genetic markers in
bination with the production of Shiga toxin (Stx) , which defi nes the hybrid
and Shiga toxin-producingE. coli (STEC), discussed below.
Currently, EAEC is considered an emerging enteropathogen, responsib
cases of acute and persistent diarrhea in children and adults worldwide, and
opmental consequences in children living in developing countries (Hebb
Jensen et al.2014 ). An increasing attention to this pathotype has arisen from t
massive outbreak caused by ahybrid hypervirulent EAEC strain (Stx2-expressing
EAEC) with severe sequelae such as the development of hemolytic uremic sy
drome (Navarro-Garcia2014 ).
1.2 General Epidemiology
Since its description in 1987, when EAEC was signifi cantly associated with a
diarrhea in children (Nataro et al.1987 ), numerous epidemiological studies of the
etiology of diarrhea searched for EAEC in an attempt to clarify its role as diarr
agent. In the early years, the association between EAEC and persistent
(≥14 days of duration) in children was well supported (Cravioto et al.1991 ).
However, the association with acute diarrhea in childhood was controversial.
In the following years, a large number of studies have reported the detect
EAEC in cases of acute diarrhea in developing and developed countries, persi
diarrhea in developing countries, and signifi cant outbreaks worldwide. In fac
and colleagues demonstrated by a meta-analysis study of the literature betw
and 2006 that EAEC was statistically associated with acute and persistent dia
developed and developing countries, to diarrhea in HIV-infected patients in d
ing countries, and adults traveler’s diarrhea (Huang et al.2006 ). Another recent
meta-analysis study of published articles between 1989 and 2011 showed as
of EAEC with acute diarrhea in children of South Asian populations (Pabalan e
2013 ). Thus, EAEC has been systematically identifi ed as an emerging enter
gen, globally distributed (Estrada-Garcia and Navarro-Garcia2012 ).
A high rate of asymptomatic young children carrying EAEC is still a
reported in several studies in the last years (Hebbelstrup Jensen et al.2014 ).
Moreover, such persistent colonization has a link with growth impairment in
dren from low socioeconomic status.
The linkage between EAEC and diarrhea in individuals living in developed c
tries became clearer in the last years. In the USA and Europe, EAEC has been
quently isolated from cases of diarrhea from children and adults. In prospecti
EAEC was the major cause of diarrhea in children in the USA (Nataro et al.2006 ).
Traveler’s diarrhea (TD) is the most frequent disease that affects individ
ing in developed countries when visiting middle and low incoming endemic a
EAEC has been systematically found among the most prevalent bacterial age
2 Enteroaggregative Escherichia coli (EAEC)
the AA pattern on HeLa or HEp-2 cells (3 or 6 h adhesion assay) and are devo
virulence markers that defi ne other types of diarrheagenicE. coli. An exception is for
strains presenting the AA pattern and other EAEC-specifi c genetic markers in
bination with the production of Shiga toxin (Stx) , which defi nes the hybrid
and Shiga toxin-producingE. coli (STEC), discussed below.
Currently, EAEC is considered an emerging enteropathogen, responsib
cases of acute and persistent diarrhea in children and adults worldwide, and
opmental consequences in children living in developing countries (Hebb
Jensen et al.2014 ). An increasing attention to this pathotype has arisen from t
massive outbreak caused by ahybrid hypervirulent EAEC strain (Stx2-expressing
EAEC) with severe sequelae such as the development of hemolytic uremic sy
drome (Navarro-Garcia2014 ).
1.2 General Epidemiology
Since its description in 1987, when EAEC was signifi cantly associated with a
diarrhea in children (Nataro et al.1987 ), numerous epidemiological studies of the
etiology of diarrhea searched for EAEC in an attempt to clarify its role as diarr
agent. In the early years, the association between EAEC and persistent
(≥14 days of duration) in children was well supported (Cravioto et al.1991 ).
However, the association with acute diarrhea in childhood was controversial.
In the following years, a large number of studies have reported the detect
EAEC in cases of acute diarrhea in developing and developed countries, persi
diarrhea in developing countries, and signifi cant outbreaks worldwide. In fac
and colleagues demonstrated by a meta-analysis study of the literature betw
and 2006 that EAEC was statistically associated with acute and persistent dia
developed and developing countries, to diarrhea in HIV-infected patients in d
ing countries, and adults traveler’s diarrhea (Huang et al.2006 ). Another recent
meta-analysis study of published articles between 1989 and 2011 showed as
of EAEC with acute diarrhea in children of South Asian populations (Pabalan e
2013 ). Thus, EAEC has been systematically identifi ed as an emerging enter
gen, globally distributed (Estrada-Garcia and Navarro-Garcia2012 ).
A high rate of asymptomatic young children carrying EAEC is still a
reported in several studies in the last years (Hebbelstrup Jensen et al.2014 ).
Moreover, such persistent colonization has a link with growth impairment in
dren from low socioeconomic status.
The linkage between EAEC and diarrhea in individuals living in developed c
tries became clearer in the last years. In the USA and Europe, EAEC has been
quently isolated from cases of diarrhea from children and adults. In prospecti
EAEC was the major cause of diarrhea in children in the USA (Nataro et al.2006 ).
Traveler’s diarrhea (TD) is the most frequent disease that affects individ
ing in developed countries when visiting middle and low incoming endemic a
EAEC has been systematically found among the most prevalent bacterial age
2 Enteroaggregative Escherichia coli (EAEC)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

30
traveler’s diarrhea since the defi nition of this category. The prevalence of EA
TD varies from 19 to 33 %, depending on the geographic region visited (Moha
et al.2011 ).
Studies linked EAEC with diarrhea in HIV-infected adults and children, a gr
usually susceptible to signifi cant cases of protracted diarrhea. EAEC was isol
the only enteropathogen in symptomatic patients presenting diarrhea for 30
(Polotsky et al.1997 ). In addition, isolation of EAEC was similar in a case/contro
study of HIV patients (Medina et al.2010 ).
Several outbreaks of gastroenteritis caused by EAEC have been reported i
and high-income countries. Some of them involving very impressive numbers
infected children or adults and associated with the consumption of contamina
food. Outbreaks in the UK (Dallman et al.2014 ), Japan (Itoh et al. 1997 ), and Italy
(Scavia et al.2008 ) show the relevance of EAEC in developed countries. In one
the Japanese outbreaks, 2697 schoolchildren were affected after consum
school lunches (Itoh et al.1997). In Italy, the outbreaks were transmitted by unpas-
teurized cheese. EAEC was also responsible for outbreaks in developing coun
(Cobeljic et al.1996 ).
