Report on Organic Chemistry: Ethene Production from Ethanol Experiment
VerifiedAdded on 2020/07/23
|18
|2606
|385
Report
AI Summary
This report details an experiment on the production of ethene from ethanol through a dehydration reaction using sulfuric acid at 170°C. It explores the reactions of alkenes, including addition reactions with halogens, hydrogen halides, steam, hydrogen, and KMnO4. The report also covers the polymerization of alkenes, focusing on the formation of polyethene and polychloroethene (PVC). Furthermore, it describes the functional groups of alkanes, alkenes, and alkynes, and discusses chemical reactions of halogen alkanes in terms of nucleophilic substitution. Finally, it compares the chemical reactions of alcohols and carbonyl compounds. The report includes observations, results, and a conclusion summarizing the experiment's findings, along with references and illustrations.

ORGANIC
CHEMISTRY
CHEMISTRY
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
PRODUCTION OF ETHENE FROM ETHANOL BY USING DEHYDRATION REACTION..1
ABSTRACT.....................................................................................................................................1
KEY TERMS...................................................................................................................................1
INTRODUCTION...........................................................................................................................1
HYPOTHESIS.................................................................................................................................1
EQUIPMENTS................................................................................................................................2
METHODS......................................................................................................................................2
RESULTS........................................................................................................................................2
CONCLUSION................................................................................................................................3
EVALUATION................................................................................................................................3
INTRODUCTION...........................................................................................................................4
TASK 2............................................................................................................................................4
2.3 Addition reaction of alkenes.............................................................................................4
2.4 Polymerisation of alkenes.................................................................................................8
TASK 3..........................................................................................................................................10
3.1 Functional Groups..........................................................................................................10
3.2 Chemical reactions of halogen alkanes in terms of nucleophilic substitution................11
3.3 Describing & comparing chemical-reactions of alcohols & carbonyl-compounds........12
CONCLUSION..............................................................................................................................14
REFERENCES..............................................................................................................................15
PRODUCTION OF ETHENE FROM ETHANOL BY USING DEHYDRATION REACTION..1
ABSTRACT.....................................................................................................................................1
KEY TERMS...................................................................................................................................1
INTRODUCTION...........................................................................................................................1
HYPOTHESIS.................................................................................................................................1
EQUIPMENTS................................................................................................................................2
METHODS......................................................................................................................................2
RESULTS........................................................................................................................................2
CONCLUSION................................................................................................................................3
EVALUATION................................................................................................................................3
INTRODUCTION...........................................................................................................................4
TASK 2............................................................................................................................................4
2.3 Addition reaction of alkenes.............................................................................................4
2.4 Polymerisation of alkenes.................................................................................................8
TASK 3..........................................................................................................................................10
3.1 Functional Groups..........................................................................................................10
3.2 Chemical reactions of halogen alkanes in terms of nucleophilic substitution................11
3.3 Describing & comparing chemical-reactions of alcohols & carbonyl-compounds........12
CONCLUSION..............................................................................................................................14
REFERENCES..............................................................................................................................15

Illustration Index
Illustration 1: Addition reaction of Ethene with Chlorine...............................................................1
Illustration 2: Addition Reaction of ethene and bromine.................................................................2
Illustration 3: Addition reaction of ethene and iodine.....................................................................2
Illustration 4: Addition Reaction of ethene with hydrogen halides.................................................3
Illustration 5: Hydration of Ethene..................................................................................................4
Illustration 6: addition reaction of ethene with hydrogen................................................................5
Illustration 7: Ethene with KMnO4.................................................................................................5
Illustration 8: Formation of polythene.............................................................................................6
Illustration 9: Polymerisation of chloroethane.................................................................................6
Illustration 10: Most common functional groups.............................................................................7
Illustration 11: chemical equation for primary-halogenalkane with water......................................8
Illustration 12: nucleophilic-substitution reaction...........................................................................8
Illustration 13: nucleophilic-substitution reaction ..........................................................................9
Illustration 14: Chemical reaction of ethanol to ethylene................................................................9
Illustration 15: Chemical reaction of 2 molecules of ethanol to diethyl ether...............................10
Illustration 16: Carbonyl-compound reaction................................................................................10
Illustration 1: Addition reaction of Ethene with Chlorine...............................................................1
Illustration 2: Addition Reaction of ethene and bromine.................................................................2
Illustration 3: Addition reaction of ethene and iodine.....................................................................2
Illustration 4: Addition Reaction of ethene with hydrogen halides.................................................3
Illustration 5: Hydration of Ethene..................................................................................................4
Illustration 6: addition reaction of ethene with hydrogen................................................................5
Illustration 7: Ethene with KMnO4.................................................................................................5
Illustration 8: Formation of polythene.............................................................................................6
Illustration 9: Polymerisation of chloroethane.................................................................................6
Illustration 10: Most common functional groups.............................................................................7
Illustration 11: chemical equation for primary-halogenalkane with water......................................8
Illustration 12: nucleophilic-substitution reaction...........................................................................8
Illustration 13: nucleophilic-substitution reaction ..........................................................................9
Illustration 14: Chemical reaction of ethanol to ethylene................................................................9
Illustration 15: Chemical reaction of 2 molecules of ethanol to diethyl ether...............................10
Illustration 16: Carbonyl-compound reaction................................................................................10
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PRODUCTION OF ETHENE FROM ETHANOL BY USING
DEHYDRATION REACTION
ABSTRACT
Ethene gas is produced when ethanol is dehydrated in presence of sulphuric acid at the
temperature of 170°c. The reaction can be
C2H5OH -----------> C2H4 + H2O
Some functional groups like Alkane, Alkene and Alkynes are showing the difference
between sigma bond, double bond and triple bond of carbons. Some reactions of alkenes were
given with halogens, bromine and iodine.
