PC702: Epidemiology & Biostatistics HIV Presentation - Summer 2019
VerifiedAdded on 2022/12/15
|10
|1128
|63
Presentation
AI Summary
This MicroSlide PowerPoint presentation, created for the PC702 Epidemiology & Biostatistics course, addresses HIV reporting requirements and biostatistical concepts. The presentation, developed by Elisabeth Nkem, Brittany Schultz, Merilee Vance, and Sarah Vialpando, covers state-level reporting laws in California, Virginia, New Mexico, and Wisconsin, including timeframes and processes. It explores HIV incidence and prevalence in New Mexico, clinical implications, and the sensitivity and specificity of HIV tests. The presentation also discusses reliability, validity, and predictive values, providing a comprehensive overview of HIV-related epidemiology and biostatistics. The presentation includes references to CDC and state health department guidelines.

PC702 - Epidemiology & Biostatistics
MicroSlide PowerPoint Presentation
By: Elisabeth Nkem
Brittany Schultz
Merilee Vance
Sarah Vialpando
Summer 2019 Term
MicroSlide PowerPoint Presentation
By: Elisabeth Nkem
Brittany Schultz
Merilee Vance
Sarah Vialpando
Summer 2019 Term
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Human Immunodeficiency Virus
The Human Immunodeficiency Virus (HIV) is a
type of disease that attacks a person’s immune
system. For the person suffering from this virus
it is tough to fight from infection.
The following slides are discussing about laws
and requirements for reporting HIV on the
state level as well as some terms and concepts
from Module 8.
The Human Immunodeficiency Virus (HIV) is a
type of disease that attacks a person’s immune
system. For the person suffering from this virus
it is tough to fight from infection.
The following slides are discussing about laws
and requirements for reporting HIV on the
state level as well as some terms and concepts
from Module 8.

Question #1
Completed by M. Vance
Preceptor asks: "How will you educate your patient about the need to report?
• In California, HIV is a reportable disease.
• People can report to the Department of Public Health within one day when
the they get know about the disease.
• California Code of Regulations states that the patient may remain
anonymous, if they choose.
• People can get involve in the Confidential test.
• The health department sends the result to the CDC and these results in the
medical record are protected by HIPAA.
Completed by M. Vance
Preceptor asks: "How will you educate your patient about the need to report?
• In California, HIV is a reportable disease.
• People can report to the Department of Public Health within one day when
the they get know about the disease.
• California Code of Regulations states that the patient may remain
anonymous, if they choose.
• People can get involve in the Confidential test.
• The health department sends the result to the CDC and these results in the
medical record are protected by HIPAA.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Requirements And Process for Reporting in Virginia
Question # 2 by E.Nkem
Time frame for Reporting and (Categories)
• 3 days to Virginia Health Department via EPI-1 Form (2)
• 24 hrs. to Local Health Department New Mexico via phone call (2)
• 72 hrs. to the State department of epidemiology of Wisconsin Via
phone call (3)
• 7 calendar days California department of health (Stage 3 Aids) via
Phone call (2)
• Acute Infection by phone call within 1 working day.
Lab Result: VA, NM, WI, CA
• CD4 count
• HIV Viral load test
• HIV antigens
• HIV -1 RNA
• California- lab result within 1 working day to the local health
department.
Lab Information and result
• Source of the specimen
• Data collected
• Lab results and finding
• name and address of the lab
• CLIA Number
Different Time Frames for Reporting:
Immediate/ 72 hrs. Reporting
Varies by State:
• Legal Basis
• State reporting Practices
• Data collection
Surveillance:
• Control Outbreak and Stop the Spread of
disease
• Prevent Future outbreaks
• Promptly identifying the source of the
infection
• Involve public safety agencies in the
investigation
Question # 2 by E.Nkem
Time frame for Reporting and (Categories)
• 3 days to Virginia Health Department via EPI-1 Form (2)
• 24 hrs. to Local Health Department New Mexico via phone call (2)
• 72 hrs. to the State department of epidemiology of Wisconsin Via
phone call (3)
• 7 calendar days California department of health (Stage 3 Aids) via
Phone call (2)
• Acute Infection by phone call within 1 working day.
