Ethical Approval Application: Maldives Swimming Pool Business Proposal
VerifiedAdded on 2023/01/05
|14
|5813
|29
Project
AI Summary
This document presents a student's application for ethical approval for a business proposal focused on developing an international standard swimming pool arena in the Maldives. The application outlines the purpose, aims, and objectives of the research, which include formulating a business idea, outlining operational, marketing, and financial plans, and researching the plan's integrity. The research will employ a positivism research philosophy and a deductive approach, using secondary data collection and analysis methods. The document details the research methodology, including data collection, location, and ethical considerations. The student confirms that the research does not involve any high-risk activities, such as questionnaires, interviews, or working with vulnerable participants. The application also includes the project's timeline, indicating a proposed start date of June 11, 2019, and an end date of August 10, 2019.
PG2 / E1 FORM
APPLICATION FOR ETHICAL APPROVAL
In order for research to result in benefit and minimise risk of harm, it must be conducted
ethically. A researcher may not be covered by the University’s insurance if ethical
approval has not been obtained prior to commencement.
The University follows the OECD Frascati manual definition of research activity: “creative work
undertaken on a systematic basis in order to increase the stock of knowledge, including
knowledge of man, culture and society, and the use of this stock of knowledge to devise new
applications”. As such this covers activities undertaken by members of staff, postgraduate
research students, and both taught postgraduate and undergraduate students working on
dissertations/projects.
The individual undertaking the research activity is known as the “principal researcher”.
Ethical approval is not required for routine audits, performance reviews, quality assurance
studies, testing within normal educational requirements, and literary or artistic criticism.
Please read the notes for guidance before completing ALL sections of the form.
This form must be completed and approved prior to undertaking any research activity.
Please see Checklist for details of process for different categories of application.
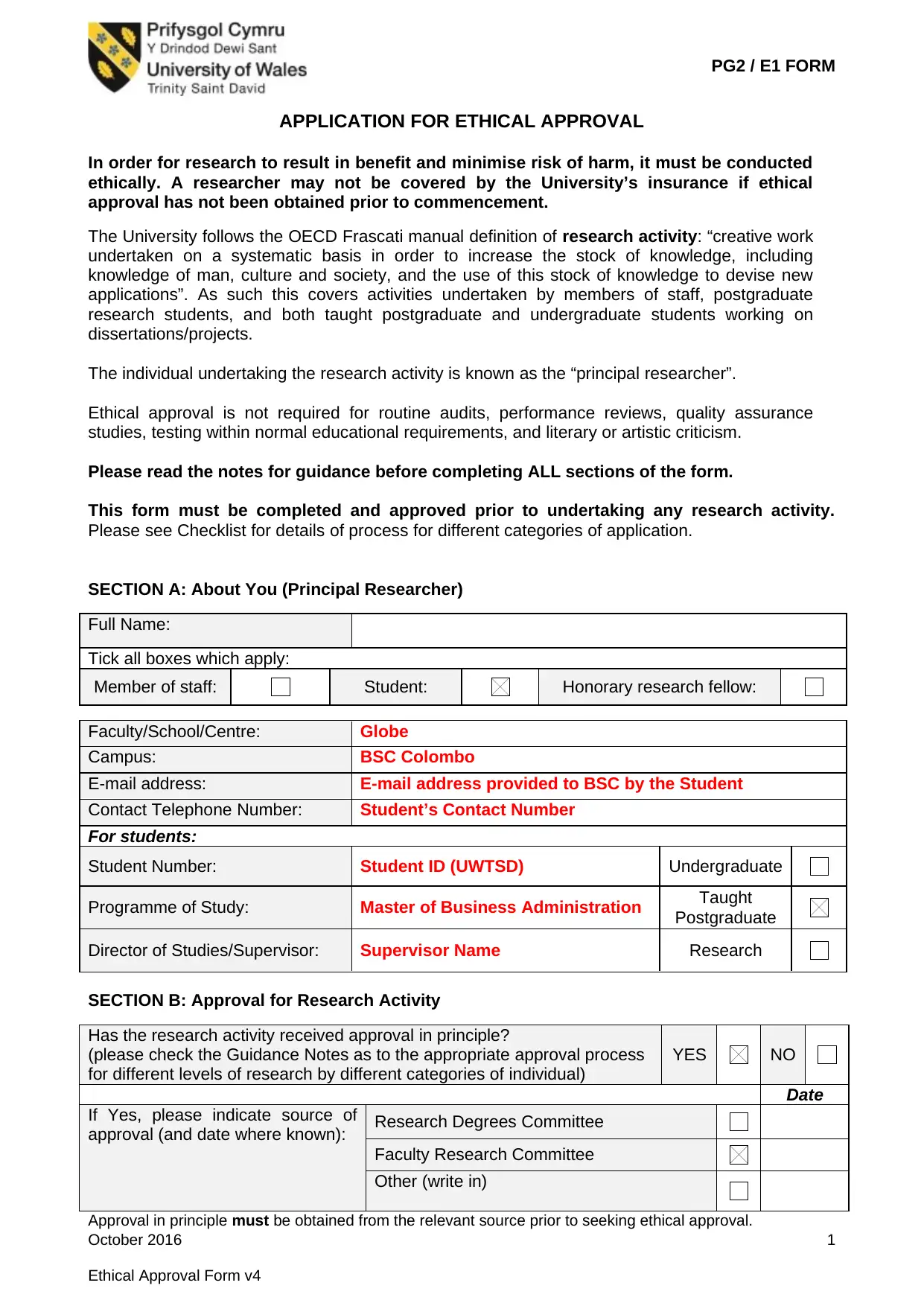
SECTION A: About You (Principal Researcher)
Full Name:
Tick all boxes which apply:
Member of staff: Student: Honorary research fellow:
Faculty/School/Centre: Globe
Campus: BSC Colombo
E-mail address: E-mail address provided to BSC by the Student
Contact Telephone Number: Student’s Contact Number
For students:
Student Number: Student ID (UWTSD) Undergraduate
Programme of Study: Master of Business Administration Taught
Postgraduate
Director of Studies/Supervisor: Supervisor Name Research
SECTION B: Approval for Research Activity
Has the research activity received approval in principle?
(please check the Guidance Notes as to the appropriate approval process
for different levels of research by different categories of individual)
YES NO
Date
If Yes, please indicate source of
approval (and date where known): Research Degrees Committee
Faculty Research Committee
Other (write in)
Approval in principle must be obtained from the relevant source prior to seeking ethical approval.
