Detailed Analysis of Environmental Bacteria and Nitrogen Cycles Report
VerifiedAdded on 2023/01/19
|10
|1637
|54
Report
AI Summary
This report provides a comprehensive overview of environmental bacteria and the nitrogen cycle. It begins with an introduction to the nitrogen cycle, explaining its importance and the interconversion of nitrogenous compounds. The report then delves into key processes such as nitrogen fixation, discussing atmospheric, industrial, and biological methods, with a focus on the Haber process. Nitrifying bacteria and their role in converting ammonia to nitrates are examined, along with the implications of nitrate presence in the soil and groundwater. The report also details denitrifying bacteria, which reverse the process, converting nitrates back into gaseous compounds. The significance of the nitrogen cycle in the ecosystem, including its impact on soil pH and the food industry, is also discussed. The report concludes by emphasizing the interplay of natural and artificial processes within the nitrogen cycle and their environmental consequences, such as global warming, with a call for controlled use of fertilizers. The report includes relevant figures and references.

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
1
ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
By Name
Course
Instructor
Institution
Location
Date
1
ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
2
Contents
INTRODUCTION...........................................................................................................................3
NITROGEN FIXING......................................................................................................................4
NITRIFYING BACTERIA.............................................................................................................5
DENITRIFYING BACTERIA........................................................................................................6
CONCLUSION................................................................................................................................7
2
Contents
INTRODUCTION...........................................................................................................................3
NITROGEN FIXING......................................................................................................................4
NITRIFYING BACTERIA.............................................................................................................5
DENITRIFYING BACTERIA........................................................................................................6
CONCLUSION................................................................................................................................7
ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
3
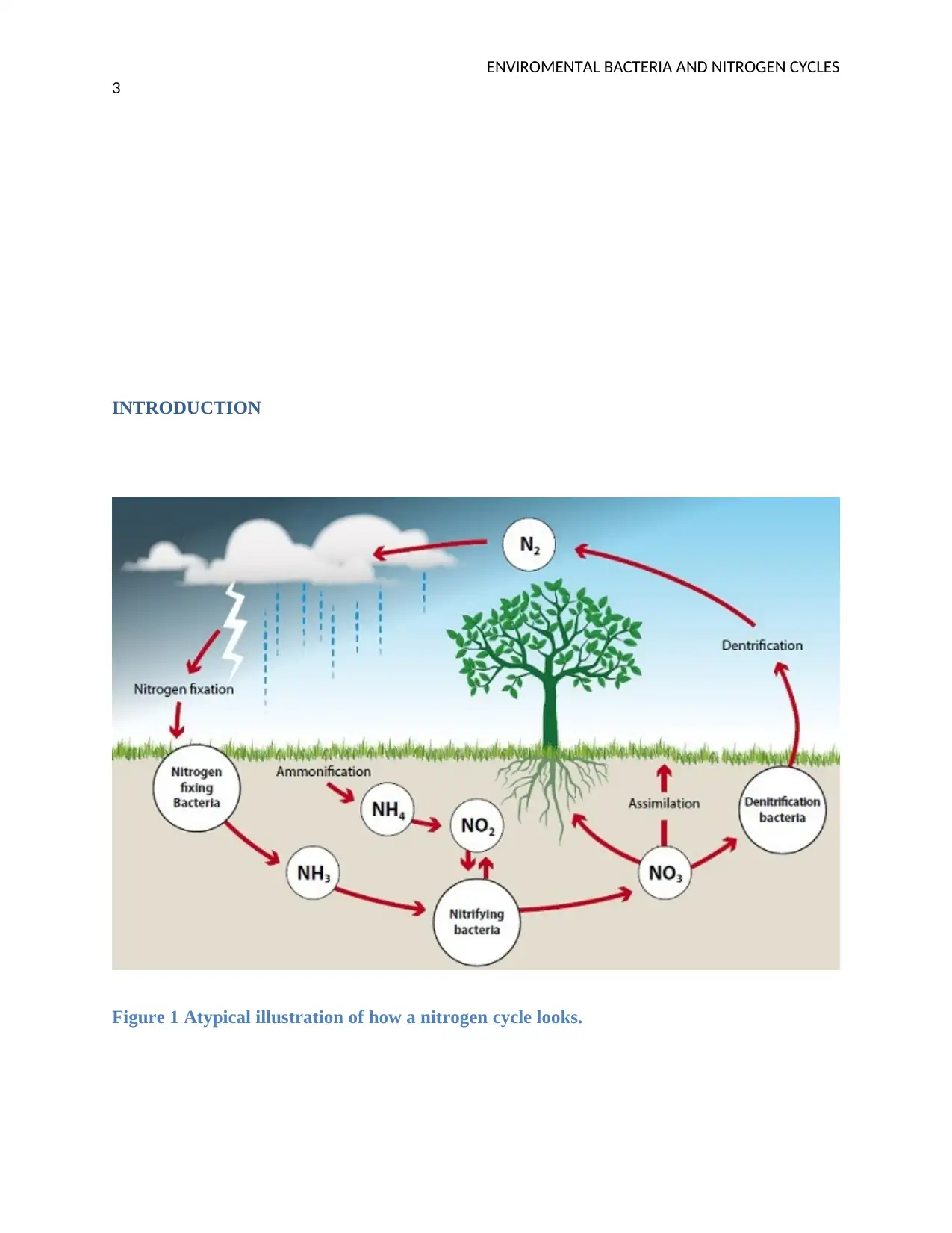
INTRODUCTION
Figure 1 Atypical illustration of how a nitrogen cycle looks.