Lab Result: VA, NM, WI, CA
• CD4 count
• HIV Viral load test
• HIV antigens
• HIV -1 RNA
• California- lab result within 1 working day to the local health
department.
Lab Information and result
• Source of the specimen
• Data collected
• Lab results and finding
• name and address of the lab
• CLIA Number
Different Time Frames for Reporting:
Immediate/ 72 hrs. Reporting
Varies by State:
• Legal Basis
• State reporting Practices
• Data collection
Surveillance:
• Control Outbreak and Stop the Spread of
disease
• Prevent Future outbreaks
• Promptly identifying the source of the
infection
• Involve public safety agencies in the
investigation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
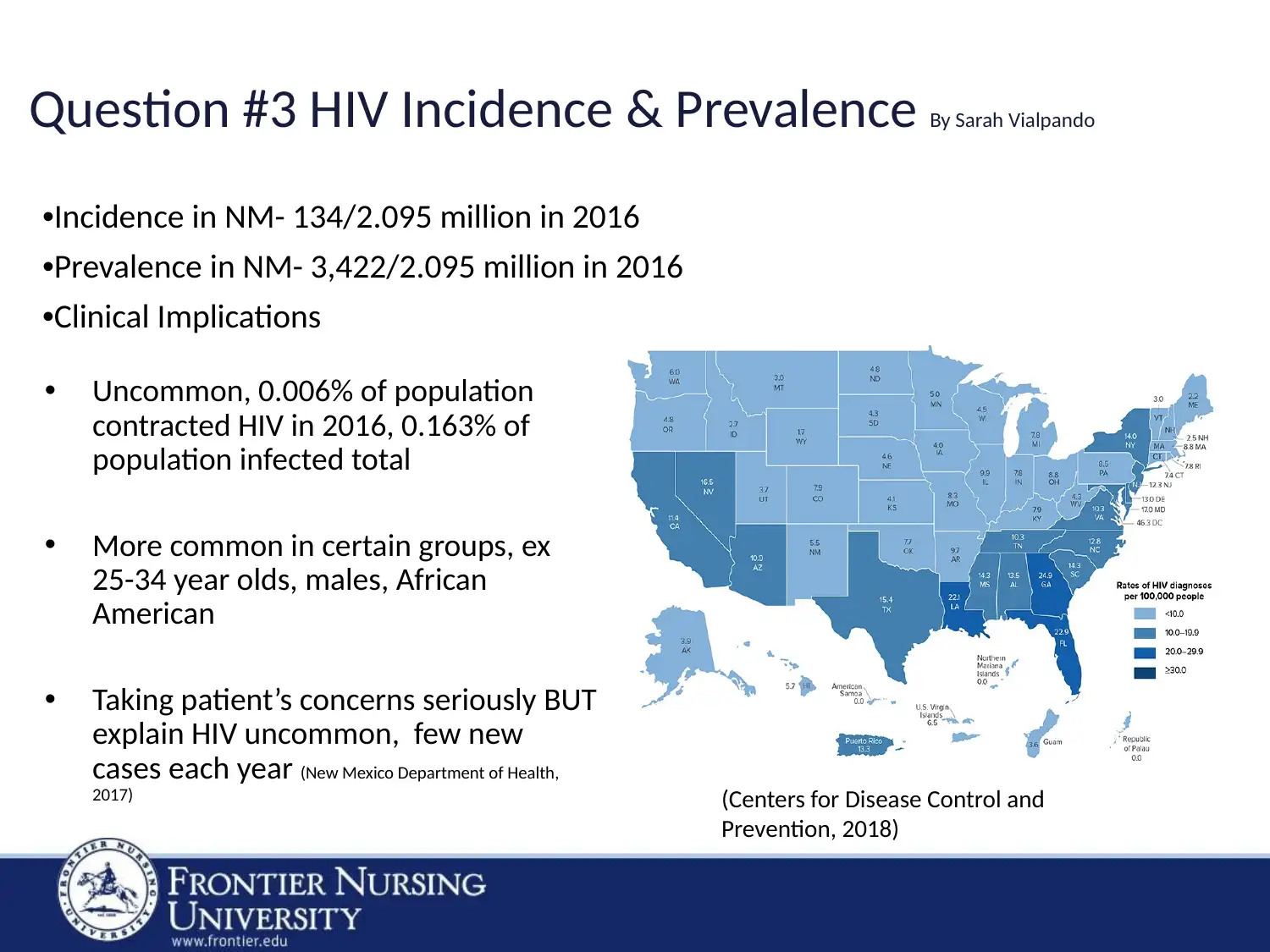
Question #3 HIV Incidence & Prevalence By Sarah Vialpando
•Incidence in NM- 134/2.095 million in 2016
•Prevalence in NM- 3,422/2.095 million in 2016
•Clinical Implications
• Uncommon, 0.006% of population
contracted HIV in 2016, 0.163% of
population infected total
• More common in certain groups, ex
25-34 year olds, males, African
American
• Taking patient’s concerns seriously BUT
explain HIV uncommon, few new
cases each year (New Mexico Department of Health,
2017) (Centers for Disease Control and
Prevention, 2018)
•Incidence in NM- 134/2.095 million in 2016
•Prevalence in NM- 3,422/2.095 million in 2016
•Clinical Implications
• Uncommon, 0.006% of population
contracted HIV in 2016, 0.163% of