KEY TERMS
Ethene
Ethanol
Bonds of carbon
Alcohol
Hydrogen
Alkene
Dehydration
INTRODUCTION
Additional reactions will include alkenes, unsaturated hydrocarbons etc. Covalent bond is
formed when an electrophilic addition includes an electron acceptor species which gets pulled
towards an electron rich atom, this causes the acceptance of electron pair resulting in covalent
bond. Certain tests can be conducted to detect the unsaturated compounds. The unsaturated
molecules will be having at least one C = C double bond.
HYPOTHESIS
All the test will show the positive results. This will show the presence of ethene, which
will be unsaturated.
Danger Risk Precautionary measures
1
DEHYDRATION REACTION
ABSTRACT
Ethene gas is produced when ethanol is dehydrated in presence of sulphuric acid at the
temperature of 170°c. The reaction can be
C2H5OH -----------> C2H4 + H2O
Some functional groups like Alkane, Alkene and Alkynes are showing the difference
between sigma bond, double bond and triple bond of carbons. Some reactions of alkenes were
given with halogens, bromine and iodine.
KEY TERMS
Ethene
Ethanol
Bonds of carbon
Alcohol
Hydrogen
Alkene
Dehydration
INTRODUCTION
Additional reactions will include alkenes, unsaturated hydrocarbons etc. Covalent bond is
formed when an electrophilic addition includes an electron acceptor species which gets pulled
towards an electron rich atom, this causes the acceptance of electron pair resulting in covalent
bond. Certain tests can be conducted to detect the unsaturated compounds. The unsaturated
molecules will be having at least one C = C double bond.
HYPOTHESIS
All the test will show the positive results. This will show the presence of ethene, which
will be unsaturated.
Danger Risk Precautionary measures
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Glassware Can cause cuts from the fragments of
broken glass.
Reducing the flow rate of gas
into tube by tilting it slightly
Bunsen burner flame Risk of catching fire from immediate
burning.
Keep all the flammable
clothing or loose clothing
away from the flames
EQUIPMENTS
Ceramic chips
Boiling tube
Mineral wool
Bunsen burner
Test tubes
Beaker
Ethanol (0.02 molar)
Bromine water solution
Pipettes
Clamp stand
Safety stand
Rubber tubing and delivery tubes
Potassium permanganate with 0.5 molar sulphuric acid
Water
METHODS
Ethanol soaked mineral is deposited into boiling tube with small chips of marble to soak
the moisture. Tube was placed at 45 degree angle and sealed with bung. This tube is placed on
burner to heat the mineral and marbles. The gas formed was allowed to displace the water which