October 2016 1
Ethical Approval Form v4
APPLICATION FOR ETHICAL APPROVAL
In order for research to result in benefit and minimise risk of harm, it must be conducted
ethically. A researcher may not be covered by the University’s insurance if ethical
approval has not been obtained prior to commencement.
The University follows the OECD Frascati manual definition of research activity: “creative work
undertaken on a systematic basis in order to increase the stock of knowledge, including
knowledge of man, culture and society, and the use of this stock of knowledge to devise new
applications”. As such this covers activities undertaken by members of staff, postgraduate
research students, and both taught postgraduate and undergraduate students working on
dissertations/projects.
The individual undertaking the research activity is known as the “principal researcher”.
Ethical approval is not required for routine audits, performance reviews, quality assurance
studies, testing within normal educational requirements, and literary or artistic criticism.
Please read the notes for guidance before completing ALL sections of the form.
This form must be completed and approved prior to undertaking any research activity.
Please see Checklist for details of process for different categories of application.
SECTION A: About You (Principal Researcher)
Full Name:
Tick all boxes which apply:
Member of staff: Student: Honorary research fellow:
Faculty/School/Centre: Globe
Campus: BSC Colombo
E-mail address: E-mail address provided to BSC by the Student
Contact Telephone Number: Student’s Contact Number
For students:
Student Number: Student ID (UWTSD) Undergraduate
Programme of Study: Master of Business Administration Taught
Postgraduate
Director of Studies/Supervisor: Supervisor Name Research
SECTION B: Approval for Research Activity
Has the research activity received approval in principle?
(please check the Guidance Notes as to the appropriate approval process
for different levels of research by different categories of individual)
YES NO
Date
If Yes, please indicate source of
approval (and date where known): Research Degrees Committee
Faculty Research Committee
Other (write in)
Approval in principle must be obtained from the relevant source prior to seeking ethical approval.
October 2016 1
Ethical Approval Form v4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
PG2 / E1 FORM
SECTION C: External Ethical Guidance Materials
Please list the core ethical guidance documents that have been referred to during the completion
of this form (including any discipline-specific codes of research ethics, and also any specific
ethical guidance relating to the proposed methodology). Please tick to confirm that your
research proposal adheres to these codes and guidelines.
http://uwtsd.ac.uk/library/research-data-management/
SECTION D: External Collaborative Research Activity
Does the research activity involve collaborators outside of
the University? YES NO
If Yes, please provide the name of the external organisation and name and contact details for the
main contact person:
Institution
Contact person name
Contact person e-mail address
Where research activity is carried out in collaboration with an external organisation
Does this organisation have its own ethics approval system? YES NO
If Yes, please attach a copy of any final approval (or interim approval) from the organisation
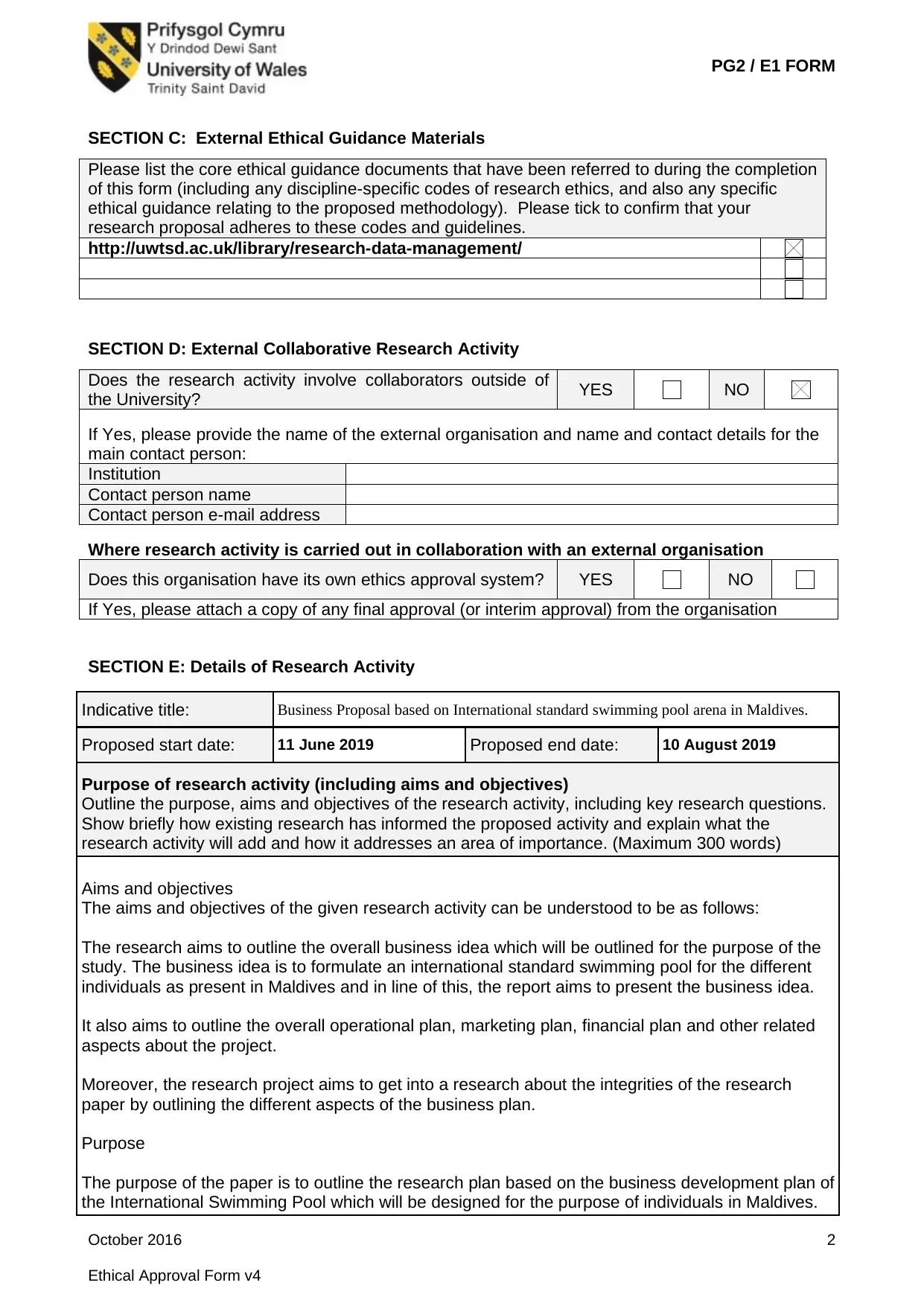
SECTION E: Details of Research Activity
Indicative title: Business Proposal based on International standard swimming pool arena in Maldives.