population infected total
• More common in certain groups, ex
25-34 year olds, males, African
American
• Taking patient’s concerns seriously BUT
explain HIV uncommon, few new
cases each year (New Mexico Department of Health,
2017) (Centers for Disease Control and
Prevention, 2018)
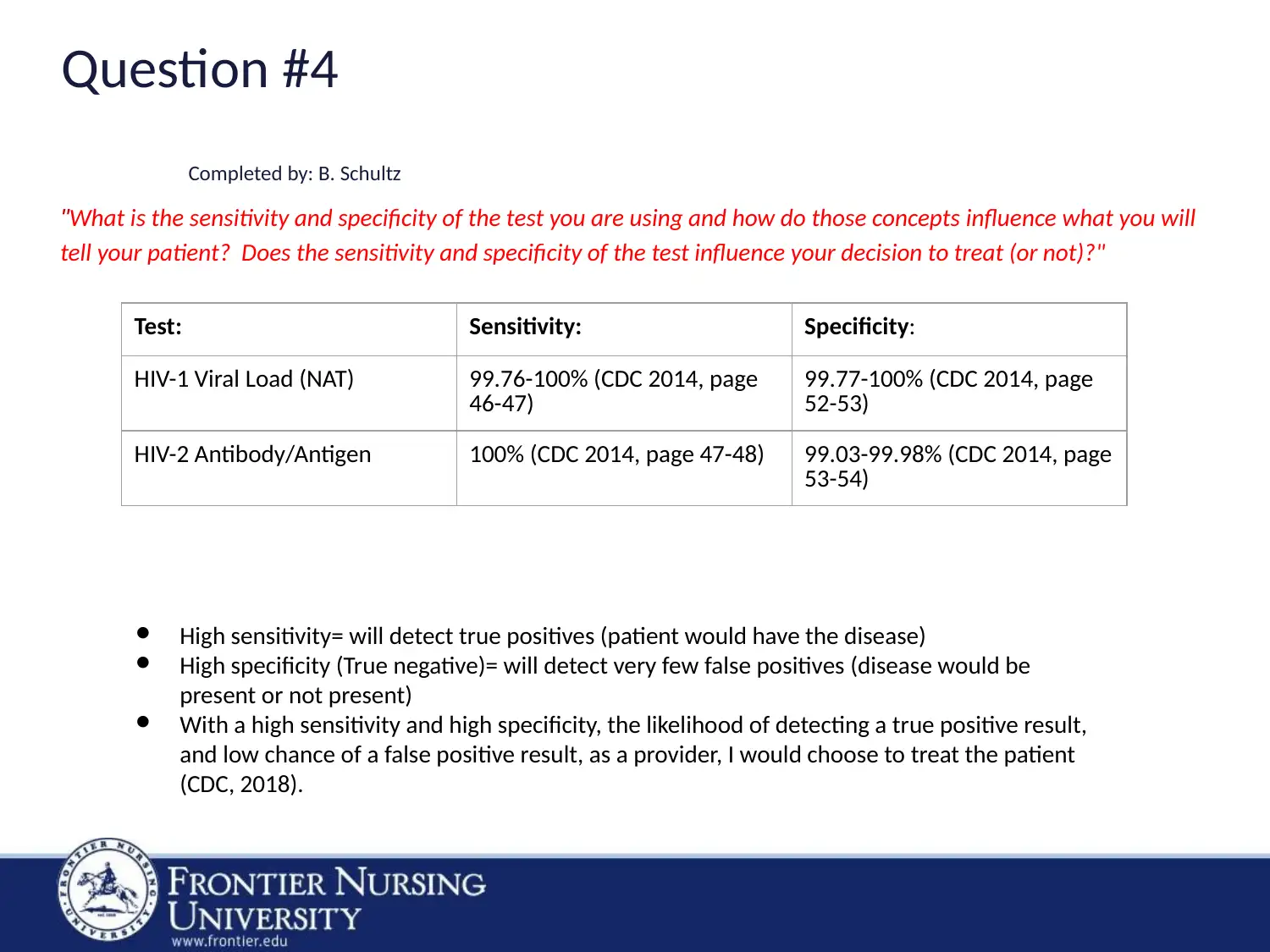
Question #4
Completed by: B. Schultz
"What is the sensitivity and specificity of the test you are using and how do those concepts influence what you will
tell your patient? Does the sensitivity and specificity of the test influence your decision to treat (or not)?"
Test: Sensitivity: Specificity:
HIV-1 Viral Load (NAT) 99.76-100% (CDC 2014, page
46-47)
99.77-100% (CDC 2014, page
52-53)
HIV-2 Antibody/Antigen 100% (CDC 2014, page 47-48) 99.03-99.98% (CDC 2014, page
53-54)
● High sensitivity= will detect true positives (patient would have the disease)
● High specificity (True negative)= will detect very few false positives (disease would be
present or not present)
● With a high sensitivity and high specificity, the likelihood of detecting a true positive result,
and low chance of a false positive result, as a provider, I would choose to treat the patient
(CDC, 2018).
Completed by: B. Schultz
"What is the sensitivity and specificity of the test you are using and how do those concepts influence what you will
tell your patient? Does the sensitivity and specificity of the test influence your decision to treat (or not)?"
Test: Sensitivity: Specificity:
HIV-1 Viral Load (NAT) 99.76-100% (CDC 2014, page
46-47)
99.77-100% (CDC 2014, page
52-53)
HIV-2 Antibody/Antigen 100% (CDC 2014, page 47-48) 99.03-99.98% (CDC 2014, page
53-54)
● High sensitivity= will detect true positives (patient would have the disease)
● High specificity (True negative)= will detect very few false positives (disease would be
present or not present)
● With a high sensitivity and high specificity, the likelihood of detecting a true positive result,
and low chance of a false positive result, as a provider, I would choose to treat the patient
(CDC, 2018).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Question #5 Completed by: B. Schultz
"Explain reliability and validity. What is the difference between reliability and validity and can you give me a
couple of examples of what might affect each one?"
● Reliability: results of the test are consistent
○ Test is able to produce same or similar test results each time
● Validity: results of the test are accurate
○ Results of test are measuring what should be measured
● A test can consistently (reliability) give you the same result and that result could
possibly be accurate or inaccurate (Validity). A test can not be validated if the
test is not reliability.
"Explain reliability and validity. What is the difference between reliability and validity and can you give me a
couple of examples of what might affect each one?"