will be submerged into beaker. The gas filled test tube was removed from the beaker and the
rubber tubing was also removed from the beaker water. The sealed gas filled test tube will be
labeled according to the test carried out.
RESULTS
The qualitative research was obtained from the following observations
2
broken glass.
Reducing the flow rate of gas
into tube by tilting it slightly
Bunsen burner flame Risk of catching fire from immediate
burning.
Keep all the flammable
clothing or loose clothing
away from the flames
EQUIPMENTS
Ceramic chips
Boiling tube
Mineral wool
Bunsen burner
Test tubes
Beaker
Ethanol (0.02 molar)
Bromine water solution
Pipettes
Clamp stand
Safety stand
Rubber tubing and delivery tubes
Potassium permanganate with 0.5 molar sulphuric acid
Water
METHODS
Ethanol soaked mineral is deposited into boiling tube with small chips of marble to soak
the moisture. Tube was placed at 45 degree angle and sealed with bung. This tube is placed on
burner to heat the mineral and marbles. The gas formed was allowed to displace the water which
will be submerged into beaker. The gas filled test tube was removed from the beaker and the
rubber tubing was also removed from the beaker water. The sealed gas filled test tube will be
labeled according to the test carried out.
RESULTS
The qualitative research was obtained from the following observations
2
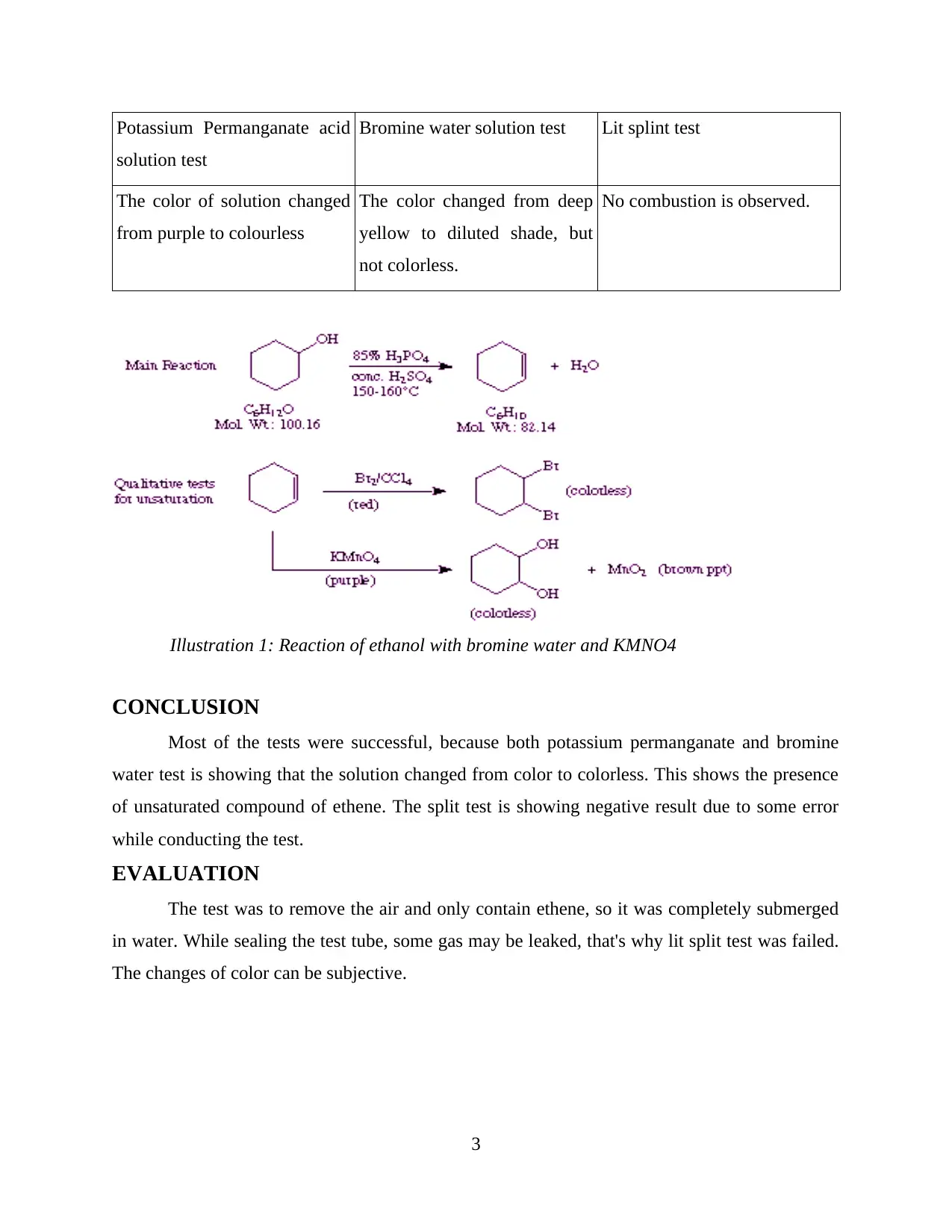
Potassium Permanganate acid
solution test
Bromine water solution test Lit splint test
The color of solution changed
from purple to colourless
The color changed from deep
yellow to diluted shade, but
not colorless.
No combustion is observed.
Illustration 1: Reaction of ethanol with bromine water and KMNO4
CONCLUSION
Most of the tests were successful, because both potassium permanganate and bromine
water test is showing that the solution changed from color to colorless. This shows the presence
of unsaturated compound of ethene. The split test is showing negative result due to some error
while conducting the test.
EVALUATION
The test was to remove the air and only contain ethene, so it was completely submerged
in water. While sealing the test tube, some gas may be leaked, that's why lit split test was failed.
The changes of color can be subjective.
3
solution test
Bromine water solution test Lit splint test
The color of solution changed
from purple to colourless
The color changed from deep
yellow to diluted shade, but
not colorless.
No combustion is observed.
Illustration 1: Reaction of ethanol with bromine water and KMNO4