Proposed start date: 11 June 2019 Proposed end date: 10 August 2019
Purpose of research activity (including aims and objectives)
Outline the purpose, aims and objectives of the research activity, including key research questions.
Show briefly how existing research has informed the proposed activity and explain what the
research activity will add and how it addresses an area of importance. (Maximum 300 words)
Aims and objectives
The aims and objectives of the given research activity can be understood to be as follows:
The research aims to outline the overall business idea which will be outlined for the purpose of the
study. The business idea is to formulate an international standard swimming pool for the different
individuals as present in Maldives and in line of this, the report aims to present the business idea.
It also aims to outline the overall operational plan, marketing plan, financial plan and other related
aspects about the project.
Moreover, the research project aims to get into a research about the integrities of the research
paper by outlining the different aspects of the business plan.
Purpose
The purpose of the paper is to outline the research plan based on the business development plan of
the International Swimming Pool which will be designed for the purpose of individuals in Maldives.
October 2016 2
Ethical Approval Form v4
SECTION C: External Ethical Guidance Materials
Please list the core ethical guidance documents that have been referred to during the completion
of this form (including any discipline-specific codes of research ethics, and also any specific
ethical guidance relating to the proposed methodology). Please tick to confirm that your
research proposal adheres to these codes and guidelines.
http://uwtsd.ac.uk/library/research-data-management/
SECTION D: External Collaborative Research Activity
Does the research activity involve collaborators outside of
the University? YES NO
If Yes, please provide the name of the external organisation and name and contact details for the
main contact person:
Institution
Contact person name
Contact person e-mail address
Where research activity is carried out in collaboration with an external organisation
Does this organisation have its own ethics approval system? YES NO
If Yes, please attach a copy of any final approval (or interim approval) from the organisation
SECTION E: Details of Research Activity
Indicative title: Business Proposal based on International standard swimming pool arena in Maldives.
Proposed start date: 11 June 2019 Proposed end date: 10 August 2019
Purpose of research activity (including aims and objectives)
Outline the purpose, aims and objectives of the research activity, including key research questions.
Show briefly how existing research has informed the proposed activity and explain what the
research activity will add and how it addresses an area of importance. (Maximum 300 words)
Aims and objectives
The aims and objectives of the given research activity can be understood to be as follows:
The research aims to outline the overall business idea which will be outlined for the purpose of the
study. The business idea is to formulate an international standard swimming pool for the different
individuals as present in Maldives and in line of this, the report aims to present the business idea.
It also aims to outline the overall operational plan, marketing plan, financial plan and other related
aspects about the project.
Moreover, the research project aims to get into a research about the integrities of the research
paper by outlining the different aspects of the business plan.
Purpose
The purpose of the paper is to outline the research plan based on the business development plan of
the International Swimming Pool which will be designed for the purpose of individuals in Maldives.
October 2016 2
Ethical Approval Form v4

PG2 / E1 FORM
There are no international standard pools as present in Maldives and with respect to this, it
becomes considerable important for the country to have a good pool design and related facilities
which will assist in ensuring that the country is able to prosper considerably and that the different
individuals are able to participate in various tournaments.
Research questions
The research questions for the purpose of the study can be understood to be as follows:
How will an international standard pool be developed in Maldives?
What resources will be required for the purpose of the business?
What will be the different financial, marketing and operational plan outline of the business
development plan?
(this box should expand as you type)
Proposed methods
Provide a brief summary of all the methods that may be used in the research activity, making it
clear what specific techniques may be used. If methods other than those listed in this section are
deemed appropriate later, additional ethical approval for those methods will be needed. (Maximum
600 words)
The Research Methodology
Research Philosophy
The research philosophy which shall be undertaken for the purpose of the research can be stated to
be the positivism research whereby secondary information shall be used for the purpose of analysis
and in order to determine the overall purpose of the business plan to be formulated.
Research Approach
The research approach which will be undertaken for the research of the paper can be stated to be
the deductive research approach. In this research approach style, the data will be actualised and
interpreted in an authentic manner based on this the analysis shall be undertaken.
Research Design
The research design which has been chosen for the purpose of the paper can be understood to be
explorative in nature. In line of this, it becomes considerably crucial to understand the impact of
external influences on the overall development of the pool and related aspects.
Data collection and Analysis
The data shall be collected and analysed using the secondary data collection and analysis method.
The internet and other such related mediums will be used to undertake the information and then
analyse it effectively.
(this box should expand as you type)
October 2016 3
Ethical Approval Form v4
There are no international standard pools as present in Maldives and with respect to this, it
becomes considerable important for the country to have a good pool design and related facilities
which will assist in ensuring that the country is able to prosper considerably and that the different
individuals are able to participate in various tournaments.
Research questions
The research questions for the purpose of the study can be understood to be as follows:
How will an international standard pool be developed in Maldives?
What resources will be required for the purpose of the business?
What will be the different financial, marketing and operational plan outline of the business
development plan?
(this box should expand as you type)
Proposed methods
Provide a brief summary of all the methods that may be used in the research activity, making it
clear what specific techniques may be used. If methods other than those listed in this section are
deemed appropriate later, additional ethical approval for those methods will be needed. (Maximum
600 words)
The Research Methodology
Research Philosophy
The research philosophy which shall be undertaken for the purpose of the research can be stated to
be the positivism research whereby secondary information shall be used for the purpose of analysis
and in order to determine the overall purpose of the business plan to be formulated.
Research Approach
The research approach which will be undertaken for the research of the paper can be stated to be
the deductive research approach. In this research approach style, the data will be actualised and
interpreted in an authentic manner based on this the analysis shall be undertaken.
Research Design
The research design which has been chosen for the purpose of the paper can be understood to be
explorative in nature. In line of this, it becomes considerably crucial to understand the impact of
external influences on the overall development of the pool and related aspects.
Data collection and Analysis
The data shall be collected and analysed using the secondary data collection and analysis method.
The internet and other such related mediums will be used to undertake the information and then
analyse it effectively.