● Reliability: results of the test are consistent
○ Test is able to produce same or similar test results each time
● Validity: results of the test are accurate
○ Results of test are measuring what should be measured
● A test can consistently (reliability) give you the same result and that result could
possibly be accurate or inaccurate (Validity). A test can not be validated if the
test is not reliability.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
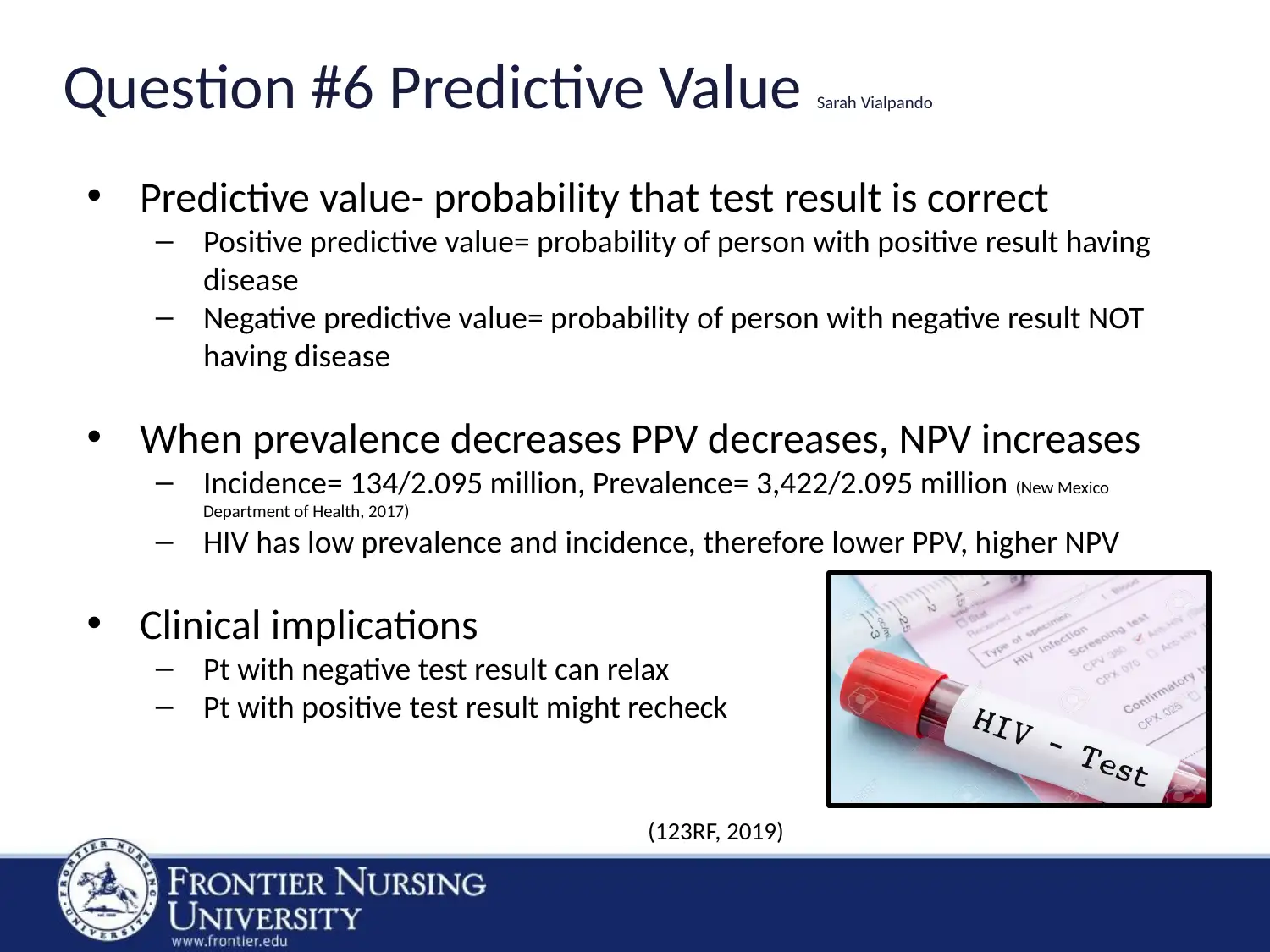
Question #6 Predictive Value Sarah Vialpando
• Predictive value- probability that test result is correct
– Positive predictive value= probability of person with positive result having
disease
– Negative predictive value= probability of person with negative result NOT
having disease
• When prevalence decreases PPV decreases, NPV increases
– Incidence= 134/2.095 million, Prevalence= 3,422/2.095 million (New Mexico
Department of Health, 2017)
– HIV has low prevalence and incidence, therefore lower PPV, higher NPV
• Clinical implications
– Pt with negative test result can relax
– Pt with positive test result might recheck
(123RF, 2019)
• Predictive value- probability that test result is correct
– Positive predictive value= probability of person with positive result having
disease
– Negative predictive value= probability of person with negative result NOT
having disease
• When prevalence decreases PPV decreases, NPV increases
– Incidence= 134/2.095 million, Prevalence= 3,422/2.095 million (New Mexico
Department of Health, 2017)
– HIV has low prevalence and incidence, therefore lower PPV, higher NPV
• Clinical implications
– Pt with negative test result can relax
– Pt with positive test result might recheck
(123RF, 2019)

References
Centers for Disease Control and Prevention. (2014). Laboratory testing for
the diagnosis of HIV infection: Updated recommendations. Retrieved from https://
stacks.cdc.gov/view/cdc/23447
Centers for Disease Control and Prevention. (2018a). Rates of HIV diagnosis
in US, 2017 . Retrieved from https://
www.cdc.gov/hiv/statistics/overview/geographicdistribution.html
Centers for Disease Control and Prevention. (2018b). 2018 Quick reference
guide: Recommended laboratory HIV testing algorithm for serum and plasma
specimens. Retrieved from https://stacks.cdc.gov/view/cdc/50872
Center for Disease Control and prevention. (2019). HIV Case reporting and
Surveillance. Retrieved from https://www.cdc.gov/hiv/guidelines/reporting.html