CONCLUSION
Most of the tests were successful, because both potassium permanganate and bromine
water test is showing that the solution changed from color to colorless. This shows the presence
of unsaturated compound of ethene. The split test is showing negative result due to some error
while conducting the test.
EVALUATION
The test was to remove the air and only contain ethene, so it was completely submerged
in water. While sealing the test tube, some gas may be leaked, that's why lit split test was failed.
The changes of color can be subjective.
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
INTRODUCTION
The following report lays emphasis upon the experiment of forming ethene from the
ethanol. Ethene is sweet-smelling and colourless gas. It is soluble in water but dissolves in
solvents which are organic. The report addresses the various alkenes, functional groups and
various chemical-reactions of alcohols involved in the experiment.
TASK 2
2.3 Addition reaction of alkenes
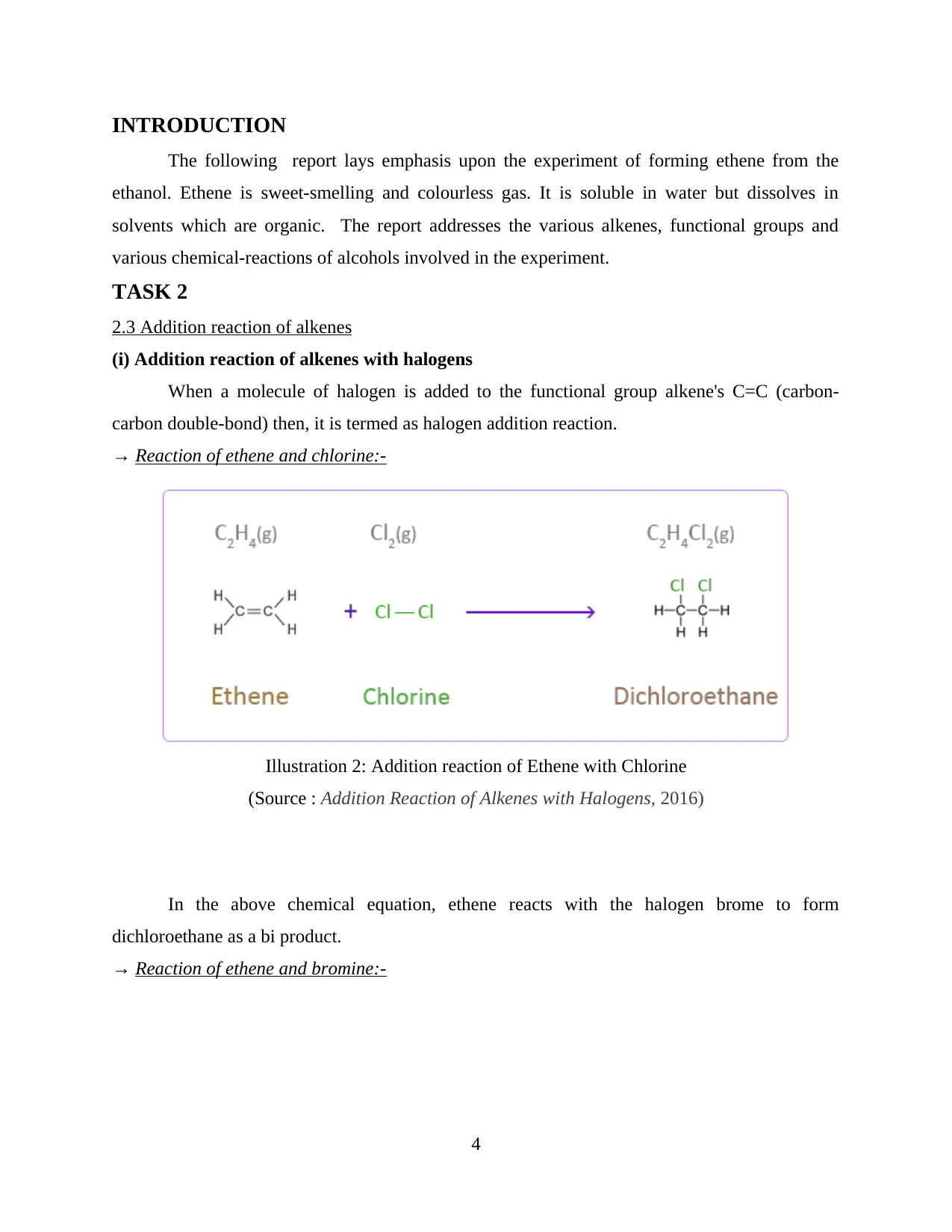
(i) Addition reaction of alkenes with halogens
When a molecule of halogen is added to the functional group alkene's C=C (carbon-
carbon double-bond) then, it is termed as halogen addition reaction.
→ Reaction of ethene and chlorine:-
In the above chemical equation, ethene reacts with the halogen brome to form
dichloroethane as a bi product.
→ Reaction of ethene and bromine:-
4
Illustration 2: Addition reaction of Ethene with Chlorine
(Source : Addition Reaction of Alkenes with Halogens, 2016)
The following report lays emphasis upon the experiment of forming ethene from the
ethanol. Ethene is sweet-smelling and colourless gas. It is soluble in water but dissolves in
solvents which are organic. The report addresses the various alkenes, functional groups and
various chemical-reactions of alcohols involved in the experiment.
TASK 2
2.3 Addition reaction of alkenes
(i) Addition reaction of alkenes with halogens
When a molecule of halogen is added to the functional group alkene's C=C (carbon-
carbon double-bond) then, it is termed as halogen addition reaction.
→ Reaction of ethene and chlorine:-
In the above chemical equation, ethene reacts with the halogen brome to form
dichloroethane as a bi product.
→ Reaction of ethene and bromine:-
4
Illustration 2: Addition reaction of Ethene with Chlorine
(Source : Addition Reaction of Alkenes with Halogens, 2016)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
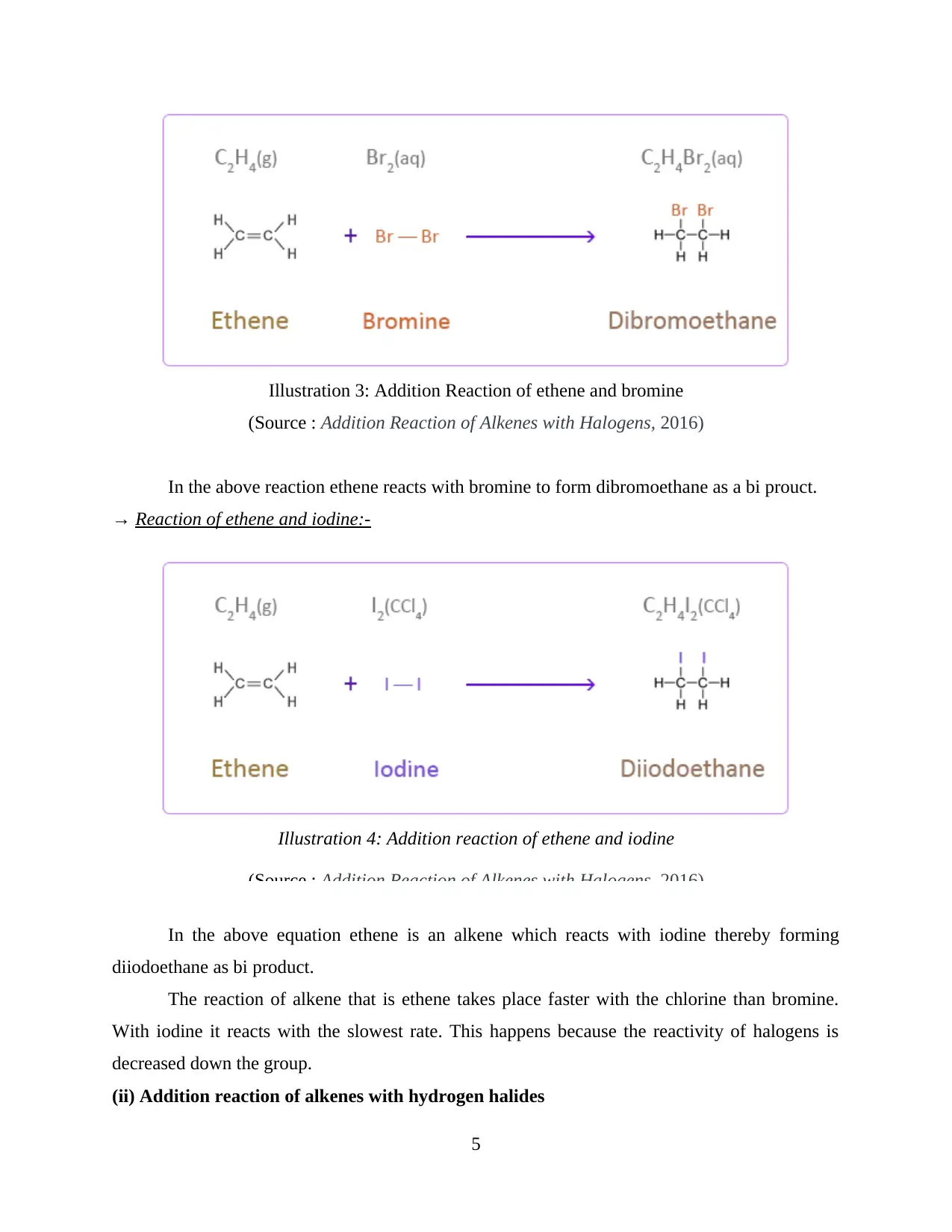
In the above reaction ethene reacts with bromine to form dibromoethane as a bi prouct.
