Biology Report: Understanding Enzymes, Inhibitors, and Reaction Rates
VerifiedAdded on 2023/04/19
|7
|888
|68
Report
AI Summary
This report delves into the realm of enzymes, crucial protein catalysts that accelerate biochemical reactions within living organisms. It elucidates the fundamental principles of enzyme function, emphasizing the lock and key and induced fit models to explain substrate specificity. The report further explores the mechanisms of enzyme inhibitors, differentiating between reversible and irreversible types, and the role of coenzymes in facilitating enzymatic reactions. Moreover, it examines various factors that influence the rate of enzymatic reactions, including temperature, substrate concentration, enzyme concentration, pH levels, and the concentration of end-products. By analyzing these elements, the report provides a comprehensive understanding of enzyme kinetics and their significance in biological processes.

Running Head: Biology Nutrition and Digestion 1
Understand enzymes
Name
Professor
Institution
Course
Date
Understand enzymes
Name
Professor
Institution
Course
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biology Nutrition and Digestion 2
1.0 Understand enzymes
1.1 Enzyme function with reference to lock and key and induced fit models
Enzymes are protein catalysts that increase the rate of chemical processes. They are produced
within the living organism where they act by decreasing activation energy and remain unchanged
at the end of the reaction. This is because only a small region of the enzyme (active site) comes
into contact with the reactant. A protein forms an enzyme-substrate complex when it joins with
the substrate (Rodrigues, Ortiz, Berenguer-Murcia, Orres, And Fernández-Lafuent, 2013)
Source: Khan Academy, (2019) Enzymes and the active site: Retrieved from:
https://www.khanacademy.org/science/biology/energy-and-enzymes/introduction-to-enzymes/a/
enzymes-and-the-active-site
Lock and Key model
This explains the exact action of an enzyme where an enzyme is a lock, and the substrate is the
key. Only the right side of the specific substrate fits into the active site of the protein to form the
complex. This means that wrongly sized or shaped substrates do not fit into the enzyme.
1.0 Understand enzymes
1.1 Enzyme function with reference to lock and key and induced fit models
Enzymes are protein catalysts that increase the rate of chemical processes. They are produced
within the living organism where they act by decreasing activation energy and remain unchanged
at the end of the reaction. This is because only a small region of the enzyme (active site) comes
into contact with the reactant. A protein forms an enzyme-substrate complex when it joins with
the substrate (Rodrigues, Ortiz, Berenguer-Murcia, Orres, And Fernández-Lafuent, 2013)
Source: Khan Academy, (2019) Enzymes and the active site: Retrieved from:
https://www.khanacademy.org/science/biology/energy-and-enzymes/introduction-to-enzymes/a/
enzymes-and-the-active-site
Lock and Key model
This explains the exact action of an enzyme where an enzyme is a lock, and the substrate is the
key. Only the right side of the specific substrate fits into the active site of the protein to form the
complex. This means that wrongly sized or shaped substrates do not fit into the enzyme.

Biology Nutrition and Digestion 3
Source: Abbas, J. Stock Photo - The Lock and Key Mechanism Labeled Diagram. Retrieved
from: https://www.123rf.com/photo_36876740_the-lock-and-key-mechanism-labeled-
diagram.html
Induced fit model
This is a modification of lock and central hypothesis. An enzyme can modify its shape for the
substrate to fit. This is because the catalyst is partly flexible and only the correct substrate can
induce the right alignment of the active site (Mustar Wender and Cheong, 2015).