(this box should expand as you type)
October 2016 3
Ethical Approval Form v4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
PG2 / E1 FORM
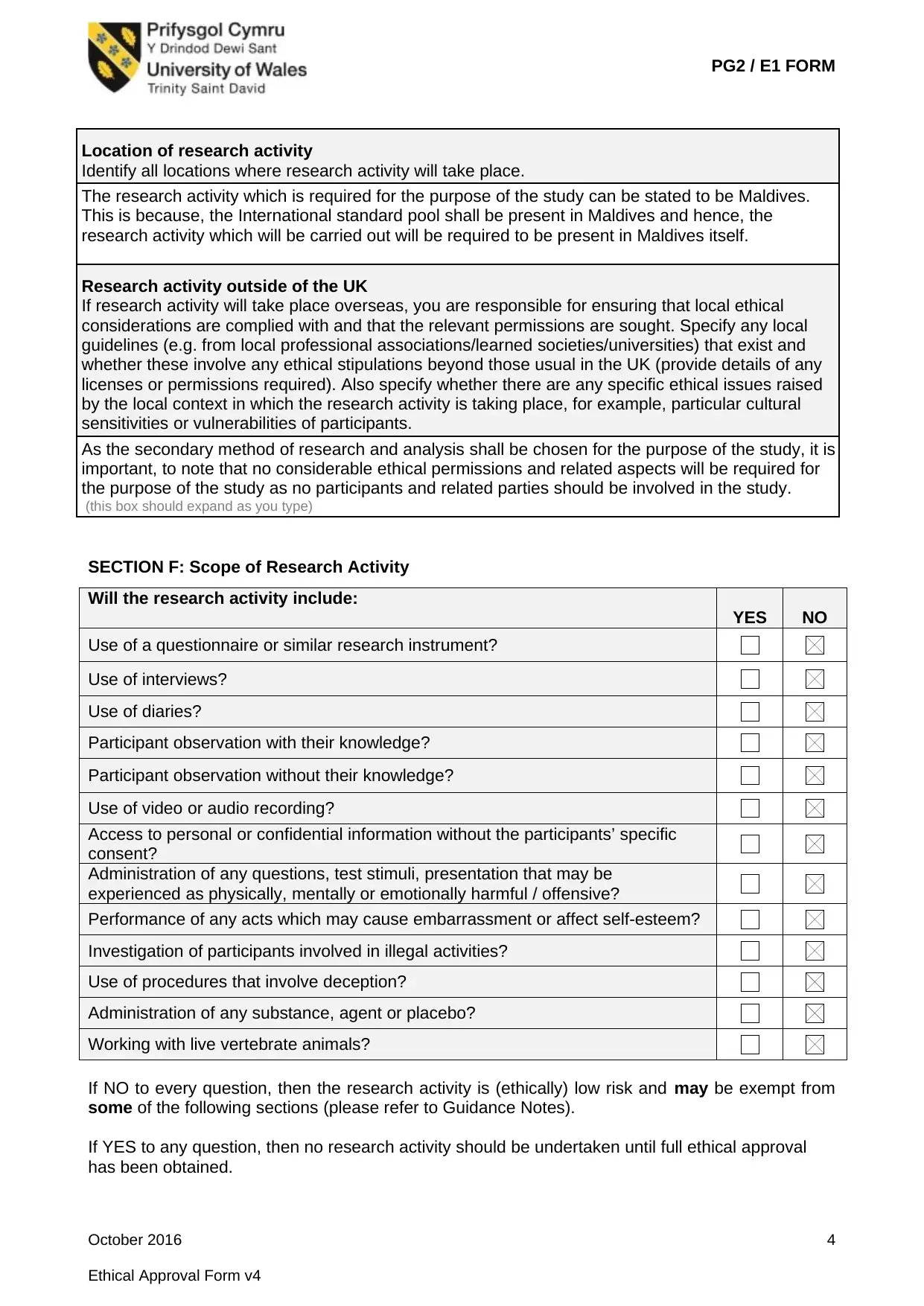
Location of research activity
Identify all locations where research activity will take place.
The research activity which is required for the purpose of the study can be stated to be Maldives.
This is because, the International standard pool shall be present in Maldives and hence, the
research activity which will be carried out will be required to be present in Maldives itself.
Research activity outside of the UK
If research activity will take place overseas, you are responsible for ensuring that local ethical
considerations are complied with and that the relevant permissions are sought. Specify any local
guidelines (e.g. from local professional associations/learned societies/universities) that exist and
whether these involve any ethical stipulations beyond those usual in the UK (provide details of any
licenses or permissions required). Also specify whether there are any specific ethical issues raised
by the local context in which the research activity is taking place, for example, particular cultural
sensitivities or vulnerabilities of participants.
As the secondary method of research and analysis shall be chosen for the purpose of the study, it is
important, to note that no considerable ethical permissions and related aspects will be required for
the purpose of the study as no participants and related parties should be involved in the study.
(this box should expand as you type)
SECTION F: Scope of Research Activity
Will the research activity include:
YES NO
Use of a questionnaire or similar research instrument?
Use of interviews?
Use of diaries?
Participant observation with their knowledge?
Participant observation without their knowledge?
Use of video or audio recording?
Access to personal or confidential information without the participants’ specific
consent?
Administration of any questions, test stimuli, presentation that may be
experienced as physically, mentally or emotionally harmful / offensive?
Performance of any acts which may cause embarrassment or affect self-esteem?
Investigation of participants involved in illegal activities?
Use of procedures that involve deception?
Administration of any substance, agent or placebo?
Working with live vertebrate animals?
If NO to every question, then the research activity is (ethically) low risk and may be exempt from
some of the following sections (please refer to Guidance Notes).
If YES to any question, then no research activity should be undertaken until full ethical approval
has been obtained.
October 2016 4
Ethical Approval Form v4
Location of research activity
Identify all locations where research activity will take place.
The research activity which is required for the purpose of the study can be stated to be Maldives.
This is because, the International standard pool shall be present in Maldives and hence, the
research activity which will be carried out will be required to be present in Maldives itself.
Research activity outside of the UK
If research activity will take place overseas, you are responsible for ensuring that local ethical
considerations are complied with and that the relevant permissions are sought. Specify any local
guidelines (e.g. from local professional associations/learned societies/universities) that exist and
whether these involve any ethical stipulations beyond those usual in the UK (provide details of any
licenses or permissions required). Also specify whether there are any specific ethical issues raised
by the local context in which the research activity is taking place, for example, particular cultural
sensitivities or vulnerabilities of participants.