1.3 Clinical Features
The most common symptoms reported in EAEC infection are watery diarrhea
mucoid, with or without blood and abdominal pain, nausea, vomiting, and low
These signs and symptoms are often self-limited but some selected patients
develop persistent diarrhea (≥14 days). The diversity of clinical symptoms in
pathotype infection may be due to heterogeneity between EAEC isolates, infe
dose, genetic susceptibility factors in the host, as well as the immune
(Harrington et al.2006 ).
In order to better characterize the virulent properties of EAEC, studi
human volunteers receiving oral inoculum of different EAEC strains were
formed (Mathewson et al.1986 ; Nataro et al.1992 , 1995 ).
Nataro and colleagues evaluated four different EAEC isolated from diff
geographic regions and different serotypes in volunteers (Nataro et al.1995 ). In this
study, the volunteers ingested dose of 1010 CFU and just EAEC O42 (serotype
O44:H18) caused diarrhea in three out of fi ve volunteers. EAEC 042 was isola
from a case of childhood diarrhea in Peru. The clinical data obtained from the
unteers who developed diarrhea suggested that EAEC 042 caused secretory d
rhea, with short incubation period, absence of fever, and leukocytes or blood
stool. Furthermore, the mucus found in the feces of two patients suggested la
intestinal secretion induced by colonization by EAEC 042. These studies, as w
others that demonstrate the virulence of EAEC for humans, have indica
EAEC is a heterogeneous category of diarrheagenicE. coli , including virulent
strains or not, what seems to depend on factors not yet fully understood.
W.P. Elias and F. Navarro-Garcia
traveler’s diarrhea since the defi nition of this category. The prevalence of EA
TD varies from 19 to 33 %, depending on the geographic region visited (Moha
et al.2011 ).
Studies linked EAEC with diarrhea in HIV-infected adults and children, a gr
usually susceptible to signifi cant cases of protracted diarrhea. EAEC was isol
the only enteropathogen in symptomatic patients presenting diarrhea for 30
(Polotsky et al.1997 ). In addition, isolation of EAEC was similar in a case/contro
study of HIV patients (Medina et al.2010 ).
Several outbreaks of gastroenteritis caused by EAEC have been reported i
and high-income countries. Some of them involving very impressive numbers
infected children or adults and associated with the consumption of contamina
food. Outbreaks in the UK (Dallman et al.2014 ), Japan (Itoh et al. 1997 ), and Italy
(Scavia et al.2008 ) show the relevance of EAEC in developed countries. In one
the Japanese outbreaks, 2697 schoolchildren were affected after consum
school lunches (Itoh et al.1997). In Italy, the outbreaks were transmitted by unpas-
teurized cheese. EAEC was also responsible for outbreaks in developing coun
(Cobeljic et al.1996 ).
1.3 Clinical Features
The most common symptoms reported in EAEC infection are watery diarrhea
mucoid, with or without blood and abdominal pain, nausea, vomiting, and low
These signs and symptoms are often self-limited but some selected patients
develop persistent diarrhea (≥14 days). The diversity of clinical symptoms in
pathotype infection may be due to heterogeneity between EAEC isolates, infe
dose, genetic susceptibility factors in the host, as well as the immune
(Harrington et al.2006 ).
In order to better characterize the virulent properties of EAEC, studi
human volunteers receiving oral inoculum of different EAEC strains were
formed (Mathewson et al.1986 ; Nataro et al.1992 , 1995 ).
Nataro and colleagues evaluated four different EAEC isolated from diff
geographic regions and different serotypes in volunteers (Nataro et al.1995 ). In this
study, the volunteers ingested dose of 1010 CFU and just EAEC O42 (serotype
O44:H18) caused diarrhea in three out of fi ve volunteers. EAEC 042 was isola
from a case of childhood diarrhea in Peru. The clinical data obtained from the
unteers who developed diarrhea suggested that EAEC 042 caused secretory d
rhea, with short incubation period, absence of fever, and leukocytes or blood
stool. Furthermore, the mucus found in the feces of two patients suggested la
intestinal secretion induced by colonization by EAEC 042. These studies, as w
others that demonstrate the virulence of EAEC for humans, have indica
EAEC is a heterogeneous category of diarrheagenicE. coli , including virulent
strains or not, what seems to depend on factors not yet fully understood.
W.P. Elias and F. Navarro-Garcia

31
1.4 Histopathology
Studies in vitro, in vivo, and ex vivo, evaluating EAEC interactions with intest
cells from animals or humans, have tried to elucidate the pathogenesis
pathotype since its description in 1987. The fi rst study about EAEC pathogen
in animal models employed ligated ileal loop of rabbit and rat intestines (Vial
1988 ), showing that EAEC 042 and 17-2 strongly adhered to the mucosa and
ing shortening of microvilli, hemorrhagic necrosis with edema, and mononucl
infi ltrates in the submucosa. Analysis by transmission electron microscopy re
no bacterial invasion and the microvilli architecture was preserved.
Other studies employing in vitro organ culture (IVOC) models have elucid
the intestinal alterations induced by EAEC in fragments of human biopsies of
denum, ileum, and colon (Hicks et al. 1996 ; Nataro et al. 1996 ). Cytotoxic e
the colon were observed, such as microvilli vesiculation, enlarged crypt open
and increased epithelial cell extrusion (Hicks et al.1996 ). Also using IVOC, Nataro
and colleagues demonstrated strong adhesion of EAEC 042 to jejunal
ileum, and more intensely to the colon, while 042 cured of the pAA2 plasmid
this ability (Nataro et al.1996 ). All together, these data strongly indicated that EA
virulence was probable due to intestinal colonization, mainly to the colonic m
in the characteristic aggregative manner, with bacteria forming strong biofi lm
increased mucus layer, followed by cytotoxic and pro-infl ammatory effects
1.5 Strain Heterogeneity
In the decades that followed its original description, EAEC strains have been
acterized in numerous studies around the world, highlighting the particular h
geneity of this category in terms of serotypes, genetic determinants lin
virulence and phylogenetic groups (Boisen et al. 2012; Chattaway et al. 2014b ;
Czeczulin et al.1999; Jenkins et al.2006 ). This heterogeneity together with the
fact that not all EAEC strains were able to cause diarrhea in experimental infe
of humans raises the idea that only a subset of EAEC strains, carrying a speci
of virulence factors, has the capacity to cause diarrhea. This set of factors ha
been determined.
After demonstrating its pathogenicity in human volunteers, EAEC 042 beca
genetically and phenotypically widely studied and considered the prototype s
for EAEC. Major advances in understanding the pathogenesis of EAEC result
from data obtained with this strain whose genome has been sequenced (Chau
et al.2010 ). Other EAEC strains have been also used as prototype in studies d
ing virulence factors and pathogenic mechanisms not present in EAEC 042. D
the variety of identifi ed virulence factors, such as enterotoxins, cytotoxins, s
proteins, outer membrane proteins, and fi mbriae (Table2.1 ), the pathogenesis of the
diarrhea caused by EAEC remains unclear.