Source: Abbas, J. Stock Photo - The Lock and Key Mechanism Labeled Diagram. Retrieved
from: https://www.123rf.com/photo_36876740_the-lock-and-key-mechanism-labeled-
diagram.html
Induced fit model
This is a modification of lock and central hypothesis. An enzyme can modify its shape for the
substrate to fit. This is because the catalyst is partly flexible and only the correct substrate can
induce the right alignment of the active site (Mustar Wender and Cheong, 2015).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Biology Nutrition and Digestion 4
Source: Jones, M., S., B.7.5 Induced fit mechanism. Retrieved from:
https://www.youtube.com/watch?v=dS2WU4_afYM
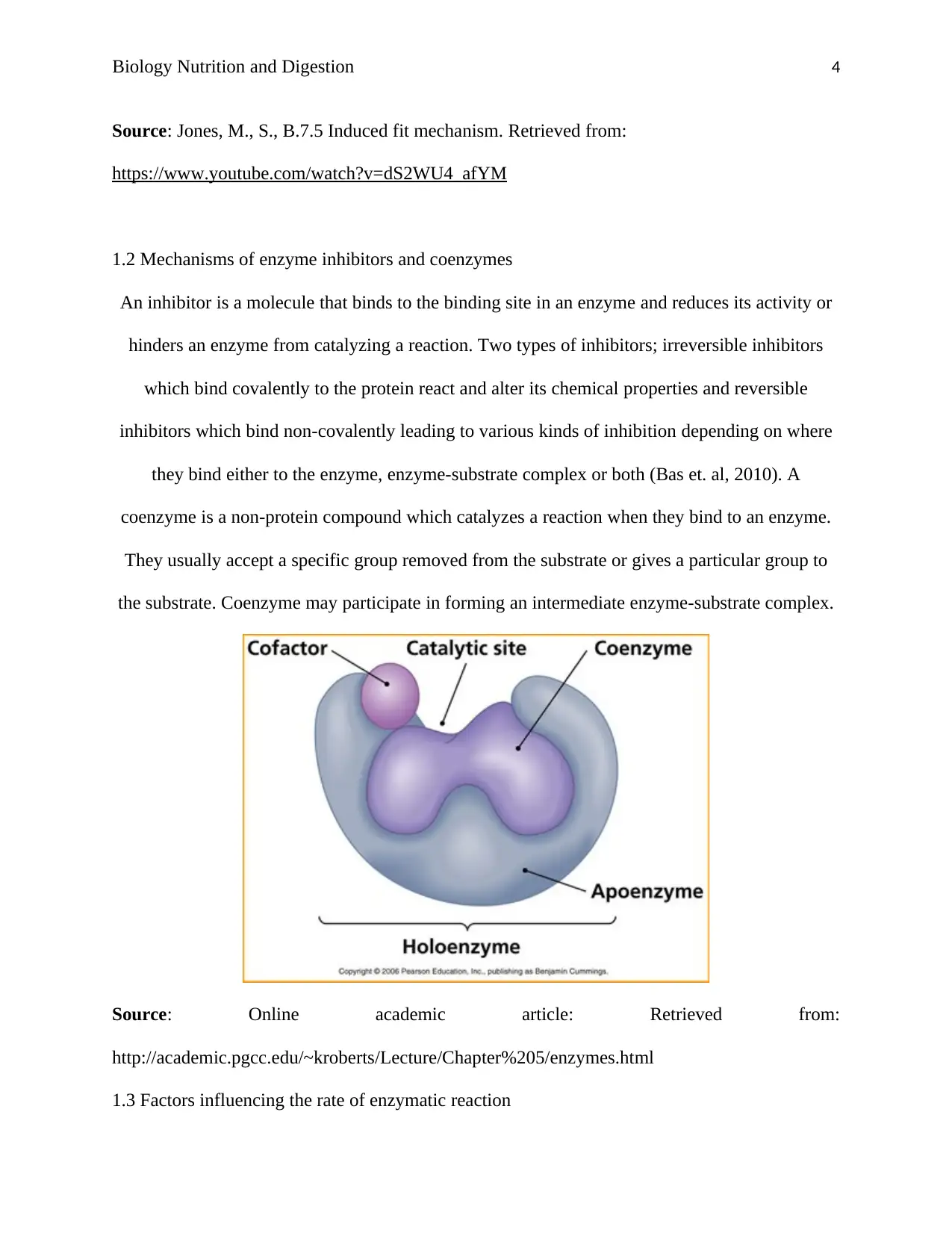
1.2 Mechanisms of enzyme inhibitors and coenzymes
An inhibitor is a molecule that binds to the binding site in an enzyme and reduces its activity or
hinders an enzyme from catalyzing a reaction. Two types of inhibitors; irreversible inhibitors
which bind covalently to the protein react and alter its chemical properties and reversible
inhibitors which bind non-covalently leading to various kinds of inhibition depending on where
they bind either to the enzyme, enzyme-substrate complex or both (Bas et. al, 2010). A
coenzyme is a non-protein compound which catalyzes a reaction when they bind to an enzyme.
They usually accept a specific group removed from the substrate or gives a particular group to
the substrate. Coenzyme may participate in forming an intermediate enzyme-substrate complex.