3
INTRODUCTION
Figure 1 Atypical illustration of how a nitrogen cycle looks.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
4
Nitrogen as a gas is seen to be the most available gas in the globe’s atmosphere making up more
than 75% of the atmospheric gasses.
The nitrogen cycle is a sequence of courses that lead to the interconversion of nitrogenous
compounds in both the atmosphere and living matter. These processes include fixation of
nitrogen, assimilation ammonification dissimilation.
The study of this series of activities cycle helps scientists understand further the importance of
nitrogen and other nitrogenous compounds in the atmosphere.
NITROGEN FIXING
Nitrogen fixation refers to all the process, industrial, biological and atmospheric through which
nitrogen in the atmosphere is converted into ammonia and other nitro group containing
compounds. (Martin, 2017, pp. 8-10)
Figure 2Chemical reaction of nitrogen fixation.
There are three main methods through which nitrogen is fixed in our biosphere.
Atmospheric fixation
Industrial fixation
Biological fixation
4
Nitrogen as a gas is seen to be the most available gas in the globe’s atmosphere making up more
than 75% of the atmospheric gasses.
The nitrogen cycle is a sequence of courses that lead to the interconversion of nitrogenous
compounds in both the atmosphere and living matter. These processes include fixation of
nitrogen, assimilation ammonification dissimilation.
The study of this series of activities cycle helps scientists understand further the importance of
nitrogen and other nitrogenous compounds in the atmosphere.
NITROGEN FIXING
Nitrogen fixation refers to all the process, industrial, biological and atmospheric through which
nitrogen in the atmosphere is converted into ammonia and other nitro group containing
compounds. (Martin, 2017, pp. 8-10)
Figure 2Chemical reaction of nitrogen fixation.
There are three main methods through which nitrogen is fixed in our biosphere.
Atmospheric fixation
Industrial fixation
Biological fixation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
5
Atmospheric fixation is also known as fixation by lightning. The energy from lightning splits
molecular bonds of nitrogen which enabling the split atoms to bond with oxygen in the air to
form various oxides of nitrogen. When these oxides of nitrogen get into rainwater they create
nitrates which get absorbed into the earth.
Less than 8% of the total nitrogen fixed is by atmospheric nitrogen fixation.
Industrial nitrogen fixation
It is also called the artificial nitrogen fixation (Development(OECD), Organisation for Economic
Co-Operation and, 2019). Haber process is the main process of industrial nitrogen fixation.
Nitrogen in the air and hydrogen derived from methane is combined to form ammonia in an
exothermic process. This can has been illustrated by the equation:
N2 (g) +3H2 (g) ⇌ 2NH3 (g)
This equation is only true under pressure at 200 atmospheres and a catalyst is comprised of iron
containing potassium hydroxide. The nitrogen to hydrogen ratio is 1:3The Haber process has
been relied on in the production of ammonium fertilizers. These fertilizers are used to fix soils
which have a low content of nitrogen and lack nitrogen fixing bacteria in them.
NITRIFYING BACTERIA
The sequence and series through which nitrates become from ammonia that has undergone
numerous reactions is called nitrification. It is achieved through the help of specialized bacteria
which perform the task. Most nitrifying bacteria are of the genus Nitrosomonas, Nitrosococcus,
Nitrobacter and nitrococcus. These bacteria are able to obtain adequate energy from the
5
Atmospheric fixation is also known as fixation by lightning. The energy from lightning splits
molecular bonds of nitrogen which enabling the split atoms to bond with oxygen in the air to
form various oxides of nitrogen. When these oxides of nitrogen get into rainwater they create
nitrates which get absorbed into the earth.