2 Enteroaggregative Escherichia coli (EAEC)
1.4 Histopathology
Studies in vitro, in vivo, and ex vivo, evaluating EAEC interactions with intest
cells from animals or humans, have tried to elucidate the pathogenesis
pathotype since its description in 1987. The fi rst study about EAEC pathogen
in animal models employed ligated ileal loop of rabbit and rat intestines (Vial
1988 ), showing that EAEC 042 and 17-2 strongly adhered to the mucosa and
ing shortening of microvilli, hemorrhagic necrosis with edema, and mononucl
infi ltrates in the submucosa. Analysis by transmission electron microscopy re
no bacterial invasion and the microvilli architecture was preserved.
Other studies employing in vitro organ culture (IVOC) models have elucid
the intestinal alterations induced by EAEC in fragments of human biopsies of
denum, ileum, and colon (Hicks et al. 1996 ; Nataro et al. 1996 ). Cytotoxic e
the colon were observed, such as microvilli vesiculation, enlarged crypt open
and increased epithelial cell extrusion (Hicks et al.1996 ). Also using IVOC, Nataro
and colleagues demonstrated strong adhesion of EAEC 042 to jejunal
ileum, and more intensely to the colon, while 042 cured of the pAA2 plasmid
this ability (Nataro et al.1996 ). All together, these data strongly indicated that EA
virulence was probable due to intestinal colonization, mainly to the colonic m
in the characteristic aggregative manner, with bacteria forming strong biofi lm
increased mucus layer, followed by cytotoxic and pro-infl ammatory effects
1.5 Strain Heterogeneity
In the decades that followed its original description, EAEC strains have been
acterized in numerous studies around the world, highlighting the particular h
geneity of this category in terms of serotypes, genetic determinants lin
virulence and phylogenetic groups (Boisen et al. 2012; Chattaway et al. 2014b ;
Czeczulin et al.1999; Jenkins et al.2006 ). This heterogeneity together with the
fact that not all EAEC strains were able to cause diarrhea in experimental infe
of humans raises the idea that only a subset of EAEC strains, carrying a speci
of virulence factors, has the capacity to cause diarrhea. This set of factors ha
been determined.
After demonstrating its pathogenicity in human volunteers, EAEC 042 beca
genetically and phenotypically widely studied and considered the prototype s
for EAEC. Major advances in understanding the pathogenesis of EAEC result
from data obtained with this strain whose genome has been sequenced (Chau
et al.2010 ). Other EAEC strains have been also used as prototype in studies d
ing virulence factors and pathogenic mechanisms not present in EAEC 042. D
the variety of identifi ed virulence factors, such as enterotoxins, cytotoxins, s
proteins, outer membrane proteins, and fi mbriae (Table2.1 ), the pathogenesis of the
diarrhea caused by EAEC remains unclear.
2 Enteroaggregative Escherichia coli (EAEC)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
32
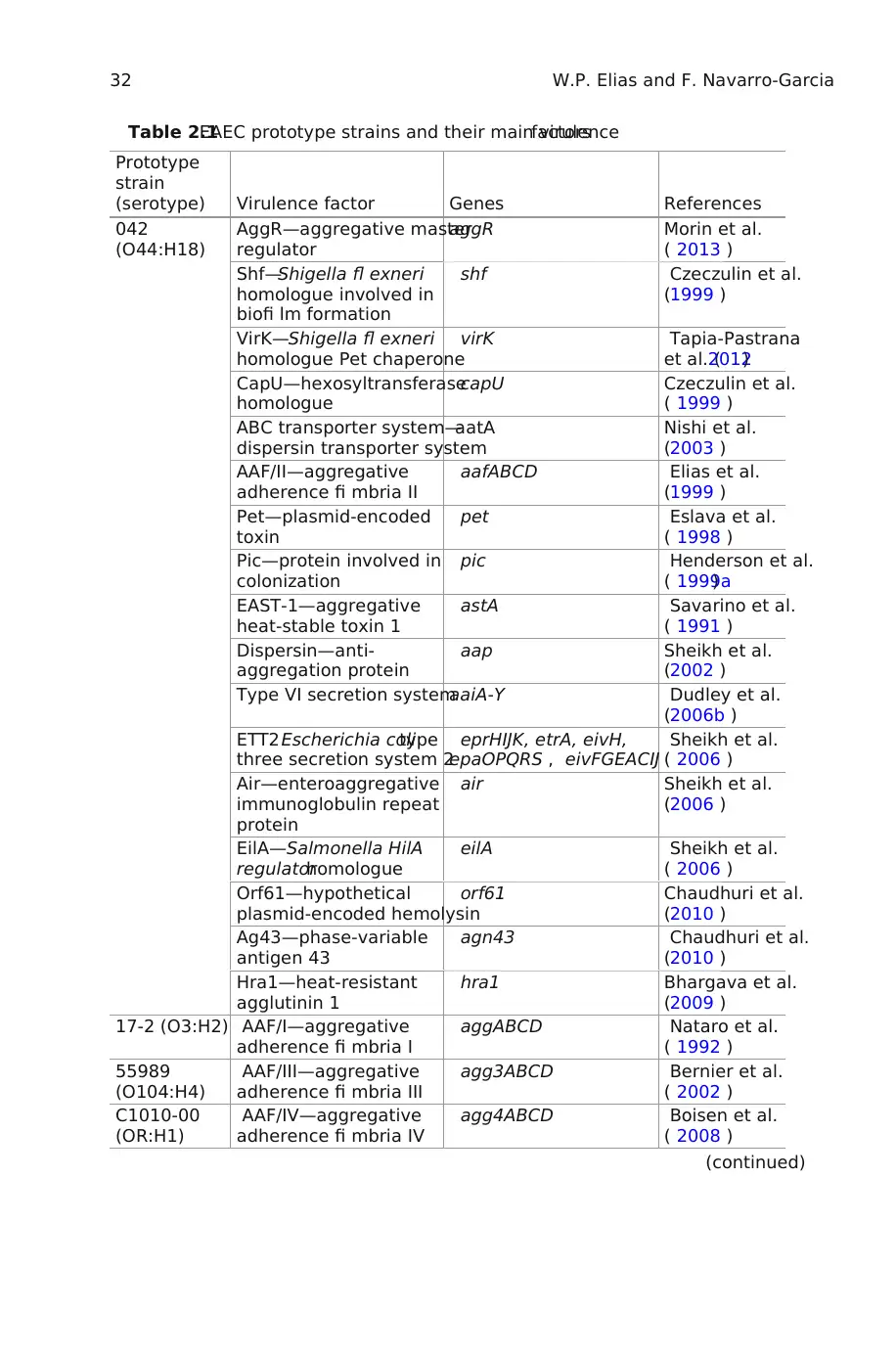
Table 2.1EAEC prototype strains and their main virulencefactors
Prototype
strain
(serotype) Virulence factor Genes References
042
(O44:H18)
AggR—aggregative master
regulator
aggR Morin et al.