Source: Online academic article: Retrieved from:
http://academic.pgcc.edu/~kroberts/Lecture/Chapter%205/enzymes.html
1.3 Factors influencing the rate of enzymatic reaction
Source: Jones, M., S., B.7.5 Induced fit mechanism. Retrieved from:
https://www.youtube.com/watch?v=dS2WU4_afYM
1.2 Mechanisms of enzyme inhibitors and coenzymes
An inhibitor is a molecule that binds to the binding site in an enzyme and reduces its activity or
hinders an enzyme from catalyzing a reaction. Two types of inhibitors; irreversible inhibitors
which bind covalently to the protein react and alter its chemical properties and reversible
inhibitors which bind non-covalently leading to various kinds of inhibition depending on where
they bind either to the enzyme, enzyme-substrate complex or both (Bas et. al, 2010). A
coenzyme is a non-protein compound which catalyzes a reaction when they bind to an enzyme.
They usually accept a specific group removed from the substrate or gives a particular group to
the substrate. Coenzyme may participate in forming an intermediate enzyme-substrate complex.
Source: Online academic article: Retrieved from:
http://academic.pgcc.edu/~kroberts/Lecture/Chapter%205/enzymes.html
1.3 Factors influencing the rate of enzymatic reaction
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Biology Nutrition and Digestion 5
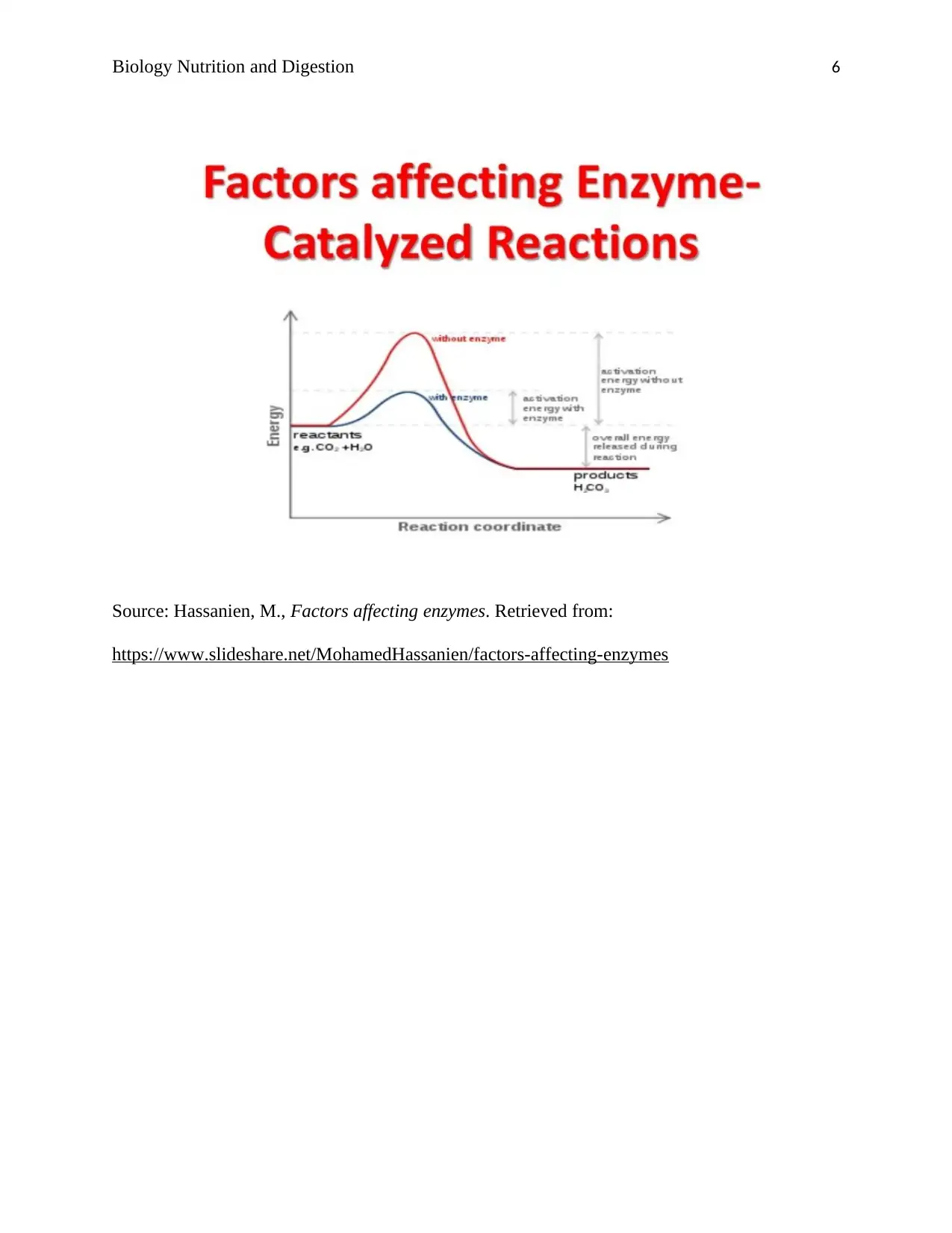
Temperature: An Increase in temperature increases the rate of enzyme activity. Further increase
changes the active site making it difficult for a substrate to fit thereby denaturing the enzymes
hence a reduction in the price of enzyme activity. A decrease in temperature reduces the rate of
enzyme activity.