As the secondary method of research and analysis shall be chosen for the purpose of the study, it is
important, to note that no considerable ethical permissions and related aspects will be required for
the purpose of the study as no participants and related parties should be involved in the study.
(this box should expand as you type)
SECTION F: Scope of Research Activity
Will the research activity include:
YES NO
Use of a questionnaire or similar research instrument?
Use of interviews?
Use of diaries?
Participant observation with their knowledge?
Participant observation without their knowledge?
Use of video or audio recording?
Access to personal or confidential information without the participants’ specific
consent?
Administration of any questions, test stimuli, presentation that may be
experienced as physically, mentally or emotionally harmful / offensive?
Performance of any acts which may cause embarrassment or affect self-esteem?
Investigation of participants involved in illegal activities?
Use of procedures that involve deception?
Administration of any substance, agent or placebo?
Working with live vertebrate animals?
If NO to every question, then the research activity is (ethically) low risk and may be exempt from
some of the following sections (please refer to Guidance Notes).
If YES to any question, then no research activity should be undertaken until full ethical approval
has been obtained.
October 2016 4
Ethical Approval Form v4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
PG2 / E1 FORM
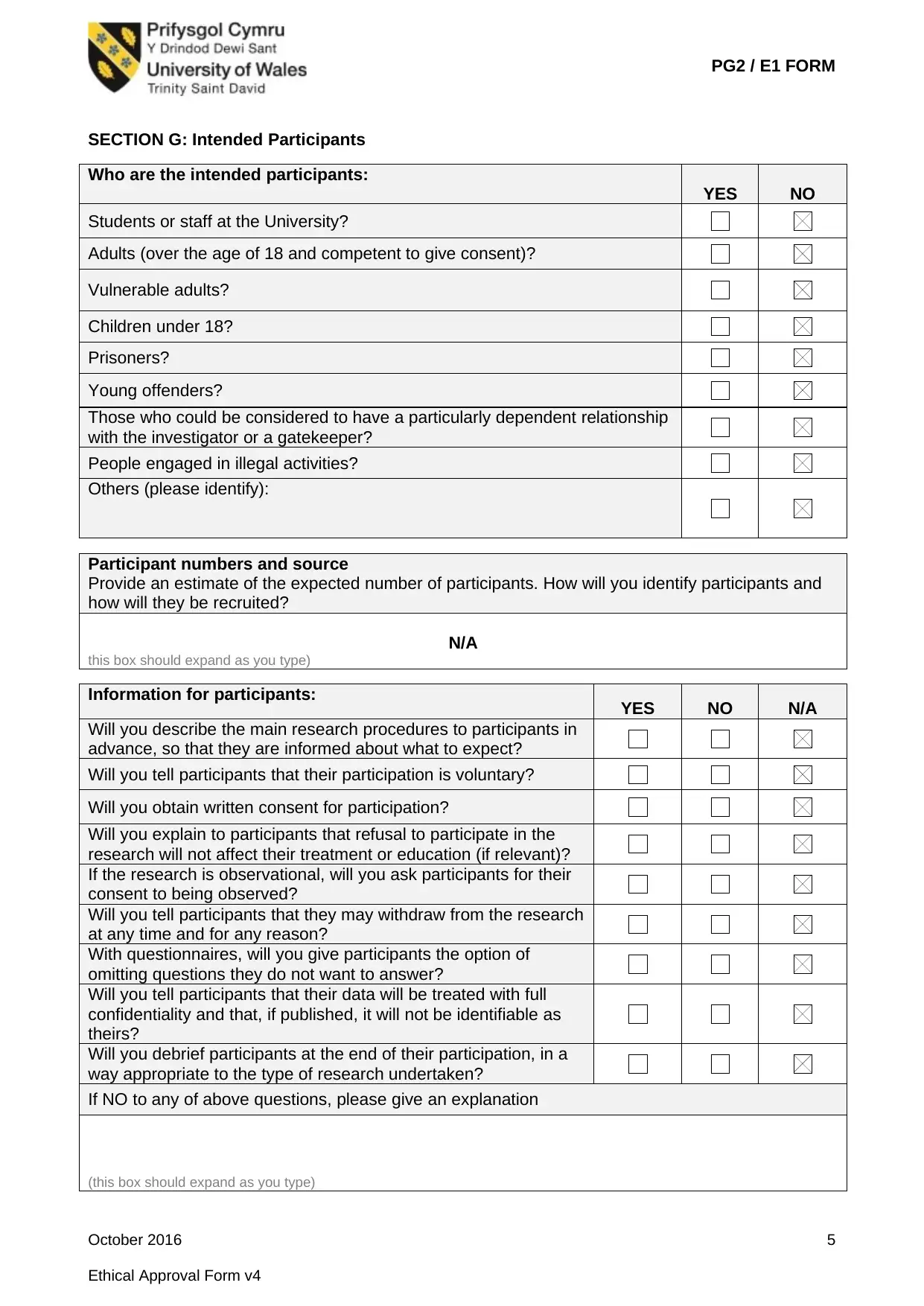
SECTION G: Intended Participants
Who are the intended participants:
YES NO
Students or staff at the University?
Adults (over the age of 18 and competent to give consent)?
Vulnerable adults?
Children under 18?
Prisoners?
Young offenders?
Those who could be considered to have a particularly dependent relationship
with the investigator or a gatekeeper?
People engaged in illegal activities?
Others (please identify):
Participant numbers and source
Provide an estimate of the expected number of participants. How will you identify participants and
how will they be recruited?
N/A
this box should expand as you type)
Information for participants: YES NO N/A
Will you describe the main research procedures to participants in
advance, so that they are informed about what to expect?
Will you tell participants that their participation is voluntary?
Will you obtain written consent for participation?
Will you explain to participants that refusal to participate in the
research will not affect their treatment or education (if relevant)?
If the research is observational, will you ask participants for their
consent to being observed?
Will you tell participants that they may withdraw from the research
at any time and for any reason?
With questionnaires, will you give participants the option of
omitting questions they do not want to answer?
Will you tell participants that their data will be treated with full
confidentiality and that, if published, it will not be identifiable as
theirs?
Will you debrief participants at the end of their participation, in a
way appropriate to the type of research undertaken?
If NO to any of above questions, please give an explanation
(this box should expand as you type)
October 2016 5
Ethical Approval Form v4
SECTION G: Intended Participants
Who are the intended participants:
YES NO
Students or staff at the University?