( 2013 )
Shf—Shigella fl exneri
homologue involved in
biofi lm formation
shf Czeczulin et al.
(1999 )
VirK—Shigella fl exneri
homologue Pet chaperone
virK Tapia-Pastrana
et al. (2012)
CapU—hexosyltransferase
homologue
capU Czeczulin et al.
( 1999 )
ABC transporter system—
dispersin transporter system
aatA Nishi et al.
(2003 )
AAF/II—aggregative
adherence fi mbria II
aafABCD Elias et al.
(1999 )
Pet—plasmid-encoded
toxin
pet Eslava et al.
( 1998 )
Pic—protein involved in
colonization
pic Henderson et al.
( 1999a)
EAST-1—aggregative
heat-stable toxin 1
astA Savarino et al.
( 1991 )
Dispersin—anti-
aggregation protein
aap Sheikh et al.
(2002 )
Type VI secretion systemaaiA-Y Dudley et al.
(2006b )
ETT2 Escherichia colitype
three secretion system 2
eprHIJK, etrA, eivH,
epaOPQRS , eivFGEACIJ
Sheikh et al.
( 2006 )
Air—enteroaggregative
immunoglobulin repeat
protein
air Sheikh et al.
(2006 )
EilA—Salmonella HilA
regulatorhomologue
eilA Sheikh et al.
( 2006 )
Orf61—hypothetical
plasmid-encoded hemolysin
orf61 Chaudhuri et al.
(2010 )
Ag43—phase-variable
antigen 43
agn43 Chaudhuri et al.
(2010 )
Hra1—heat-resistant
agglutinin 1
hra1 Bhargava et al.
(2009 )
17-2 (O3:H2) AAF/I—aggregative
adherence fi mbria I
aggABCD Nataro et al.
( 1992 )
55989
(O104:H4)
AAF/III—aggregative
adherence fi mbria III
agg3ABCD Bernier et al.
( 2002 )
C1010-00
(OR:H1)
AAF/IV—aggregative
adherence fi mbria IV
agg4ABCD Boisen et al.
( 2008 )
(continued)
W.P. Elias and F. Navarro-Garcia
Table 2.1EAEC prototype strains and their main virulencefactors
Prototype
strain
(serotype) Virulence factor Genes References
042
(O44:H18)
AggR—aggregative master
regulator
aggR Morin et al.
( 2013 )
Shf—Shigella fl exneri
homologue involved in
biofi lm formation
shf Czeczulin et al.
(1999 )
VirK—Shigella fl exneri
homologue Pet chaperone
virK Tapia-Pastrana
et al. (2012)
CapU—hexosyltransferase
homologue
capU Czeczulin et al.
( 1999 )
ABC transporter system—
dispersin transporter system
aatA Nishi et al.
(2003 )
AAF/II—aggregative
adherence fi mbria II
aafABCD Elias et al.
(1999 )
Pet—plasmid-encoded
toxin
pet Eslava et al.
( 1998 )
Pic—protein involved in
colonization
pic Henderson et al.
( 1999a)
EAST-1—aggregative
heat-stable toxin 1
astA Savarino et al.
( 1991 )
Dispersin—anti-
aggregation protein
aap Sheikh et al.
(2002 )
Type VI secretion systemaaiA-Y Dudley et al.
(2006b )
ETT2 Escherichia colitype
three secretion system 2
eprHIJK, etrA, eivH,
epaOPQRS , eivFGEACIJ
Sheikh et al.
( 2006 )
Air—enteroaggregative
immunoglobulin repeat
protein
air Sheikh et al.
(2006 )
EilA—Salmonella HilA
regulatorhomologue
eilA Sheikh et al.
( 2006 )
Orf61—hypothetical
plasmid-encoded hemolysin
orf61 Chaudhuri et al.
(2010 )
Ag43—phase-variable
antigen 43
agn43 Chaudhuri et al.
(2010 )
Hra1—heat-resistant
agglutinin 1
hra1 Bhargava et al.
(2009 )
17-2 (O3:H2) AAF/I—aggregative
adherence fi mbria I
aggABCD Nataro et al.
( 1992 )
55989
(O104:H4)
AAF/III—aggregative
adherence fi mbria III
agg3ABCD Bernier et al.
( 2002 )
C1010-00
(OR:H1)
AAF/IV—aggregative
adherence fi mbria IV
agg4ABCD Boisen et al.
( 2008 )
(continued)
W.P. Elias and F. Navarro-Garcia
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

33
Due to the association of AA phenotype with high-molecular weight plasm
carrying large number of plasmid-encoded virulence factors in EAEC, these
mids are called aggregative virulence plasmids or pAA (Harrington et al.2006 ).
From the pAA1 plasmid present in the prototype EAEC 17-2 (serotype O3:H
Baudry et al. ( 1990 ) isolated the CVD432 probe fragment, widely us
molecular diagnosis of EAEC. Plasmid pAA2, present in the EAEC 042, also ha
approximately 100 kb of genetic information and encodes many well-charact
virulence factors (Czeczulin et al.1999 ).
AggR is a transcriptional activator and regulates the expression of various
lence factors present in the chromosome and pAA2 plasmid of EAEC 042, defi
the AggR regulon (Harrington et al.2006 ). At least 44 genes are regulated byaggR ,
including the genes for AAF/II biogenesis, dispersin and its secretion system,
CapU, theaai type VI secretion system, andaagR itself (Morin et al.2013 ; Dudley
et al.2006b ). Not all EAEC strains harbor aggR and, consequently, the pAA p
mid. Thus, the classifi cation of EAEC was proposed into two subgroups, e.g.,
cal and atypical, taking into account the presence or absence ofaggR , respectively
(Harrington et al. 2006 ). This classifi cation defi nes two groups of strains, on
them consisting of typical strains with higher pathogenic potential due to the
ence of the AggR regulon and the pAA virulence plasmid (Estrada-Gar
Navarro-Garcia2012 ). However, atypical EAEC strains are commonly isolat
from cases of diarrhea, as the sole pathogen (Huang et al.2007 ; Jiang et al. 2002 ).
In one epidemiological study on the etiology of acute diarrhea in childr
Espírito Santo (Brazil), atypical strains were more frequent than typical
(Isabel Scaletsky, unpublished data). In addition, at least two outbreaks of dia
were caused by atypical EAEC (Cobeljic et al. 1996 ), one of them affected m
than two thousand children (Itoh et al.1997 ).