Less than 8% of the total nitrogen fixed is by atmospheric nitrogen fixation.
Industrial nitrogen fixation
It is also called the artificial nitrogen fixation (Development(OECD), Organisation for Economic
Co-Operation and, 2019). Haber process is the main process of industrial nitrogen fixation.
Nitrogen in the air and hydrogen derived from methane is combined to form ammonia in an
exothermic process. This can has been illustrated by the equation:
N2 (g) +3H2 (g) ⇌ 2NH3 (g)
This equation is only true under pressure at 200 atmospheres and a catalyst is comprised of iron
containing potassium hydroxide. The nitrogen to hydrogen ratio is 1:3The Haber process has
been relied on in the production of ammonium fertilizers. These fertilizers are used to fix soils
which have a low content of nitrogen and lack nitrogen fixing bacteria in them.
NITRIFYING BACTERIA
The sequence and series through which nitrates become from ammonia that has undergone
numerous reactions is called nitrification. It is achieved through the help of specialized bacteria
which perform the task. Most nitrifying bacteria are of the genus Nitrosomonas, Nitrosococcus,
Nitrobacter and nitrococcus. These bacteria are able to obtain adequate energy from the

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
6
oxidation of ammonia to support them in doing the essential metabolic processes. In return the
bacteria get rid of ammonia which is toxic to plants. (David W. Emerich, 2012, p. 65)
Some of the nitrifying bacteria like rhizobium develop a symbiotic relationship with leguminous
plants to help in nitrogen fixation in the root nodules of plants. (Golterman, 2013, p. 161) The
bacteria provide nitrates and nitrogen to the plant which in return offers amino acids to the
bacteria.
The bacteria of genus Nitrosonomas oxidize the ammonia into nitrates and the bacteria of genus
that oxidizes nitrites to nitrates. The two types of bacteria are autotrophic (a bacteria with the
ability to make its own food) and are called nitrifying bacteria. Their action provides them with
energy while making nitrogen available for the plants via their roots. Nitrates are the only form
in which the plants take nitrogen from the atmosphere for nutrition and growth.
The soil is not able to contain the nitrate anions produced by the bacteria since they are highly
soluble in water, so they end up in ground water (Rattan Lal, 2018, p. 298). This makes the
ground water unsafe for drinking especially for infants. The anions interfere with oxygen levels
in infant blood which causes methemoglobinemia. The water also being enriched with nitrogen if
it reaches water sources causes abnormal growth of plants in water. This can be dangerous to fish
population especially for the plants in the water are oxygen consuming like water hyacinths.
Plants that grow in water tend to consume much of the water oxygen which suffocates the fish
and reduces their population.
The nitrification is a two-step reaction which relies on bacteria. Ammonia has to be oxidized
first; this is the rate limiting step of bacterial nitrification. Firstly, ammonia is oxidized to
nitrinous acid with the aid of monooxygenase. This reaction takes place with absorption of
6
oxidation of ammonia to support them in doing the essential metabolic processes. In return the
bacteria get rid of ammonia which is toxic to plants. (David W. Emerich, 2012, p. 65)
Some of the nitrifying bacteria like rhizobium develop a symbiotic relationship with leguminous
plants to help in nitrogen fixation in the root nodules of plants. (Golterman, 2013, p. 161) The
bacteria provide nitrates and nitrogen to the plant which in return offers amino acids to the
bacteria.
The bacteria of genus Nitrosonomas oxidize the ammonia into nitrates and the bacteria of genus
that oxidizes nitrites to nitrates. The two types of bacteria are autotrophic (a bacteria with the
ability to make its own food) and are called nitrifying bacteria. Their action provides them with
energy while making nitrogen available for the plants via their roots. Nitrates are the only form
in which the plants take nitrogen from the atmosphere for nutrition and growth.
The soil is not able to contain the nitrate anions produced by the bacteria since they are highly
soluble in water, so they end up in ground water (Rattan Lal, 2018, p. 298). This makes the
ground water unsafe for drinking especially for infants. The anions interfere with oxygen levels
in infant blood which causes methemoglobinemia. The water also being enriched with nitrogen if
it reaches water sources causes abnormal growth of plants in water. This can be dangerous to fish
population especially for the plants in the water are oxygen consuming like water hyacinths.