Adults (over the age of 18 and competent to give consent)?
Vulnerable adults?
Children under 18?
Prisoners?
Young offenders?
Those who could be considered to have a particularly dependent relationship
with the investigator or a gatekeeper?
People engaged in illegal activities?
Others (please identify):
Participant numbers and source
Provide an estimate of the expected number of participants. How will you identify participants and
how will they be recruited?
N/A
this box should expand as you type)
Information for participants: YES NO N/A
Will you describe the main research procedures to participants in
advance, so that they are informed about what to expect?
Will you tell participants that their participation is voluntary?
Will you obtain written consent for participation?
Will you explain to participants that refusal to participate in the
research will not affect their treatment or education (if relevant)?
If the research is observational, will you ask participants for their
consent to being observed?
Will you tell participants that they may withdraw from the research
at any time and for any reason?
With questionnaires, will you give participants the option of
omitting questions they do not want to answer?
Will you tell participants that their data will be treated with full
confidentiality and that, if published, it will not be identifiable as
theirs?
Will you debrief participants at the end of their participation, in a
way appropriate to the type of research undertaken?
If NO to any of above questions, please give an explanation
(this box should expand as you type)
October 2016 5
Ethical Approval Form v4
PG2 / E1 FORM
Information for participants: YES NO N/A
Will participants be paid?
Is specialist electrical or other equipment to be used with
participants?
Are there any financial or other interests to the investigator or
University arising from this study?
Will the research activity involve deliberately misleading
participants in any way, or the partial or full concealment of the
specific study aims?
If YES to any question, please provide full details
(this box should expand as you type)
October 2016 6
Ethical Approval Form v4
Information for participants: YES NO N/A
Will participants be paid?
Is specialist electrical or other equipment to be used with
participants?
Are there any financial or other interests to the investigator or
University arising from this study?
Will the research activity involve deliberately misleading
participants in any way, or the partial or full concealment of the
specific study aims?
If YES to any question, please provide full details
(this box should expand as you type)
October 2016 6
Ethical Approval Form v4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
PG2 / E1 FORM
SECTION H: Anticipated Risks
Outline any anticipated risks that may adversely affect any of the participants, the
researchers and/or the University, and the steps that will be taken to address them.
Risks to participants
For example: emotional distress, financial disclosure, physical harm, transfer of personal data,
sensitive organisational information
N/A
(this box should expand as you type)
If research activity may include sensitive, embarrassing or upsetting topics (e.g. sexual activity,
drug use) or issues likely to disclose information requiring further action (e.g. criminal activity), give
details of the procedures to deal with these issues, including any support/advice (e.g. helpline
numbers) to be offered to participants. Note that where applicable, consent procedures should
make it clear that if something potentially or actually illegal is discovered in the course of a project,
it may need to be disclosed to the proper authorities
N/A
(this box should expand as you type)
Risks to investigator
For example: personal safety, physical harm, emotional distress, risk of accusation of
harm/impropriety, conflict of interest
N/A
(this box should expand as you type)
University/institutional risks
For example: adverse publicity, financial loss, data protection
N/A
(this box should expand as you type)
Adverse outcomes
List measures put in place to limit any adverse effects or outcomes of research activity where
appropriate. Include any emergency protocols.
N/A
(this box should expand as you type)
Disclosure and Barring Service
If the research activity involves children or vulnerable adults, a
Disclosure and Barring Service (DBS) certificate must be obtained
before any contact with such participants. YES NO N/A
Has a DBS certificate been obtained?
October 2016 7
Ethical Approval Form v4
SECTION H: Anticipated Risks
Outline any anticipated risks that may adversely affect any of the participants, the
researchers and/or the University, and the steps that will be taken to address them.
Risks to participants
For example: emotional distress, financial disclosure, physical harm, transfer of personal data,
sensitive organisational information
N/A
(this box should expand as you type)
If research activity may include sensitive, embarrassing or upsetting topics (e.g. sexual activity,
drug use) or issues likely to disclose information requiring further action (e.g. criminal activity), give
details of the procedures to deal with these issues, including any support/advice (e.g. helpline
numbers) to be offered to participants. Note that where applicable, consent procedures should
make it clear that if something potentially or actually illegal is discovered in the course of a project,
it may need to be disclosed to the proper authorities
N/A
(this box should expand as you type)
Risks to investigator
For example: personal safety, physical harm, emotional distress, risk of accusation of
harm/impropriety, conflict of interest
N/A
(this box should expand as you type)
University/institutional risks
For example: adverse publicity, financial loss, data protection
N/A
(this box should expand as you type)
Adverse outcomes
List measures put in place to limit any adverse effects or outcomes of research activity where
appropriate. Include any emergency protocols.
N/A
(this box should expand as you type)
Disclosure and Barring Service
If the research activity involves children or vulnerable adults, a
Disclosure and Barring Service (DBS) certificate must be obtained
before any contact with such participants. YES NO N/A
Has a DBS certificate been obtained?
October 2016 7
Ethical Approval Form v4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
PG2 / E1 FORM
SECTION I: Feedback, Consent and Confidentiality
Feedback
What feedback will be provided to participants, how will this be done and when?
N/A
(this box should expand as you type)
Informed consent
Describe the arrangements to inform potential participants, before providing consent, of what is
involved in participating. Describe the arrangements for participants to provide full consent before
data collection begins. If gaining consent in this way is inappropriate, explain how consent will be
obtained and recorded.
N/A
(this box should expand as you type)
Confidentiality / Anonymity
Set out how anonymity of participants and confidentiality will be ensured in any outputs. If
anonymity is not being offered, explain why this is the case.
N/A
(this box should expand as you type)
October 2016 8
Ethical Approval Form v4
SECTION I: Feedback, Consent and Confidentiality
Feedback
What feedback will be provided to participants, how will this be done and when?
N/A
(this box should expand as you type)
Informed consent
Describe the arrangements to inform potential participants, before providing consent, of what is
involved in participating. Describe the arrangements for participants to provide full consent before
data collection begins. If gaining consent in this way is inappropriate, explain how consent will be
obtained and recorded.
N/A
(this box should expand as you type)
Confidentiality / Anonymity
Set out how anonymity of participants and confidentiality will be ensured in any outputs. If
anonymity is not being offered, explain why this is the case.