Table 2.1(continued)
Prototype
strain
(serotype) Virulence factor Genes References
C338-14
(O55:H19)
AAF/V—aggregative
adherence fi mbria V
Agg5ABCD Jonsson et al.
(2015 )
C1096
(O4:HNT)
Pil—type IV pilus pilLMNOPQRSTUV Dudley et al.
( 2006a)
JM221
(O92:H33)
AAF/I—aggregative
adherence fi mbria I
aggABCD Mathewson et al.
( 1986 )
60A (ND) Hra2—heat-resistant
agglutinin 2
hra2 Mancini et al.
( 2011)
OR O rough,NT non-typable,ND not determined
2 Enteroaggregative Escherichia coli (EAEC)
Due to the association of AA phenotype with high-molecular weight plasm
carrying large number of plasmid-encoded virulence factors in EAEC, these
mids are called aggregative virulence plasmids or pAA (Harrington et al.2006 ).
From the pAA1 plasmid present in the prototype EAEC 17-2 (serotype O3:H
Baudry et al. ( 1990 ) isolated the CVD432 probe fragment, widely us
molecular diagnosis of EAEC. Plasmid pAA2, present in the EAEC 042, also ha
approximately 100 kb of genetic information and encodes many well-charact
virulence factors (Czeczulin et al.1999 ).
AggR is a transcriptional activator and regulates the expression of various
lence factors present in the chromosome and pAA2 plasmid of EAEC 042, defi
the AggR regulon (Harrington et al.2006 ). At least 44 genes are regulated byaggR ,
including the genes for AAF/II biogenesis, dispersin and its secretion system,
CapU, theaai type VI secretion system, andaagR itself (Morin et al.2013 ; Dudley
et al.2006b ). Not all EAEC strains harbor aggR and, consequently, the pAA p
mid. Thus, the classifi cation of EAEC was proposed into two subgroups, e.g.,
cal and atypical, taking into account the presence or absence ofaggR , respectively
(Harrington et al. 2006 ). This classifi cation defi nes two groups of strains, on
them consisting of typical strains with higher pathogenic potential due to the
ence of the AggR regulon and the pAA virulence plasmid (Estrada-Gar
Navarro-Garcia2012 ). However, atypical EAEC strains are commonly isolat
from cases of diarrhea, as the sole pathogen (Huang et al.2007 ; Jiang et al. 2002 ).
In one epidemiological study on the etiology of acute diarrhea in childr
Espírito Santo (Brazil), atypical strains were more frequent than typical
(Isabel Scaletsky, unpublished data). In addition, at least two outbreaks of dia
were caused by atypical EAEC (Cobeljic et al. 1996 ), one of them affected m
than two thousand children (Itoh et al.1997 ).
Table 2.1(continued)
Prototype
strain
(serotype) Virulence factor Genes References
C338-14
(O55:H19)
AAF/V—aggregative
adherence fi mbria V
Agg5ABCD Jonsson et al.
(2015 )
C1096
(O4:HNT)
Pil—type IV pilus pilLMNOPQRSTUV Dudley et al.
( 2006a)
JM221
(O92:H33)
AAF/I—aggregative
adherence fi mbria I
aggABCD Mathewson et al.
( 1986 )
60A (ND) Hra2—heat-resistant
agglutinin 2
hra2 Mancini et al.
( 2011)
OR O rough,NT non-typable,ND not determined
2 Enteroaggregative Escherichia coli (EAEC)

34
1.6 Pathogenesis in Three Steps
EAEC ability to mediate diarrhea was clearly established through the
study with EAEC strain 042 (Nataro et al.1995 ). However, this study and others
have left clear that the pathogenesis of EAEC is complex, and EAEC strains ar
very heterogeneous.
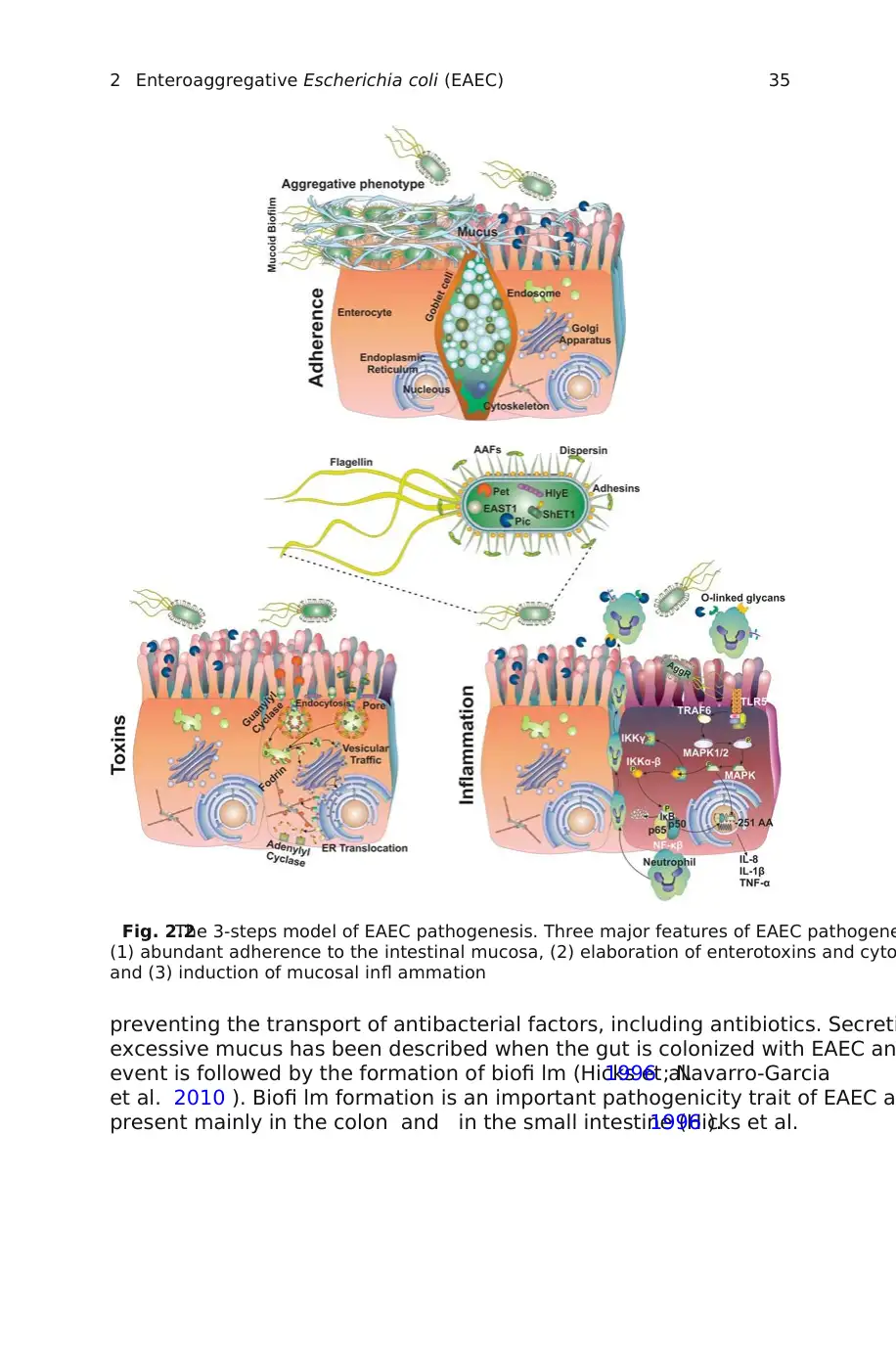
Data accumulated from several studies have suggested three major featur
EAEC pathogenesis : (1) abundant adherence to the intestinal mucosa, (2) e
tion of enterotoxins and cytotoxins, and (3) induction of mucosal infl am
(Fig.2.2 ). These stages of EAEC pathogenicity have been obtained from stud
in vitro in cell cultures, animal models, and patients infected with EAEC (Hicks
et al. 1996; Navarro-Garcia and Elias2011 ). Heterogeneity is also found in EAEC
colonization, because once ingested, the location of infection in the gastroint
tract has not been well defi ned. Studies done on endoscopic intestinal specim
demonstrate that EAEC can bind to jejunal, ileal, and colonic epithelium (Nata
et al.1996 ). These fi ndings were recently validated in fragments from termina
and colon that were excised from pediatric patients undergoing intestinal sur
and from adult patients that underwent colonoscopy treatment; such intestin
ments were used to defi ne interaction with three EAEC strains. These bacter
nized ileal and colonic mucosa in the typical stacked-brick confi guration. In b
regions, the strains were seen over a great amount of mucus and sometimes
intact epithelium. It was possible to see adhered bacteria to the intestinal sur
with visualization of fi mbrial structures that could be responsible for the adh
process (Andrade et al.2011 ). Although a great diversity of adhesins, toxins, and