Centers for Disease Control and Prevention. (2014). Laboratory testing for
the diagnosis of HIV infection: Updated recommendations. Retrieved from https://
stacks.cdc.gov/view/cdc/23447
Centers for Disease Control and Prevention. (2018a). Rates of HIV diagnosis
in US, 2017 . Retrieved from https://
www.cdc.gov/hiv/statistics/overview/geographicdistribution.html
Centers for Disease Control and Prevention. (2018b). 2018 Quick reference
guide: Recommended laboratory HIV testing algorithm for serum and plasma
specimens. Retrieved from https://stacks.cdc.gov/view/cdc/50872
Center for Disease Control and prevention. (2019). HIV Case reporting and
Surveillance. Retrieved from https://www.cdc.gov/hiv/guidelines/reporting.html
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References
New Mexico Department of Health. (2017). Human immunodeficiency virus and
acquired immunodeficiency syndrome among adults and adolescents in New Mexico--
2016. Retrieved from https://nmhealth.org/data/view/infectious/2139/
Pumila, P. (2019). Sample blood collection tube with HIV test label on HIV
infection screening test form . Retrieved from
https://www.123rf.com/photo_46805256_sample-blood-collection-tube-with-hiv-tes
t-label-on-hiv-infection-screening-test-form-.html
Virginia Department of Health. (2019). Division Of Surveillance And Investigation.
Retrieved from http://www.vdh.virginia.gov/surveillance-and-investigation/
Wisconsin Department of Health Services. (2015). Guide for wisconsin PrEP
providers. Retrieved from https://www.dhs.wisconsin.gov/publications/p01197.pdf
New Mexico Department of Health. (2017). Human immunodeficiency virus and
acquired immunodeficiency syndrome among adults and adolescents in New Mexico--
2016. Retrieved from https://nmhealth.org/data/view/infectious/2139/
Pumila, P. (2019). Sample blood collection tube with HIV test label on HIV
infection screening test form . Retrieved from
https://www.123rf.com/photo_46805256_sample-blood-collection-tube-with-hiv-tes
t-label-on-hiv-infection-screening-test-form-.html
Virginia Department of Health. (2019). Division Of Surveillance And Investigation.
Retrieved from http://www.vdh.virginia.gov/surveillance-and-investigation/
Wisconsin Department of Health Services. (2015). Guide for wisconsin PrEP
providers. Retrieved from https://www.dhs.wisconsin.gov/publications/p01197.pdf
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.