→ Reaction of ethene and iodine:-
In the above equation ethene is an alkene which reacts with iodine thereby forming
diiodoethane as bi product.
The reaction of alkene that is ethene takes place faster with the chlorine than bromine.
With iodine it reacts with the slowest rate. This happens because the reactivity of halogens is
decreased down the group.
(ii) Addition reaction of alkenes with hydrogen halides
5
Illustration 3: Addition Reaction of ethene and bromine
(Source : Addition Reaction of Alkenes with Halogens, 2016)
Illustration 4: Addition reaction of ethene and iodine
(Source : Addition Reaction of Alkenes with Halogens, 2016)
→ Reaction of ethene and iodine:-
In the above equation ethene is an alkene which reacts with iodine thereby forming
diiodoethane as bi product.
The reaction of alkene that is ethene takes place faster with the chlorine than bromine.
With iodine it reacts with the slowest rate. This happens because the reactivity of halogens is
decreased down the group.
(ii) Addition reaction of alkenes with hydrogen halides
5
Illustration 3: Addition Reaction of ethene and bromine
(Source : Addition Reaction of Alkenes with Halogens, 2016)
Illustration 4: Addition reaction of ethene and iodine
(Source : Addition Reaction of Alkenes with Halogens, 2016)
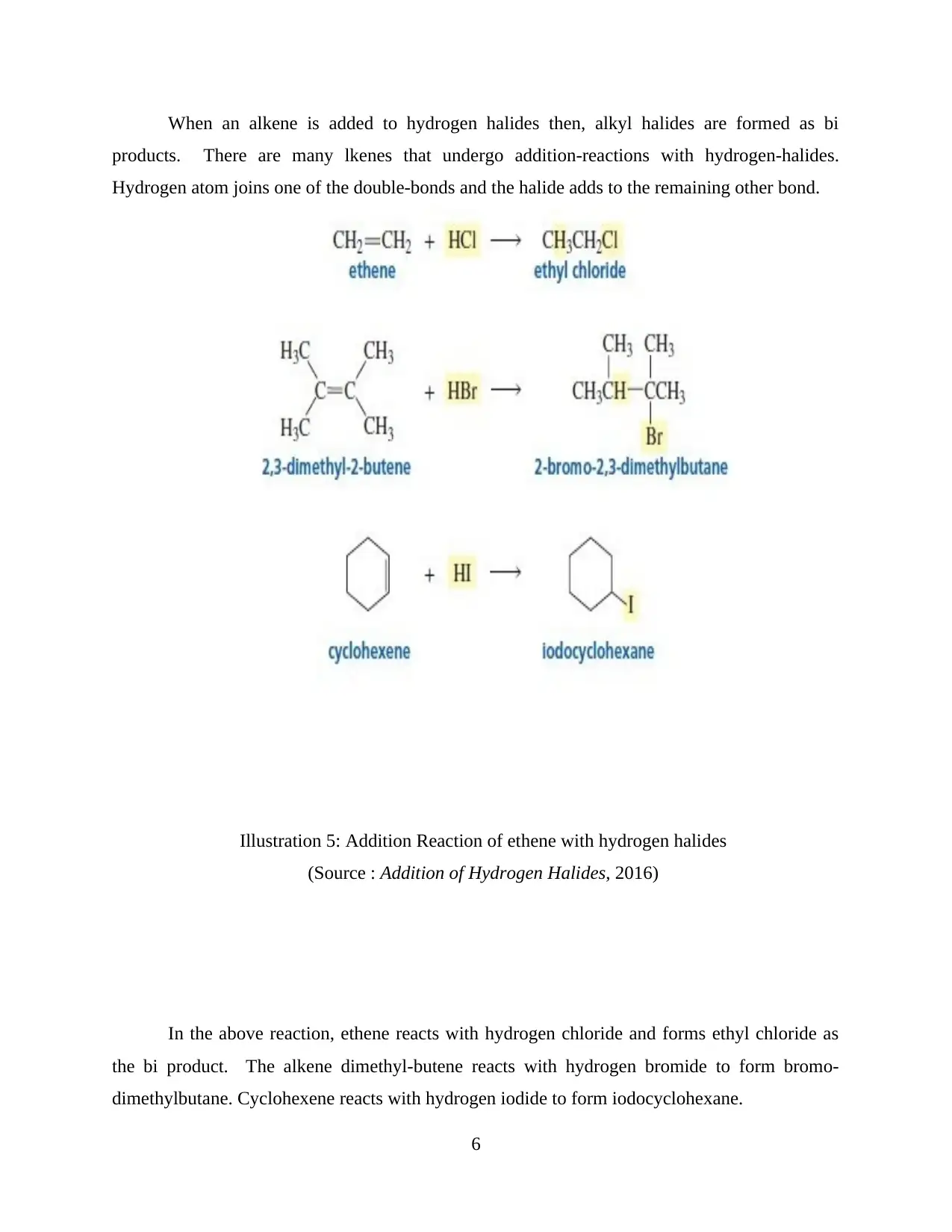
When an alkene is added to hydrogen halides then, alkyl halides are formed as bi
products. There are many lkenes that undergo addition-reactions with hydrogen-halides.