proteins involved in EAEC pathogenesis has been described, the prevalence o
virulence factors-encoding genes is highly variable and none of these h
found present in all EAEC strains (Czeczulin et al.1999 ; Jenkins et al.2006 ).
1.6.1 Adherence
Adhesion to the intestinal epithelium is facilitated by fi mbriae and is the fi rs
the bacterial colonization of the gut. Several authors demonstrated that the A
is associated with the presence of fi mbrial and afi mbrial adhesins in EAEC st
(Bhargava et al.2009 ; Boisen et al.2008 ; Czeczulin et al.1997 ; Hicks et al.1996 ).
However, the genes encoding these adhesion factors are found in low prevale
indicating a high diversity of adhesive structures responsible for the AA patte
fi mbriae bind to components of the extracellular matrix of intestinal epithelia
(Farfan et al. 2008 ; Izquierdo et al. 2014b ), and the AA pattern is thought t
from binding to the epithelial cell surface and binding to adjacent EAEC bacte
Abundant adherence of EAEC to the intestinal mucosa includes mucoid bi
formation . Biofi lm formation is a potential important contributor in persisten
tion by allowing the bacteria population to evade the local immune system an
W.P. Elias and F. Navarro-Garcia
1.6 Pathogenesis in Three Steps
EAEC ability to mediate diarrhea was clearly established through the
study with EAEC strain 042 (Nataro et al.1995 ). However, this study and others
have left clear that the pathogenesis of EAEC is complex, and EAEC strains ar
very heterogeneous.
Data accumulated from several studies have suggested three major featur
EAEC pathogenesis : (1) abundant adherence to the intestinal mucosa, (2) e
tion of enterotoxins and cytotoxins, and (3) induction of mucosal infl am
(Fig.2.2 ). These stages of EAEC pathogenicity have been obtained from stud
in vitro in cell cultures, animal models, and patients infected with EAEC (Hicks
et al. 1996; Navarro-Garcia and Elias2011 ). Heterogeneity is also found in EAEC
colonization, because once ingested, the location of infection in the gastroint
tract has not been well defi ned. Studies done on endoscopic intestinal specim
demonstrate that EAEC can bind to jejunal, ileal, and colonic epithelium (Nata
et al.1996 ). These fi ndings were recently validated in fragments from termina
and colon that were excised from pediatric patients undergoing intestinal sur
and from adult patients that underwent colonoscopy treatment; such intestin
ments were used to defi ne interaction with three EAEC strains. These bacter
nized ileal and colonic mucosa in the typical stacked-brick confi guration. In b
regions, the strains were seen over a great amount of mucus and sometimes
intact epithelium. It was possible to see adhered bacteria to the intestinal sur
with visualization of fi mbrial structures that could be responsible for the adh
process (Andrade et al.2011 ). Although a great diversity of adhesins, toxins, and
proteins involved in EAEC pathogenesis has been described, the prevalence o
virulence factors-encoding genes is highly variable and none of these h
found present in all EAEC strains (Czeczulin et al.1999 ; Jenkins et al.2006 ).
1.6.1 Adherence
Adhesion to the intestinal epithelium is facilitated by fi mbriae and is the fi rs
the bacterial colonization of the gut. Several authors demonstrated that the A
is associated with the presence of fi mbrial and afi mbrial adhesins in EAEC st
(Bhargava et al.2009 ; Boisen et al.2008 ; Czeczulin et al.1997 ; Hicks et al.1996 ).
However, the genes encoding these adhesion factors are found in low prevale
indicating a high diversity of adhesive structures responsible for the AA patte
fi mbriae bind to components of the extracellular matrix of intestinal epithelia
(Farfan et al. 2008 ; Izquierdo et al. 2014b ), and the AA pattern is thought t
from binding to the epithelial cell surface and binding to adjacent EAEC bacte
Abundant adherence of EAEC to the intestinal mucosa includes mucoid bi
formation . Biofi lm formation is a potential important contributor in persisten
tion by allowing the bacteria population to evade the local immune system an
W.P. Elias and F. Navarro-Garcia
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
35
preventing the transport of antibacterial factors, including antibiotics. Secreti
excessive mucus has been described when the gut is colonized with EAEC an
event is followed by the formation of biofi lm (Hicks et al.1996 ; Navarro-Garcia
et al. 2010 ). Biofi lm formation is an important pathogenicity trait of EAEC a
present mainly in the colon and in the small intestine (Hicks et al.1996 ).