Plants that grow in water tend to consume much of the water oxygen which suffocates the fish
and reduces their population.
The nitrification is a two-step reaction which relies on bacteria. Ammonia has to be oxidized
first; this is the rate limiting step of bacterial nitrification. Firstly, ammonia is oxidized to
nitrinous acid with the aid of monooxygenase. This reaction takes place with absorption of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
7
energy and oxygen. Oxygen is then added to the nitrinous acid to convert it to nitrite. This
reaction requires two electrons and the rest are passed down the electron transport cycle (Silvia
Pajares, 2016).
The reactions are shown below.
NH3 (aq) +O2 (g) +2H++2e-→NH2OH (aq) + H2O(l)
NH2OH (aq) + H2O (l) →NO-2+5H++4e-
DENITRIFYING BACTERIA
Denitrifying bacteria play the role of reversing the converted nitrates into gaseous compounds
like nitric oxide nitrous oxide and atmospheric nitrogen. This happens when anaerobic
respiration occurs in the absence of oxygen forcing them to use nitrate instead of oxygen as the
bridging receiver of the electrons.
In bacterial dentrification nitrate/nitrite used as a receiver of the electrons is lost together with
the energy source.
C6H12O6 + 6O2 = 6CO2 + 6H2O + Energy
Denitrification is usually unfavorable for farmers since it takes nitrates away from the soil which
depriving crops of the essential nutrient. The whole denitrification process is essential for sewage
treatment to prevent the nitrate effect of causing eutrophication.
Denitrification leads to production of nitrous oxide, a greenhouse gas that is responsible for
global warming.
7
energy and oxygen. Oxygen is then added to the nitrinous acid to convert it to nitrite. This
reaction requires two electrons and the rest are passed down the electron transport cycle (Silvia
Pajares, 2016).
The reactions are shown below.
NH3 (aq) +O2 (g) +2H++2e-→NH2OH (aq) + H2O(l)
NH2OH (aq) + H2O (l) →NO-2+5H++4e-
DENITRIFYING BACTERIA
Denitrifying bacteria play the role of reversing the converted nitrates into gaseous compounds
like nitric oxide nitrous oxide and atmospheric nitrogen. This happens when anaerobic
respiration occurs in the absence of oxygen forcing them to use nitrate instead of oxygen as the
bridging receiver of the electrons.
In bacterial dentrification nitrate/nitrite used as a receiver of the electrons is lost together with
the energy source.
C6H12O6 + 6O2 = 6CO2 + 6H2O + Energy
Denitrification is usually unfavorable for farmers since it takes nitrates away from the soil which
depriving crops of the essential nutrient. The whole denitrification process is essential for sewage
treatment to prevent the nitrate effect of causing eutrophication.
Denitrification leads to production of nitrous oxide, a greenhouse gas that is responsible for
global warming.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
8
SIGNIFICANCE OF CYCLING IN THE ECOSYSTEM
Nitrification has numerous significances in the ecosystem. “The main sources of nitrogen
derivatives in the ground are defecation and decay of biological nitrogen resultant from animals
and plants, and the use of ammonium fertilizers. Many microbes and plants need this ammonium
for growth, while others integrate nitrate”. (Silvia Pajares, 2016) . “For both it is important in
regulating the amount of nitrogen in the atmosphere. Ammonia has the natural tendency to fix to
soil particles, its alteration to nitrate causes substantial loss of the nitrogen present in the soil
through percolating and transformation to its gas forms through denitrification”. (Silvia Pajares,
2016). Nitrification furthermore leads to the decrease of pH levels in the soil, with consequential
intensifications in activating heavy metals present in soils which contain a lot of added fertilizers
and not treated for neutralization of acidity.
With the increasing human population in the world the food industry is faced with a high food
demand. This has brought the need for artificial nitrogen to compensate for the soils insufficient
in crucial crop growth enhancing elements like potassium calcium and phosphorous. (Arvin
Mosier, 2013, pp. 245-261) Addition of industrially manufactured ammonia to barren lands
increases the availability of nitrogen to plants which enhances growth.