Hydrogen atom joins one of the double-bonds and the halide adds to the remaining other bond.
In the above reaction, ethene reacts with hydrogen chloride and forms ethyl chloride as
the bi product. The alkene dimethyl-butene reacts with hydrogen bromide to form bromo-
dimethylbutane. Cyclohexene reacts with hydrogen iodide to form iodocyclohexane.
6
Illustration 5: Addition Reaction of ethene with hydrogen halides
(Source : Addition of Hydrogen Halides, 2016)
products. There are many lkenes that undergo addition-reactions with hydrogen-halides.
Hydrogen atom joins one of the double-bonds and the halide adds to the remaining other bond.
In the above reaction, ethene reacts with hydrogen chloride and forms ethyl chloride as
the bi product. The alkene dimethyl-butene reacts with hydrogen bromide to form bromo-
dimethylbutane. Cyclohexene reacts with hydrogen iodide to form iodocyclohexane.
6
Illustration 5: Addition Reaction of ethene with hydrogen halides
(Source : Addition of Hydrogen Halides, 2016)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
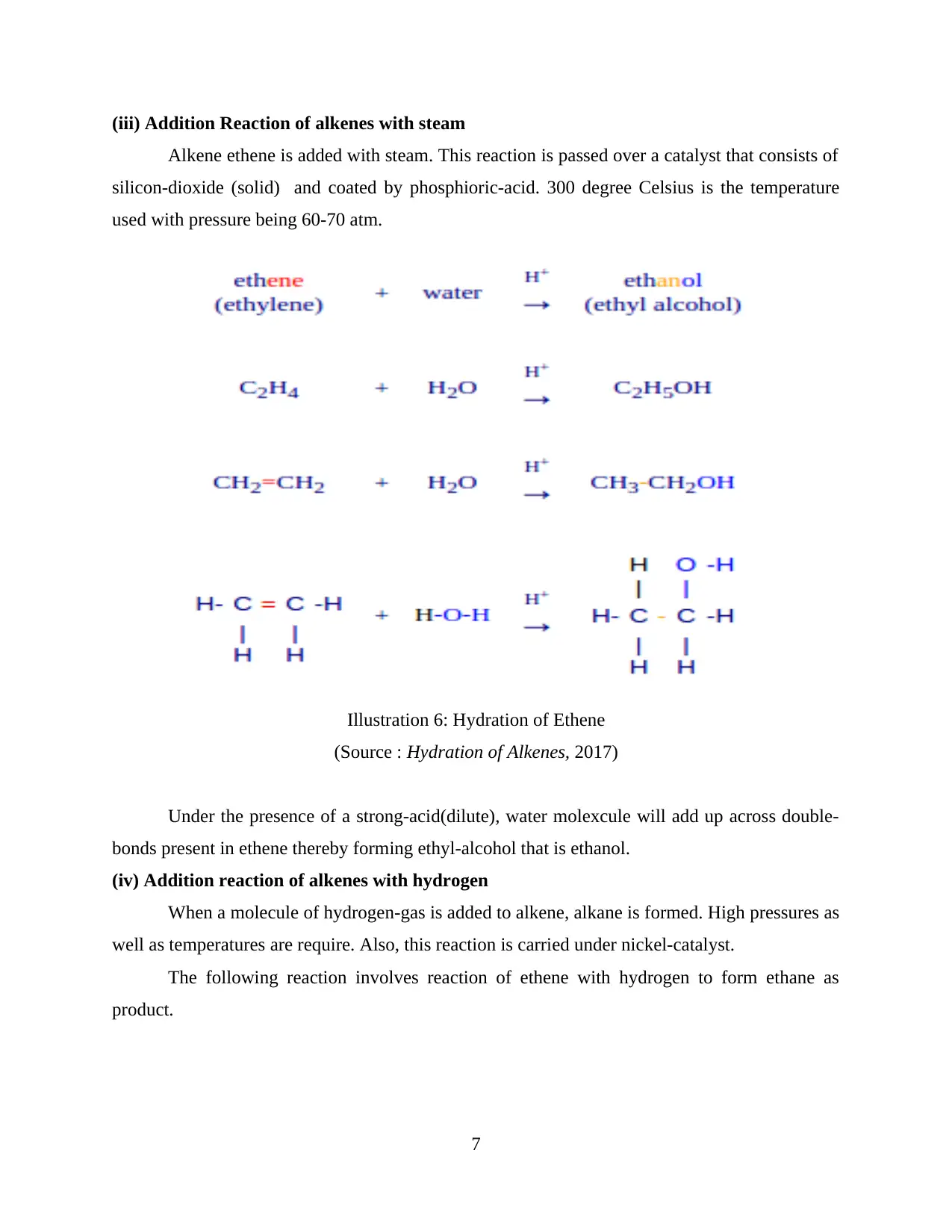
(iii) Addition Reaction of alkenes with steam
Alkene ethene is added with steam. This reaction is passed over a catalyst that consists of
silicon-dioxide (solid) and coated by phosphioric-acid. 300 degree Celsius is the temperature
used with pressure being 60-70 atm.