N/A
(this box should expand as you type)
October 2016 8
Ethical Approval Form v4
PG2 / E1 FORM
SECTION J: Data Protection and Storage
In completing this section refer to the University’s Research Data Management Policy and
the extensive resources on the University’s Research Data Management web pages
(http://uwtsd.ac.uk/library/research-data-management/).
YES NO
Does the research activity involve personal data (as defined by the Data
Protection Act)?
If YES, provide a description of the data and explain why this data needs to be collected:
(this box should expand as you type)
Does it involve sensitive personal data (as defined by the Data Protection
Act)?
If YES, provide a description of the data and explain why this data needs to be collected:
(this box should expand as you type)
Will the research activity involve any of the following activities: YES NO
Electronic transfer of data in any form?
Sharing of data with others at the University?
Sharing of data with other organisations?
Export of data outside the European Union or importing of data from outside
the UK?
Use of personal addresses, postcodes, faxes, emails or telephone numbers?
Publication of data that might allow identification of individuals?
Use of data management system?
Data archiving?
If YES to any question, please provide full details, explaining how this will be conducted in
accordance with the Data Protection Act (and/or any international equivalent):
(this box should expand as you type)
October 2016 9
Ethical Approval Form v4
SECTION J: Data Protection and Storage
In completing this section refer to the University’s Research Data Management Policy and
the extensive resources on the University’s Research Data Management web pages
(http://uwtsd.ac.uk/library/research-data-management/).
YES NO
Does the research activity involve personal data (as defined by the Data
Protection Act)?
If YES, provide a description of the data and explain why this data needs to be collected:
(this box should expand as you type)
Does it involve sensitive personal data (as defined by the Data Protection
Act)?
If YES, provide a description of the data and explain why this data needs to be collected:
(this box should expand as you type)
Will the research activity involve any of the following activities: YES NO
Electronic transfer of data in any form?
Sharing of data with others at the University?
Sharing of data with other organisations?
Export of data outside the European Union or importing of data from outside
the UK?
Use of personal addresses, postcodes, faxes, emails or telephone numbers?
Publication of data that might allow identification of individuals?
Use of data management system?
Data archiving?
If YES to any question, please provide full details, explaining how this will be conducted in
accordance with the Data Protection Act (and/or any international equivalent):
(this box should expand as you type)
October 2016 9
Ethical Approval Form v4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
PG2 / E1 FORM
Will the research activity involve storing personal data on one of the
following: YES NO
Manual files (i.e. in paper form)?
University computers?
Private company computers?
Home or other personal computers?
Laptop computers/ CDs/ Portable disk-drives/ memory sticks?
“Cloud” storage or websites?
Other – specify:
For all stored data, explain the measures in place to ensure data confidentiality, including details of
password protection, encryption and anonymisation:
N/A
(this box should expand as you type)
List all who will have access to the data generated by the research activity:
N/A
(this box should expand as you type)
List who will have control of, and act as custodian(s) for, data generated by the research activity:
N/A
(this box should expand as you type)
Give details of data storage arrangements, including where data will be stored, how long for, and in
what form. Will data be archived – if so how and if not why not.
N/A
(this box should expand as you type)
October 2016 10
Ethical Approval Form v4
Will the research activity involve storing personal data on one of the
following: YES NO
Manual files (i.e. in paper form)?
University computers?
Private company computers?
Home or other personal computers?
Laptop computers/ CDs/ Portable disk-drives/ memory sticks?
“Cloud” storage or websites?
Other – specify:
For all stored data, explain the measures in place to ensure data confidentiality, including details of
password protection, encryption and anonymisation:
N/A
(this box should expand as you type)
List all who will have access to the data generated by the research activity:
N/A
(this box should expand as you type)
List who will have control of, and act as custodian(s) for, data generated by the research activity:
N/A
(this box should expand as you type)
Give details of data storage arrangements, including where data will be stored, how long for, and in
what form. Will data be archived – if so how and if not why not.
N/A
(this box should expand as you type)
October 2016 10
Ethical Approval Form v4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
PG2 / E1 FORM
SECTION K: Declaration
The information which I have provided is correct and complete to the best of my knowledge. I have
attempted to identify any risks and issues related to the research activity and acknowledge my
obligations and the rights of the participants.
In submitting this application I hereby confirm that I undertake to ensure that the above named
research activity will meet the University’s Research Ethics and Integrity Code of Practice
Signature of applicant: Date:
Type the Ethics
form final
submission date
given in the
schedule.
For students:
Director of Studies/Supervisor: Type your supervisor’s name
Signature:
Date:
For staff:
Head of School/Assistant
Dean:
Signature:
Date:
Checklist: Please complete the checklist below to ensure that you have completed the form
according to the guidelines and attached any required documentation:
I have read the guidance notes supplied before completing the form.
I have completed ALL RELEVANT sections of the form in full.
I confirm that the research activity has received approval in principle
N/A I have attached a copy of final/interim approval from external organisation (where appropriate)
I understand that it is my responsibility to ensure that the above named research activity will
meet the University’s Research Ethics and Integrity Code of Practice.
N/A
I understand that before commencing data collection all documents aimed at respondents
(including information sheets, consent forms, questionnaires, interview schedules etc.) must
be confirmed by the DoS/Supervisor, module tutor or Head of School.
RESEARCH STUDENTS AND STAFF ONLY
All communications relating to this application during its processing must be in writing and emailed to
pgresearch@uwtsd.ac.uk , with the title ‘Ethical Approval’ followed by your name.
You will be informed of the outcome of your claim by email; therefore it is important that you check
October 2016 11
Ethical Approval Form v4
SECTION K: Declaration
The information which I have provided is correct and complete to the best of my knowledge. I have
attempted to identify any risks and issues related to the research activity and acknowledge my
obligations and the rights of the participants.
In submitting this application I hereby confirm that I undertake to ensure that the above named
research activity will meet the University’s Research Ethics and Integrity Code of Practice
Signature of applicant: Date:
Type the Ethics
form final
submission date
given in the
schedule.
For students:
Director of Studies/Supervisor: Type your supervisor’s name
Signature:
Date:
For staff:
Head of School/Assistant
Dean:
Signature:
Date:
Checklist: Please complete the checklist below to ensure that you have completed the form
according to the guidelines and attached any required documentation:
I have read the guidance notes supplied before completing the form.
I have completed ALL RELEVANT sections of the form in full.
