Detailed Report on Enzyme Structure, Function, and Influencing Factors
VerifiedAdded on 2023/06/08
|7
|745
|134
Report
AI Summary
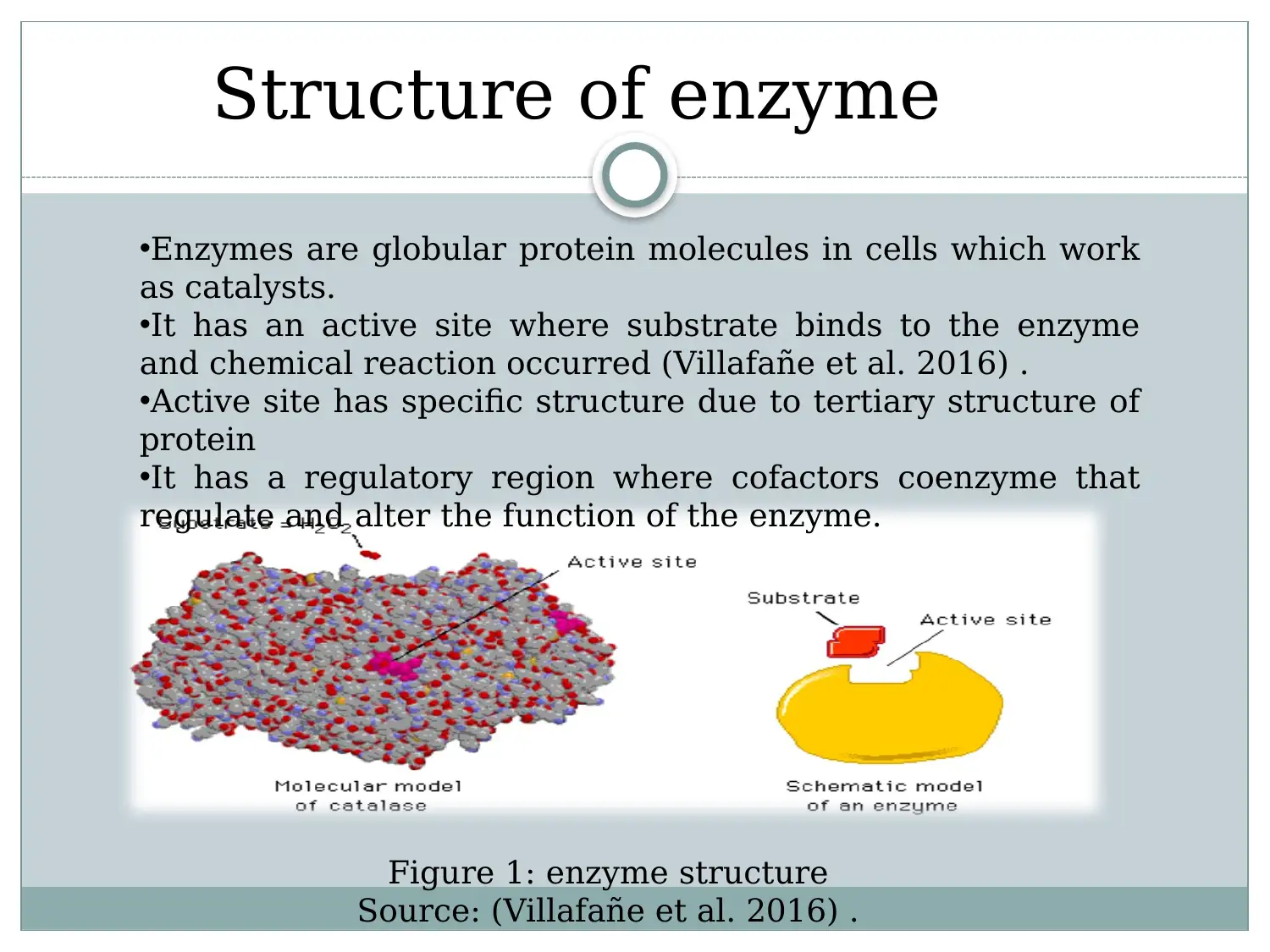
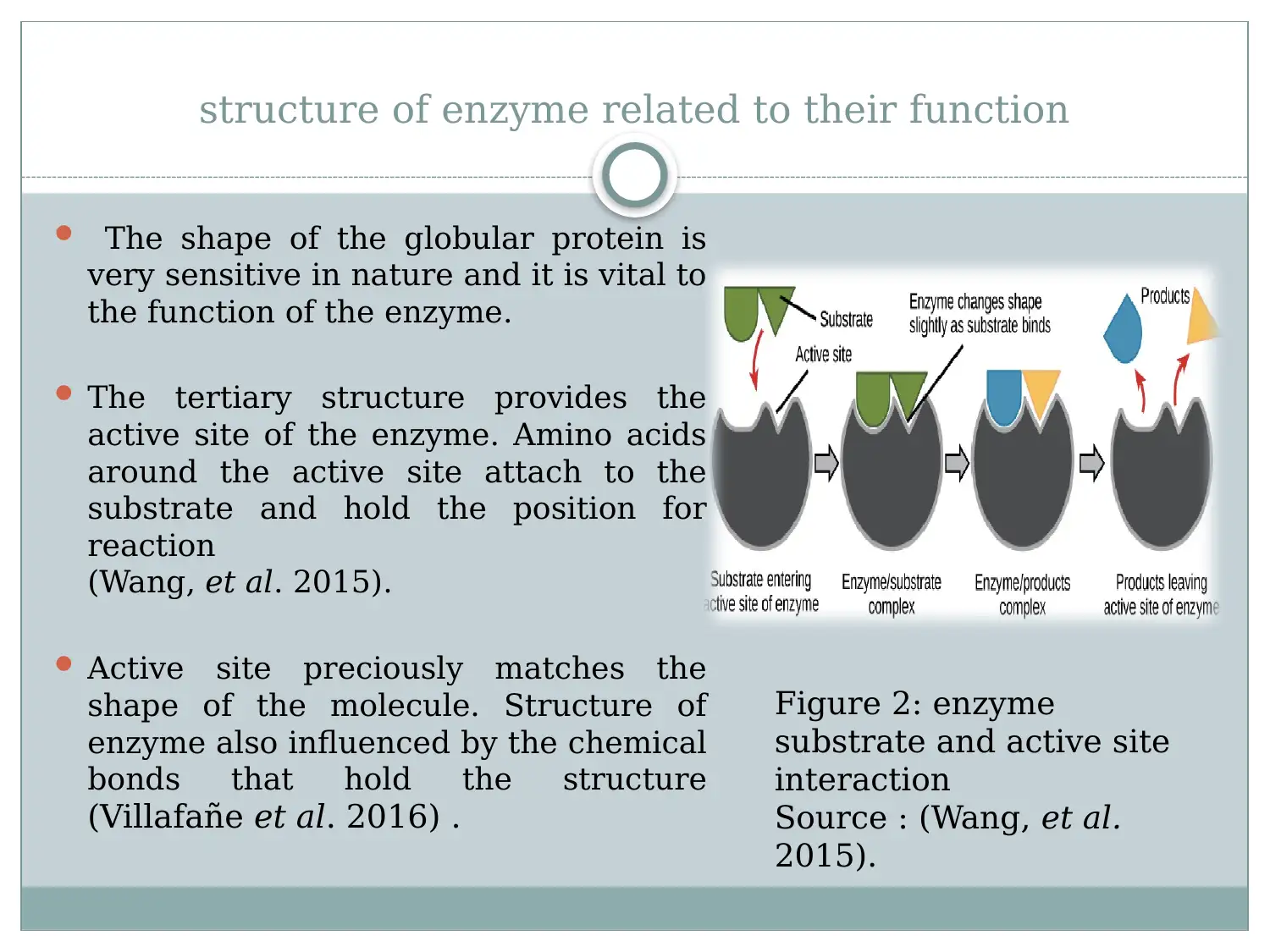
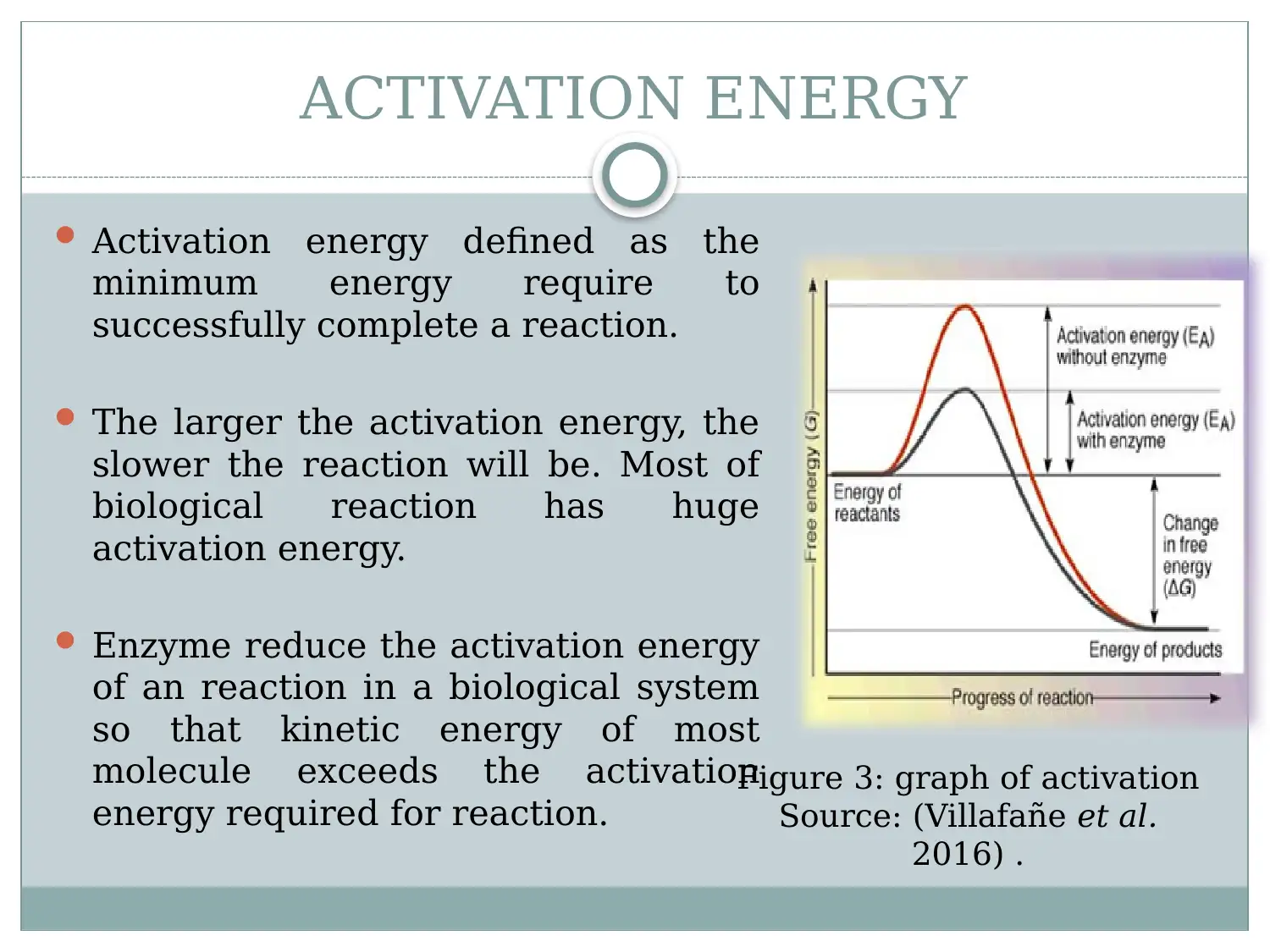