CONCLUSION
The nitrogen cycle relies on both natural and artificial processes. The natural processes are
lightening fixation and dependence on biological processes which include nitrification and
denitrification. This is a clear sign that the naturally existing nitrogen is constantly and
consistently being converted to its oxides and other compounds while being broken down back to
nitrogen gas. With the increasing demand of artificial nitrogen the natural balance by nature is
8
SIGNIFICANCE OF CYCLING IN THE ECOSYSTEM
Nitrification has numerous significances in the ecosystem. “The main sources of nitrogen
derivatives in the ground are defecation and decay of biological nitrogen resultant from animals
and plants, and the use of ammonium fertilizers. Many microbes and plants need this ammonium
for growth, while others integrate nitrate”. (Silvia Pajares, 2016) . “For both it is important in
regulating the amount of nitrogen in the atmosphere. Ammonia has the natural tendency to fix to
soil particles, its alteration to nitrate causes substantial loss of the nitrogen present in the soil
through percolating and transformation to its gas forms through denitrification”. (Silvia Pajares,
2016). Nitrification furthermore leads to the decrease of pH levels in the soil, with consequential
intensifications in activating heavy metals present in soils which contain a lot of added fertilizers
and not treated for neutralization of acidity.
With the increasing human population in the world the food industry is faced with a high food
demand. This has brought the need for artificial nitrogen to compensate for the soils insufficient
in crucial crop growth enhancing elements like potassium calcium and phosphorous. (Arvin
Mosier, 2013, pp. 245-261) Addition of industrially manufactured ammonia to barren lands
increases the availability of nitrogen to plants which enhances growth.
CONCLUSION
The nitrogen cycle relies on both natural and artificial processes. The natural processes are
lightening fixation and dependence on biological processes which include nitrification and
denitrification. This is a clear sign that the naturally existing nitrogen is constantly and
consistently being converted to its oxides and other compounds while being broken down back to
nitrogen gas. With the increasing demand of artificial nitrogen the natural balance by nature is

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
9
being interfered with which is causing increase in greenhouse gases which are involved in the
cycle (nitrous oxide). Experts urge that this will is one of the major causes of global warming
and climate change this has led to the controlled use of ammonium fertilizers in various
countries.
REFERENCES
Arvin Mosier, J. K. (2013). Agriculture and the Nitrogen Cycle: Assessing the Impacts of
Fertilizer Use on Food Production and the Environment. Washington D.C: Island Press.
David W. Emerich, H. B. (2012). Nitrogen Fixation In Crop Production. Madison: ASA-CSSA-
SSSA.
Development(OECD), Organisation for Economic Co-Operation and. (2019). Human
Acceleration of the Nitrogen Cycle: Managing Risks and Uncertainty. London: IWA
Publishing.
Golterman, H. (2013). Denitrification in the Nitrogen Cycle. Berlin: Springer Science &
Business Media.
Martin, B. (2017). The Nitrogen Cycle. New York : The Rosen Publishing Group, Inc.
Rattan Lal, B. S. (2018). Soil Nitrogen Uses and Enviromental Impact. Florida: CRC Press.
Silvia Pajares, B. J. (2016). Ecology of Nitrogen Fixing, Nitrifying, and Denitrifying
Microorganisms in Tropical Forest Soils. Front Microbial, 20.
9
being interfered with which is causing increase in greenhouse gases which are involved in the
cycle (nitrous oxide). Experts urge that this will is one of the major causes of global warming
and climate change this has led to the controlled use of ammonium fertilizers in various
countries.
REFERENCES
Arvin Mosier, J. K. (2013). Agriculture and the Nitrogen Cycle: Assessing the Impacts of
Fertilizer Use on Food Production and the Environment. Washington D.C: Island Press.
David W. Emerich, H. B. (2012). Nitrogen Fixation In Crop Production. Madison: ASA-CSSA-
SSSA.
Development(OECD), Organisation for Economic Co-Operation and. (2019). Human
Acceleration of the Nitrogen Cycle: Managing Risks and Uncertainty. London: IWA
Publishing.
Golterman, H. (2013). Denitrification in the Nitrogen Cycle. Berlin: Springer Science &
Business Media.
Martin, B. (2017). The Nitrogen Cycle. New York : The Rosen Publishing Group, Inc.
Rattan Lal, B. S. (2018). Soil Nitrogen Uses and Enviromental Impact. Florida: CRC Press.
Silvia Pajares, B. J. (2016). Ecology of Nitrogen Fixing, Nitrifying, and Denitrifying
Microorganisms in Tropical Forest Soils. Front Microbial, 20.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ENVIROMENTAL BACTERIA AND NITROGEN CYCLES
10
10
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