Fig. 2.2The 3-steps model of EAEC pathogenesis. Three major features of EAEC pathogene
(1) abundant adherence to the intestinal mucosa, (2) elaboration of enterotoxins and cyto
and (3) induction of mucosal infl ammation
2 Enteroaggregative Escherichia coli (EAEC)
preventing the transport of antibacterial factors, including antibiotics. Secreti
excessive mucus has been described when the gut is colonized with EAEC an
event is followed by the formation of biofi lm (Hicks et al.1996 ; Navarro-Garcia
et al. 2010 ). Biofi lm formation is an important pathogenicity trait of EAEC a
present mainly in the colon and in the small intestine (Hicks et al.1996 ).
Fig. 2.2The 3-steps model of EAEC pathogenesis. Three major features of EAEC pathogene
(1) abundant adherence to the intestinal mucosa, (2) elaboration of enterotoxins and cyto
and (3) induction of mucosal infl ammation
2 Enteroaggregative Escherichia coli (EAEC)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

36
1.6.2 Toxins
Once the biofi lm has been established, EAEC produces cytotoxic effects such
microvillus vesiculation, enlarged crypt openings, and increased epithelia
extrusion (Harrington et al.2006 ). It is thought that the secretion of toxins plays a
important role in secretory diarrhea, which is a typical clinical manifest
EAEC infection. Numerous putative EAEC virulence factors, such as Pet, EAST
and ShET1 toxins, and Pic, have been associated to these cytotoxic effects.
1.6.3 Infl ammation
EAEC is an infl ammatory pathogen, as demonstrated both in clinical (Greenb
2002 ) and laboratory (Steiner et al.1998 ) studies. It is clear that multiple factors con
tribute to EAEC-induced infl ammation, and further characterization of the na
EAEC pro-infl ammatory factors will greatly advance the understanding of this
ing pathogen. The initial host infl ammatory response to EAEC infection is dep
on the host innate immune system and the type of EAEC strain causing infect
role of putative virulence genes and clinical outcomes is not well understood;
ever, the presence of several EAEC virulence factors correlate with fi ndings i
ing that levels of fecal cytokines and infl ammatory markers in stools of adult
children with diarrhea are elevated, including interleukin (IL)-1 receptor
IL-1β, IL-8, interferon (INF)-γ, lactoferrin, fecal leukocytes, and occult blood (Ji
et al. 2002 ). The IL-8 infl ammatory response appears to be partially caused
(FliC) in a Caco-2 cell assay, as it was found that an afl agellated mutant of EA
not produce the same infl ammatory response (Steiner et al.2000 ).
Besides the pathogenic EAEC mechanisms, host factors are also determina
EAEC infl ammation. It was found that single nucleotide polymorphisms in the
moter region of the gene encoding the lipopolysaccharide receptor CD14 ar
ated with bacterial diarrhea in US and Canadian travelers to Mexico (Mohame
et al. 2011 ). The CD14 gene encodes a crucial step in the infl ammatory res
bacterial lipopolysaccharide stimulation mediated by the innate immune
Thus, this study found that one SNP in the promoter region of the CD14 gene
associated with an increased risk of EAEC-induced diarrhea. Patients wit
CD14 − 159 TT genotype were signifi cantly associated with EAEC-induced
rhea compared with healthy controls.
1.7 Main Virulence Factors
In the initial stage of EAEC colonization, the role of fi mbrial and afi mbrial ad
is fundamental. Five aggregative adherence fi mbriae (AAF/I–AAF/V) have bee
characterized in EAEC (Boisen et al. 2008 ; Czeczulin et al. 1997 ; Nataro et
Jonsson et al. 2015 ). All AAF fi mbriae are encoded by genes located in the p
W.P. Elias and F. Navarro-Garcia
1.6.2 Toxins
Once the biofi lm has been established, EAEC produces cytotoxic effects such
microvillus vesiculation, enlarged crypt openings, and increased epithelia
extrusion (Harrington et al.2006 ). It is thought that the secretion of toxins plays a
important role in secretory diarrhea, which is a typical clinical manifest
EAEC infection. Numerous putative EAEC virulence factors, such as Pet, EAST
and ShET1 toxins, and Pic, have been associated to these cytotoxic effects.
1.6.3 Infl ammation
EAEC is an infl ammatory pathogen, as demonstrated both in clinical (Greenb
2002 ) and laboratory (Steiner et al.1998 ) studies. It is clear that multiple factors con
tribute to EAEC-induced infl ammation, and further characterization of the na
EAEC pro-infl ammatory factors will greatly advance the understanding of this
ing pathogen. The initial host infl ammatory response to EAEC infection is dep
on the host innate immune system and the type of EAEC strain causing infect
role of putative virulence genes and clinical outcomes is not well understood;
ever, the presence of several EAEC virulence factors correlate with fi ndings i
ing that levels of fecal cytokines and infl ammatory markers in stools of adult
children with diarrhea are elevated, including interleukin (IL)-1 receptor
IL-1β, IL-8, interferon (INF)-γ, lactoferrin, fecal leukocytes, and occult blood (Ji
et al. 2002 ). The IL-8 infl ammatory response appears to be partially caused
(FliC) in a Caco-2 cell assay, as it was found that an afl agellated mutant of EA
not produce the same infl ammatory response (Steiner et al.2000 ).
Besides the pathogenic EAEC mechanisms, host factors are also determina
EAEC infl ammation. It was found that single nucleotide polymorphisms in the
moter region of the gene encoding the lipopolysaccharide receptor CD14 ar
ated with bacterial diarrhea in US and Canadian travelers to Mexico (Mohame
et al. 2011 ). The CD14 gene encodes a crucial step in the infl ammatory res
bacterial lipopolysaccharide stimulation mediated by the innate immune
Thus, this study found that one SNP in the promoter region of the CD14 gene
associated with an increased risk of EAEC-induced diarrhea. Patients wit
CD14 − 159 TT genotype were signifi cantly associated with EAEC-induced
rhea compared with healthy controls.
1.7 Main Virulence Factors
In the initial stage of EAEC colonization, the role of fi mbrial and afi mbrial ad
is fundamental. Five aggregative adherence fi mbriae (AAF/I–AAF/V) have bee
characterized in EAEC (Boisen et al. 2008 ; Czeczulin et al. 1997 ; Nataro et
Jonsson et al. 2015 ). All AAF fi mbriae are encoded by genes located in the p
W.P. Elias and F. Navarro-Garcia

37
plasmids, which regulated by AggR and their biogenesis follows the usher- ch
pathway. Also, a type IV fi mbrial, calledPil pilus, is responsible for the AA pattern in
cultured epithelial cells and abiotic surface and it’s exhibited by an atypical E
strain isolated in the outbreak in Serbia (Cobeljic et al.1996 ; Dudley et al.2006a ).