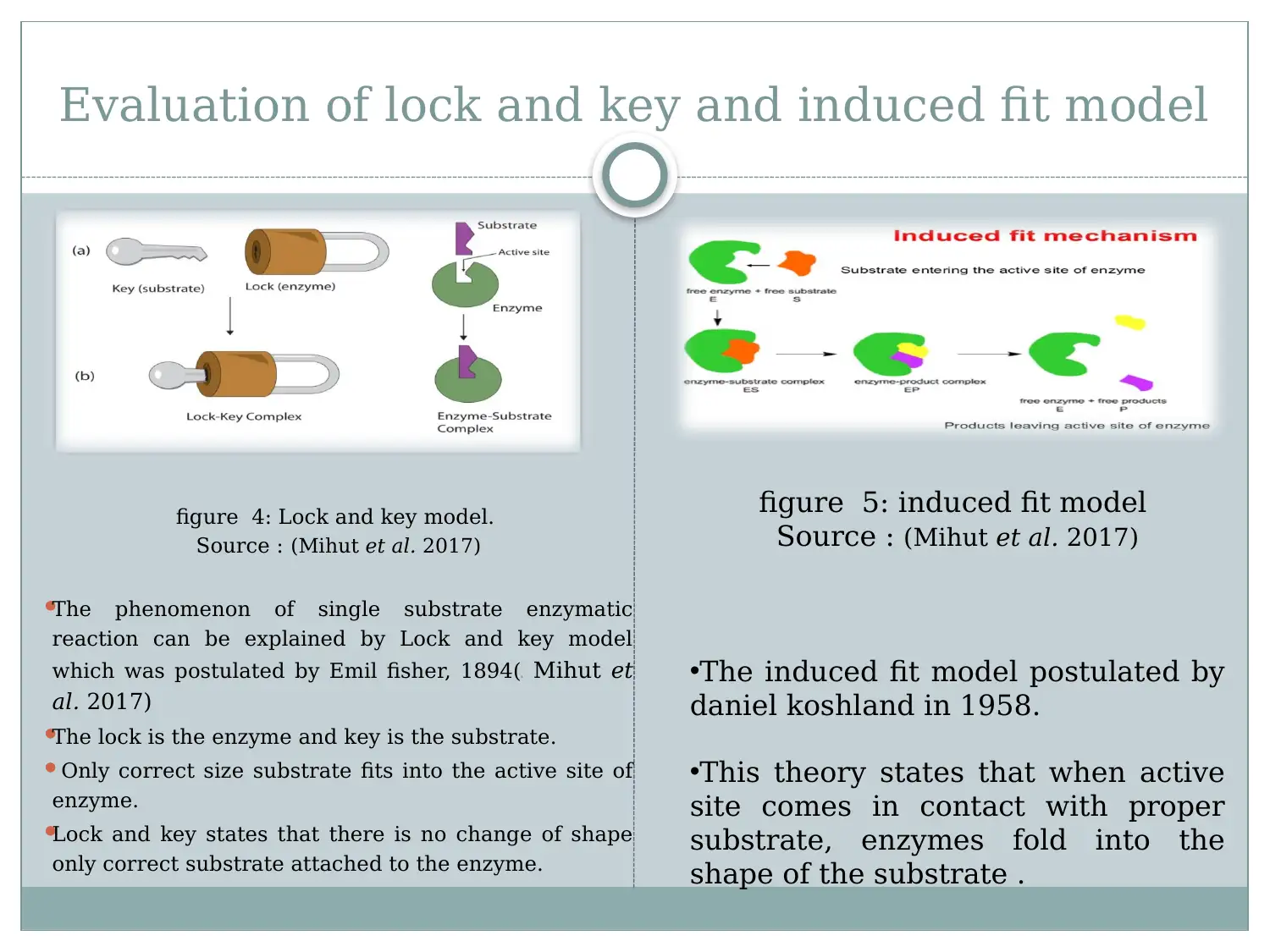
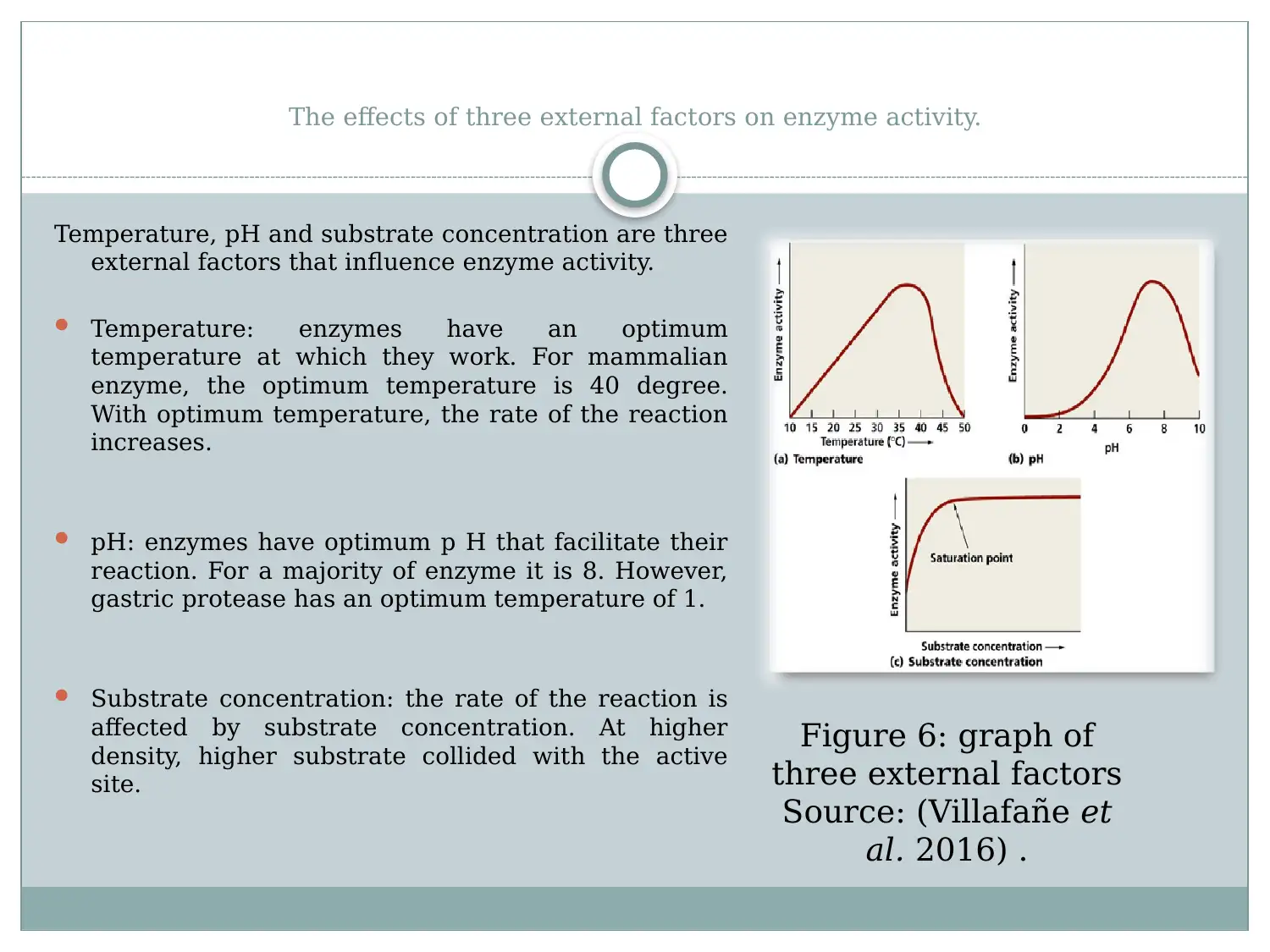
This report provides a detailed overview of enzymes, starting with their structure as globular proteins with active sites and regulatory regions. It explains how the enzyme's structure, particularly its tertiary structure, is crucial for its function, especially in substrate binding. The report discusses activation energy and how enzymes reduce it to facilitate biological reactions. It evaluates the lock and key and induced fit models, explaining substrate enzymatic reaction. Furthermore, the report examines the effects of external factors such as temperature, pH, and substrate concentration on enzyme activity, highlighting optimum conditions for enzyme function. The document references relevant studies to support its explanations of enzyme mechanisms and influencing factors.
1 out of 7







![[object Object]](/_next/static/media/star-bottom.7253800d.svg)