Under the presence of a strong-acid(dilute), water molexcule will add up across double-
bonds present in ethene thereby forming ethyl-alcohol that is ethanol.
(iv) Addition reaction of alkenes with hydrogen
When a molecule of hydrogen-gas is added to alkene, alkane is formed. High pressures as
well as temperatures are require. Also, this reaction is carried under nickel-catalyst.
The following reaction involves reaction of ethene with hydrogen to form ethane as
product.
7
Illustration 6: Hydration of Ethene
(Source : Hydration of Alkenes, 2017)
Alkene ethene is added with steam. This reaction is passed over a catalyst that consists of
silicon-dioxide (solid) and coated by phosphioric-acid. 300 degree Celsius is the temperature
used with pressure being 60-70 atm.
Under the presence of a strong-acid(dilute), water molexcule will add up across double-
bonds present in ethene thereby forming ethyl-alcohol that is ethanol.
(iv) Addition reaction of alkenes with hydrogen
When a molecule of hydrogen-gas is added to alkene, alkane is formed. High pressures as
well as temperatures are require. Also, this reaction is carried under nickel-catalyst.
The following reaction involves reaction of ethene with hydrogen to form ethane as
product.
7
Illustration 6: Hydration of Ethene
(Source : Hydration of Alkenes, 2017)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
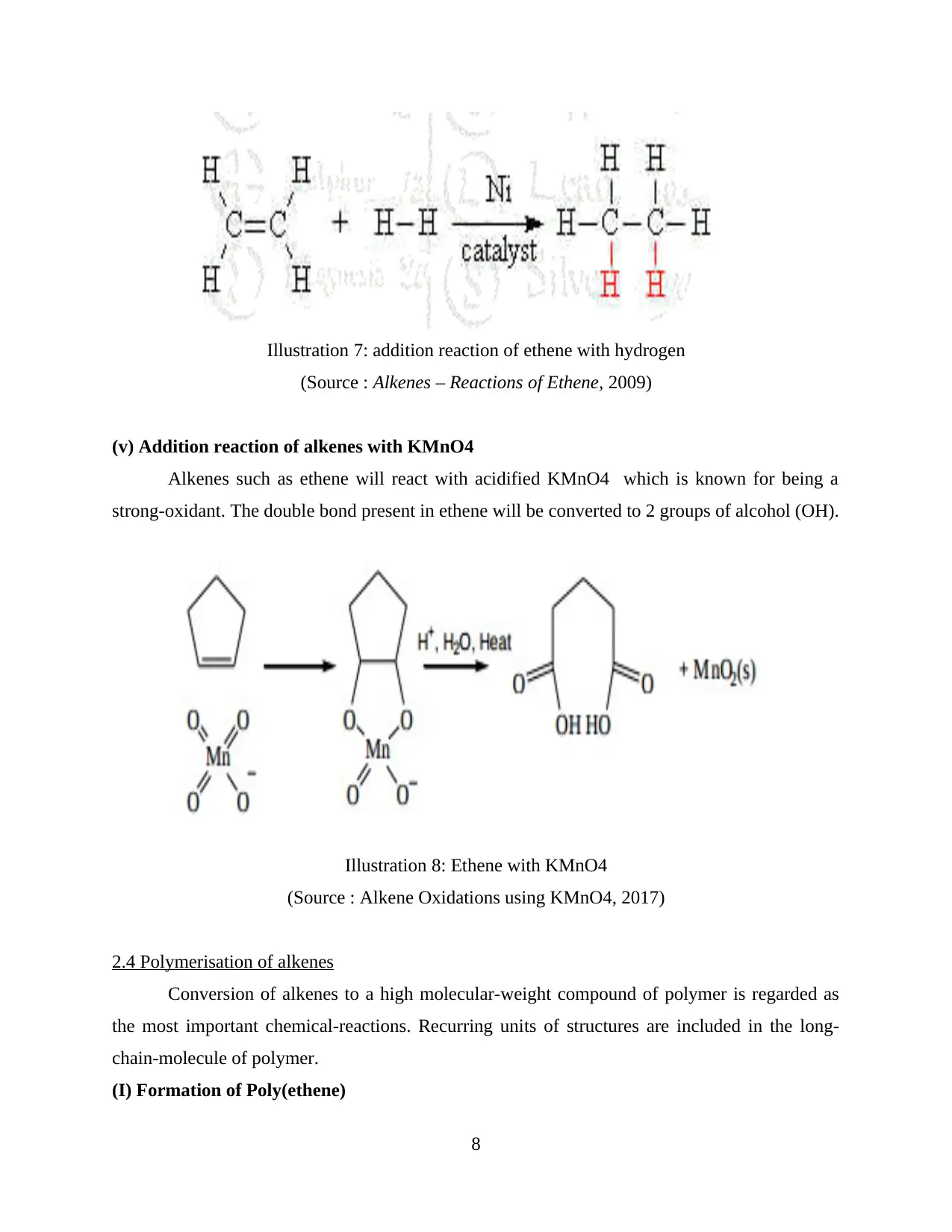
(v) Addition reaction of alkenes with KMnO4
Alkenes such as ethene will react with acidified KMnO4 which is known for being a
strong-oxidant. The double bond present in ethene will be converted to 2 groups of alcohol (OH).
2.4 Polymerisation of alkenes
Conversion of alkenes to a high molecular-weight compound of polymer is regarded as
the most important chemical-reactions. Recurring units of structures are included in the long-
chain-molecule of polymer.