I confirm that the research activity has received approval in principle
N/A I have attached a copy of final/interim approval from external organisation (where appropriate)
I understand that it is my responsibility to ensure that the above named research activity will
meet the University’s Research Ethics and Integrity Code of Practice.
N/A
I understand that before commencing data collection all documents aimed at respondents
(including information sheets, consent forms, questionnaires, interview schedules etc.) must
be confirmed by the DoS/Supervisor, module tutor or Head of School.
RESEARCH STUDENTS AND STAFF ONLY
All communications relating to this application during its processing must be in writing and emailed to
pgresearch@uwtsd.ac.uk , with the title ‘Ethical Approval’ followed by your name.
You will be informed of the outcome of your claim by email; therefore it is important that you check
October 2016 11
Ethical Approval Form v4

PG2 / E1 FORM
your University and personal email accounts regularly.
STUDENTS ON UNDERGRADUATE OR TAUGHT MASTERS PROGRAMMES should submit
this form (and receive the outcome) via systems explained to you by the supervisor/module leader.
This form is available electronically from the Academic Office web pages:
http://www.uwtsd.ac.uk/academic-office/
Application Process
All staff research projects and all research students must submit the Ethical Approval Form to the
University Ethics Committee via the Postgraduate Research office (pgresearch@uwtsd.ac.uk). Staff
research directly in relation to personal study for taught undergraduate or Masters programmes should be
submitted via the Faculty procedures explained below.
Taught masters and taught undergraduate research Ethical Approval Forms are considered within
Faculties. Faculties will provide details of the specific processes for this. Where the Ethical issues within
any single ethical application are of particular concern the Faculty will refer these to the University Ethics
Committee. Any student activity that involves the collection of primary data needs to undergo Ethical
approval, this includes assignment work as well as dissertations.
Notes for guidance in completion of this form
Section A: About You
Please complete all relevant sections
Section B: Approval for research activity
Research proposals must be approved in principle before applying for Ethical Approval. The proposal
approval only becomes final when the ethical approval is received.
The process for proposal approval varies according the individual and programme of study:
Research students, by application on form PG1 to the Research Degrees Committee
Taught students by review of research proposal within Faculties (Faculties provide specific details of
these processes)
Staff, by agreement by the Head of School/Assistant Dean
Section C: External Ethical Guidance materials
Many discipline areas are required to operate with the discipline specific codes of research ethics (for
example health, psychology, education etc.), any such codes must be listed and you must tick to confirm that
you have consulted with these.
Section D: External Collaborative Research Activity
Provide details of the external collaborative partners, where appropriate you might want to submit a copy of
the external collaboration agreement with the Ethical Approval Form. If the partner requires the research to
be subject to its own internal Ethical approval process then please provide details of that process and a copy
of any final (or interim) approvals received from the organisation.
Section E: Details of Research Activity
Remember that the individuals reviewing this Ethical Approval Form may not have seen your research
proposal, and also may not be experts in the specific area of your research. The information provided should
therefore be jargon free and clearly stated.
Indicative Title: please use the same title as used on the research proposal.
Purpose: the Ethical approval process will want to ensure that the methods you propose are adequate and
appropriate to address the research aims and objectives. Excessive additional data collection can be seen
as unethical.
Proposed Methods: the Ethical approval process seeks to ensure that you understand the methods that
are intended, and that the implementation of those methods will be appropriate and without unnecessary
impact on respondents. Please be specific.
Location: this needs to mention geographical location and also local situation (for example, within Local
Authority Offices in Cardiff, using a private room but close to other individuals). If you are collecting data
within an organisational setting then you need to explain the permissions that you have obtained to do this.
October 2016 12
Ethical Approval Form v4
your University and personal email accounts regularly.
STUDENTS ON UNDERGRADUATE OR TAUGHT MASTERS PROGRAMMES should submit
this form (and receive the outcome) via systems explained to you by the supervisor/module leader.
This form is available electronically from the Academic Office web pages:
http://www.uwtsd.ac.uk/academic-office/
Application Process
All staff research projects and all research students must submit the Ethical Approval Form to the
University Ethics Committee via the Postgraduate Research office (pgresearch@uwtsd.ac.uk). Staff
research directly in relation to personal study for taught undergraduate or Masters programmes should be
submitted via the Faculty procedures explained below.
Taught masters and taught undergraduate research Ethical Approval Forms are considered within
Faculties. Faculties will provide details of the specific processes for this. Where the Ethical issues within
any single ethical application are of particular concern the Faculty will refer these to the University Ethics
Committee. Any student activity that involves the collection of primary data needs to undergo Ethical
approval, this includes assignment work as well as dissertations.
Notes for guidance in completion of this form
Section A: About You
Please complete all relevant sections
Section B: Approval for research activity
Research proposals must be approved in principle before applying for Ethical Approval. The proposal
approval only becomes final when the ethical approval is received.
The process for proposal approval varies according the individual and programme of study:
Research students, by application on form PG1 to the Research Degrees Committee
Taught students by review of research proposal within Faculties (Faculties provide specific details of
these processes)
Staff, by agreement by the Head of School/Assistant Dean
Section C: External Ethical Guidance materials
Many discipline areas are required to operate with the discipline specific codes of research ethics (for
example health, psychology, education etc.), any such codes must be listed and you must tick to confirm that
you have consulted with these.
Section D: External Collaborative Research Activity
Provide details of the external collaborative partners, where appropriate you might want to submit a copy of
the external collaboration agreement with the Ethical Approval Form. If the partner requires the research to
be subject to its own internal Ethical approval process then please provide details of that process and a copy
of any final (or interim) approvals received from the organisation.
Section E: Details of Research Activity
Remember that the individuals reviewing this Ethical Approval Form may not have seen your research
proposal, and also may not be experts in the specific area of your research. The information provided should
therefore be jargon free and clearly stated.
Indicative Title: please use the same title as used on the research proposal.
Purpose: the Ethical approval process will want to ensure that the methods you propose are adequate and
appropriate to address the research aims and objectives. Excessive additional data collection can be seen
as unethical.
Proposed Methods: the Ethical approval process seeks to ensure that you understand the methods that
are intended, and that the implementation of those methods will be appropriate and without unnecessary
impact on respondents. Please be specific.
Location: this needs to mention geographical location and also local situation (for example, within Local
Authority Offices in Cardiff, using a private room but close to other individuals). If you are collecting data
within an organisational setting then you need to explain the permissions that you have obtained to do this.
October 2016 12
Ethical Approval Form v4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