Non-fi mbrial adhesins, or outer membrane proteins with molecular w
between 30 and 58 kDa, and associated with AA pattern have been described
various EAEC strains (Bhargava et al.2009 ). In addition, Hra1 and Hra2 are heat-
resistant agglutinins involved in autoaggregation, biofi lm formation, and agg
tive adherence phenotypes (Bhargava et al.2009 ; Mancini et al. 2011 ). The presence
of agn43gene, encoding the autotransporter protein antigen 43, was associate
biofi lm formation and autoaggregation in EAEC 042 (Chaudhuri et al.2010 ). The
multifactorial characteristic of the AA phenotype is clear from studies with str
expressing multiple adhesins. Furthermore, the low prevalence of genes enco
these adhesins highlights the great diversity of adhesive structures in EAEC.
The dispersin protein (anti-aggregation protein) is an important EAEC vir
factor that mediates the dispersion of EAEC along the intestinal mucosa (She
et al.2002 ). Dispersin neutralizes the negative charge on the surface of the ba
cell and allows AAF/II fi mbrial projection , leading to anti-aggregation and d
sal of bacteria in the intestinal wall. This protein requires an ABC-type transpo
system encoded by the aatPABCD operon, which is present in the pAA2 plas
(Nishi et al.2003 ).
As described before, EAEC produces cytotoxic effects evident during in vit
and in vivo studies. Several putative virulence factors associated to those cyt
effects have been identifi ed and characterized in EAEC.
The fi rst toxin described in EAEC was the enteroaggregative heat-stabl
1 (EAST-1) , which is related to the heat-stable toxin (STa) of enterotoxigenic
(Savarino et al.1991 ). EAST-1 activates adenylate cyclase inducing increased cy
GMP levels in enterocytes, generating a secretory response (Savarino et al. 1
EAST-1 is a 38 amino acids peptide (4.1 kDa) encoded by astA gene,
located in pAA2 of EAEC 042 (Czeczulin et al.1999 ). Since STa toxin causes
secretory diarrhea, it was believed que EAST-1 was responsible for this effect
EAEC-induced diarrhea. However, the presence of EAST-1 in EAEC 17-2 was n
suffi cient to provoke diarrhea in volunteers (Nataro et al.1995 ).
Another toxin encoded by a chromosomal gene in EAEC 042 is called ShET
(Shigella enterotoxin 1), which is encoded by setAB located in the antisense str
of pic (Henderson et al.1999a ; Navarro-Garcia and Elias2011). ShET1 is an A:B
type toxin that causes accumulation of fl uid in rabbit ileal loops. The enterot
mechanism of ShET1 is independent of cAMP, cGMP, and calcium. However, t
precise mechanism of ShET1 action remains unclear.
Amongst the virulence factors produced by EAEC, the autotransporter prot
have a relevant role in the EAEC pathogenesis. Initially, two autotransporter p
teins were identifi ed in EAEC, comprising high-molecular weight protein
104 kDa for Pet and 109 kDa for Pic (Eslava et al.1998 ; Henderson et al.1999a ).
Autotransporter proteins are currently assigned to the type 5 secretio
(T5SS), which is described in detail in Chap. 10. Autotransporters contain three
2 Enteroaggregative Escherichia coli (EAEC)
plasmids, which regulated by AggR and their biogenesis follows the usher- ch
pathway. Also, a type IV fi mbrial, calledPil pilus, is responsible for the AA pattern in
cultured epithelial cells and abiotic surface and it’s exhibited by an atypical E
strain isolated in the outbreak in Serbia (Cobeljic et al.1996 ; Dudley et al.2006a ).
Non-fi mbrial adhesins, or outer membrane proteins with molecular w
between 30 and 58 kDa, and associated with AA pattern have been described
various EAEC strains (Bhargava et al.2009 ). In addition, Hra1 and Hra2 are heat-
resistant agglutinins involved in autoaggregation, biofi lm formation, and agg
tive adherence phenotypes (Bhargava et al.2009 ; Mancini et al. 2011 ). The presence
of agn43gene, encoding the autotransporter protein antigen 43, was associate
biofi lm formation and autoaggregation in EAEC 042 (Chaudhuri et al.2010 ). The
multifactorial characteristic of the AA phenotype is clear from studies with str
expressing multiple adhesins. Furthermore, the low prevalence of genes enco
these adhesins highlights the great diversity of adhesive structures in EAEC.
The dispersin protein (anti-aggregation protein) is an important EAEC vir
factor that mediates the dispersion of EAEC along the intestinal mucosa (She
et al.2002 ). Dispersin neutralizes the negative charge on the surface of the ba
cell and allows AAF/II fi mbrial projection , leading to anti-aggregation and d
sal of bacteria in the intestinal wall. This protein requires an ABC-type transpo
system encoded by the aatPABCD operon, which is present in the pAA2 plas
(Nishi et al.2003 ).
As described before, EAEC produces cytotoxic effects evident during in vit
and in vivo studies. Several putative virulence factors associated to those cyt
effects have been identifi ed and characterized in EAEC.
The fi rst toxin described in EAEC was the enteroaggregative heat-stabl
1 (EAST-1) , which is related to the heat-stable toxin (STa) of enterotoxigenic
(Savarino et al.1991 ). EAST-1 activates adenylate cyclase inducing increased cy
GMP levels in enterocytes, generating a secretory response (Savarino et al. 1
EAST-1 is a 38 amino acids peptide (4.1 kDa) encoded by astA gene,
located in pAA2 of EAEC 042 (Czeczulin et al.1999 ). Since STa toxin causes
secretory diarrhea, it was believed que EAST-1 was responsible for this effect
EAEC-induced diarrhea. However, the presence of EAST-1 in EAEC 17-2 was n
suffi cient to provoke diarrhea in volunteers (Nataro et al.1995 ).
Another toxin encoded by a chromosomal gene in EAEC 042 is called ShET
(Shigella enterotoxin 1), which is encoded by setAB located in the antisense str
of pic (Henderson et al.1999a ; Navarro-Garcia and Elias2011). ShET1 is an A:B
type toxin that causes accumulation of fl uid in rabbit ileal loops. The enterot
mechanism of ShET1 is independent of cAMP, cGMP, and calcium. However, t
precise mechanism of ShET1 action remains unclear.
Amongst the virulence factors produced by EAEC, the autotransporter prot
have a relevant role in the EAEC pathogenesis. Initially, two autotransporter p
teins were identifi ed in EAEC, comprising high-molecular weight protein
104 kDa for Pet and 109 kDa for Pic (Eslava et al.1998 ; Henderson et al.1999a ).
Autotransporter proteins are currently assigned to the type 5 secretio
(T5SS), which is described in detail in Chap. 10. Autotransporters contain three
2 Enteroaggregative Escherichia coli (EAEC)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 32
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.