(I) Formation of Poly(ethene)
8
Illustration 7: addition reaction of ethene with hydrogen
(Source : Alkenes – Reactions of Ethene, 2009)
Illustration 8: Ethene with KMnO4
(Source : Alkene Oxidations using KMnO4, 2017)
Alkenes such as ethene will react with acidified KMnO4 which is known for being a
strong-oxidant. The double bond present in ethene will be converted to 2 groups of alcohol (OH).
2.4 Polymerisation of alkenes
Conversion of alkenes to a high molecular-weight compound of polymer is regarded as
the most important chemical-reactions. Recurring units of structures are included in the long-
chain-molecule of polymer.
(I) Formation of Poly(ethene)
8
Illustration 7: addition reaction of ethene with hydrogen
(Source : Alkenes – Reactions of Ethene, 2009)
Illustration 8: Ethene with KMnO4
(Source : Alkene Oxidations using KMnO4, 2017)
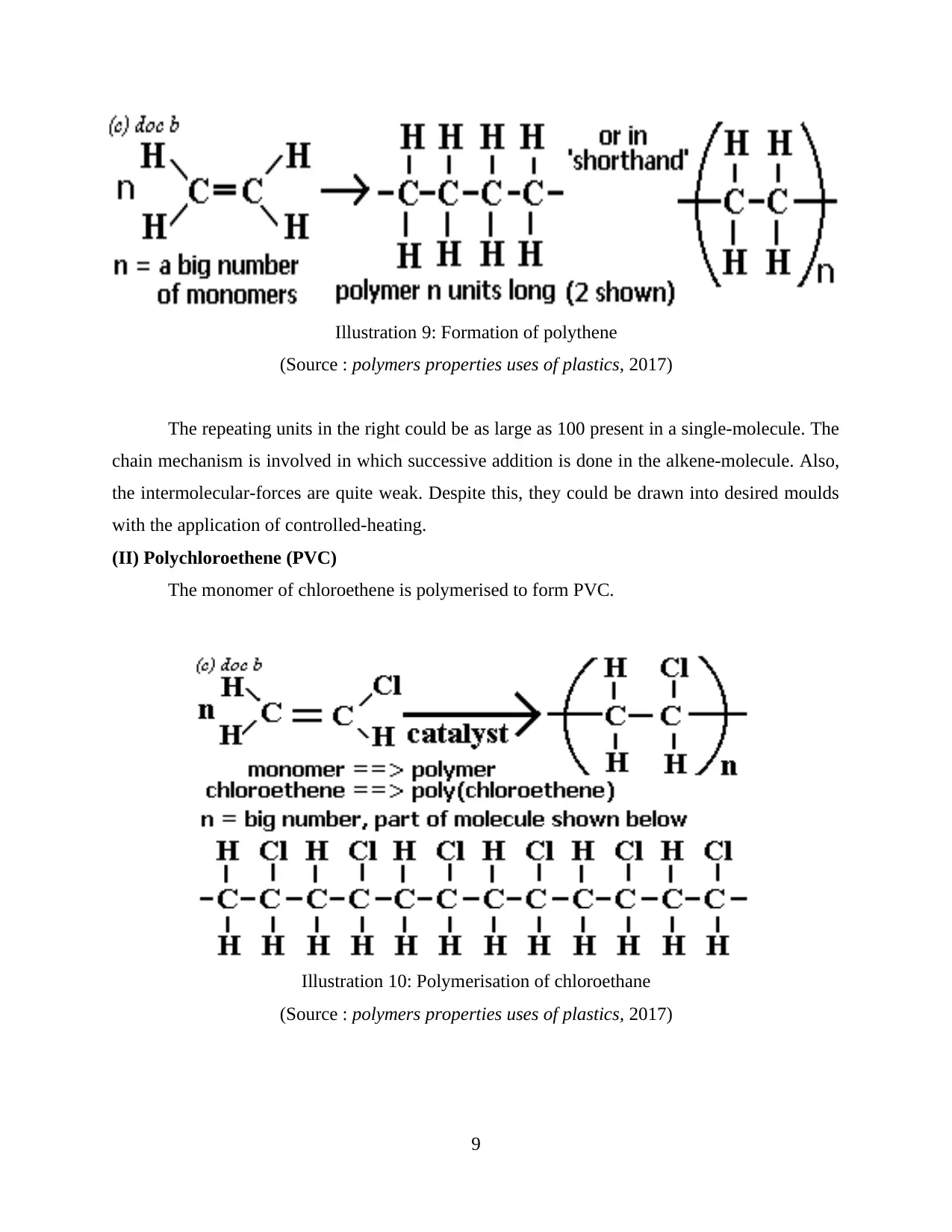
The repeating units in the right could be as large as 100 present in a single-molecule. The
chain mechanism is involved in which successive addition is done in the alkene-molecule. Also,
the intermolecular-forces are quite weak. Despite this, they could be drawn into desired moulds
with the application of controlled-heating.
(II) Polychloroethene (PVC)
The monomer of chloroethene is polymerised to form PVC.
9
Illustration 9: Formation of polythene
(Source : polymers properties uses of plastics, 2017)
Illustration 10: Polymerisation of chloroethane
(Source : polymers properties uses of plastics, 2017)
chain mechanism is involved in which successive addition is done in the alkene-molecule. Also,
the intermolecular-forces are quite weak. Despite this, they could be drawn into desired moulds
with the application of controlled-heating.
(II) Polychloroethene (PVC)
The monomer of chloroethene is polymerised to form PVC.
9
Illustration 9: Formation of polythene
(Source : polymers properties uses of plastics, 2017)
Illustration 10: Polymerisation of chloroethane
(Source : polymers properties uses of plastics, 2017)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 18
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.