Substrate concentration: Increase in substrate concentration increases the rate of enzyme
activity up to a saturation point, but the extreme increase does not affect the price of enzyme
activity. At this point, all the binding sites are occupied and both dissociation time and enzyme
concentration are the limiting factors. At a reduced level of the product, there is decreased
enzyme activity because all the active sites are not occupied.
Enzyme concentration: The speed of enzyme activity increases with an increase in enzyme
concentration when other factors such as pressure and temperature are kept constant.
Changes in pH affect the basic and acidic group ionic charges. An increase or decrease in pH
under constant pressure and temperature decreases enzyme activity. Extreme rise or fall in pH
denatures the enzyme.
Concentration of End-products: Accumulation of end-products decreases the rate of enzyme
reaction. This is because the enzyme can combine with the products with a shape similar to the
substrate thereby inhibiting enzyme activity.
Temperature: An Increase in temperature increases the rate of enzyme activity. Further increase
changes the active site making it difficult for a substrate to fit thereby denaturing the enzymes
hence a reduction in the price of enzyme activity. A decrease in temperature reduces the rate of
enzyme activity.
Substrate concentration: Increase in substrate concentration increases the rate of enzyme
activity up to a saturation point, but the extreme increase does not affect the price of enzyme
activity. At this point, all the binding sites are occupied and both dissociation time and enzyme
concentration are the limiting factors. At a reduced level of the product, there is decreased
enzyme activity because all the active sites are not occupied.
Enzyme concentration: The speed of enzyme activity increases with an increase in enzyme
concentration when other factors such as pressure and temperature are kept constant.
Changes in pH affect the basic and acidic group ionic charges. An increase or decrease in pH
under constant pressure and temperature decreases enzyme activity. Extreme rise or fall in pH
denatures the enzyme.
Concentration of End-products: Accumulation of end-products decreases the rate of enzyme
reaction. This is because the enzyme can combine with the products with a shape similar to the
substrate thereby inhibiting enzyme activity.
Biology Nutrition and Digestion 6
Source: Hassanien, M., Factors affecting enzymes. Retrieved from:
https://www.slideshare.net/MohamedHassanien/factors-affecting-enzymes
Source: Hassanien, M., Factors affecting enzymes. Retrieved from:
https://www.slideshare.net/MohamedHassanien/factors-affecting-enzymes
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Biology Nutrition and Digestion 7
References
Bas, M., Greve, J., Stelter, K., Bier, H., Stark, T., Hoffmann, T. K., & Kojda, G. (2010).
Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme
inhibitors: a case series. Annals of emergency medicine, 56(3), 278-282.
Mustard, T.J., Wender, P.A. and Cheong, P.H.Y., 2015. Catalytic efficiency is a function of how
rhodium (I)(5+ 2) catalysts accommodate a conserved substrate transition state geometry:
induced fit model for explaining transition metal catalysis. ACS catalysis, 5(3),1758-1763.
Rodrigues, R.C., Ortiz, C., Berenguer-Murcia, Á., Torres, R. and
Fernández-Lafuente, R., 2013. Modifying enzyme activity and
selectivity by immobilization. Chemical Society Reviews, 42(15),
6290-6307.
References
Bas, M., Greve, J., Stelter, K., Bier, H., Stark, T., Hoffmann, T. K., & Kojda, G. (2010).
Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme
inhibitors: a case series. Annals of emergency medicine, 56(3), 278-282.
Mustard, T.J., Wender, P.A. and Cheong, P.H.Y., 2015. Catalytic efficiency is a function of how
rhodium (I)(5+ 2) catalysts accommodate a conserved substrate transition state geometry:
induced fit model for explaining transition metal catalysis. ACS catalysis, 5(3),1758-1763.
Rodrigues, R.C., Ortiz, C., Berenguer-Murcia, Á., Torres, R. and
Fernández-Lafuente, R., 2013. Modifying enzyme activity and
selectivity by immobilization. Chemical Society Reviews, 42(15),
6290-6307.
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





