ETHICS FORM C: Finance Activities and Corporate Strategies Alignment
VerifiedAdded on 2019/12/03
|19
|5088
|1132
Homework Assignment
AI Summary
This assignment, submitted by a student, presents a comprehensive analysis of how financial activities can be aligned with corporate strategies to achieve long-term organizational goals. The research employs a qualitative approach, utilizing both primary and secondary data collected through questionnaires and literature review. The study focuses on UK retail organizations, examining the integration of financial functions with corporate strategies. The findings emphasize the importance of a competent finance workforce and the development of a long-term vision with performance indicators. The student's work also addresses ethical considerations, including the need for ethical review and adherence to data protection regulations. The assignment provides valuable insights into the strategic importance of finance within a corporate setting, emphasizing the necessity of aligning financial activities with broader organizational objectives for sustainable growth and success. The study concludes with practical recommendations for corporations to ensure proper alignment of financial function with corporate strategies.

ETHICS FORM C: FULL APPLICATION FORM
CORE APPLICATION FORM FOR ETHICAL REVIEW AND APPROVAL
OF WORK WHICH INVOLVES HUMAN PARTICIPANTS AND/OR GIVES RISE TO
ETHICAL ISSUES
Please note: the length of the form is designed to ensure coverage of all ethical aspects - if the
form is completed in full. If unsure of a particular question, then please refer to the Core
Checklist.
INTRODUCTION:
All undergraduate, postgraduate student or staff proposals for University work which involves
human participants and/or gives rise to ethical issues must be assessed for ethics approval,
whether it is for teaching, learning-related or research purposes. (The term "participant" is
used to cover any volunteers involved in the project, with the exclusion of the applicant and
his/her supervisor).
Applications should normally be submitted one month before the intended project start
date.
Please complete all sections of the form, as a word-processed document, marking as not
applicable [N/A] those questions which do not apply to your study. The spacing between
questions is for guidance only; you may need to type more text than is indicated by the
spaces.
Annexes to your signed application form for ethical review may include:
Letter of invitation to participate;
Information sheet/leaflet for all relevant parties, written in accessible/layperson’s
language which the recipients will understand;
The letter to parents/guardians, key carers/the Social Services to explain the project in
understandable language, and to request informed consent for participation;
Consent form for all relevant parties, including for head-teachers/parents where children
are involved to ensure full, informed consent is given [An example consent form is
included at the end of this form, and this should be followed as closely as possible];
Agreement from the manager of the agency/agencies/head-teacher involved, where
children/others within the care of the agency/agencies are involved;
Questionnaire/questions to be put to participants ;
Arrangements for confidentiality, security of data, and compliance with the data
protection legislation.
Submission of the Form:
Please ensure the form is signed by the applicant, the academic supervisor/term leader and
the Head of Department/School, before submission to the Secretary to your Sub-Committee.
Returned applications must be either typed or word-processed. It would assist members if
you could also forward your form to the Secretary as an e-mail attachment - it is understood
that this additional copy would be unsigned.
Referrals:
Here, and in any country where it is intended to undertake work in health/social care which
falls within the Department of Health’s Research Governance Framework for Health and
Social Care, in particular where it involves patients, tissue sampling, invasive procedures, or
any clinical trial, full prior permission must be sought and obtained from the NHS Research
Ethics Committee - using the NHS’ application form, to be submitted in compliance with the
NHS Regulations and Procedures (www.corec.org.uk) or its authorised equivalent.
The document: “Notes on Referrals to external Committees/organisations” gives further
examples of when applications for ethical review must be referred externally.
CORE APPLICATION FORM FOR ETHICAL REVIEW AND APPROVAL
OF WORK WHICH INVOLVES HUMAN PARTICIPANTS AND/OR GIVES RISE TO
ETHICAL ISSUES
Please note: the length of the form is designed to ensure coverage of all ethical aspects - if the
form is completed in full. If unsure of a particular question, then please refer to the Core
Checklist.
INTRODUCTION:
All undergraduate, postgraduate student or staff proposals for University work which involves
human participants and/or gives rise to ethical issues must be assessed for ethics approval,
whether it is for teaching, learning-related or research purposes. (The term "participant" is
used to cover any volunteers involved in the project, with the exclusion of the applicant and
his/her supervisor).
Applications should normally be submitted one month before the intended project start
date.
Please complete all sections of the form, as a word-processed document, marking as not
applicable [N/A] those questions which do not apply to your study. The spacing between
questions is for guidance only; you may need to type more text than is indicated by the
spaces.
Annexes to your signed application form for ethical review may include:
Letter of invitation to participate;
Information sheet/leaflet for all relevant parties, written in accessible/layperson’s
language which the recipients will understand;
The letter to parents/guardians, key carers/the Social Services to explain the project in
understandable language, and to request informed consent for participation;
Consent form for all relevant parties, including for head-teachers/parents where children
are involved to ensure full, informed consent is given [An example consent form is
included at the end of this form, and this should be followed as closely as possible];
Agreement from the manager of the agency/agencies/head-teacher involved, where
children/others within the care of the agency/agencies are involved;
Questionnaire/questions to be put to participants ;
Arrangements for confidentiality, security of data, and compliance with the data
protection legislation.
Submission of the Form:
Please ensure the form is signed by the applicant, the academic supervisor/term leader and
the Head of Department/School, before submission to the Secretary to your Sub-Committee.
Returned applications must be either typed or word-processed. It would assist members if
you could also forward your form to the Secretary as an e-mail attachment - it is understood
that this additional copy would be unsigned.
Referrals:
Here, and in any country where it is intended to undertake work in health/social care which
falls within the Department of Health’s Research Governance Framework for Health and
Social Care, in particular where it involves patients, tissue sampling, invasive procedures, or
any clinical trial, full prior permission must be sought and obtained from the NHS Research
Ethics Committee - using the NHS’ application form, to be submitted in compliance with the
NHS Regulations and Procedures (www.corec.org.uk) or its authorised equivalent.
The document: “Notes on Referrals to external Committees/organisations” gives further
examples of when applications for ethical review must be referred externally.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser


A copy of the application form to, and response from, the external ethical
committee/organisation, including the NHS Research Ethics Committee, should be lodged
with the secretary to the Ethics Advisory Sub-Committee and the Insurance Officer.
Please consult with the Research and Economic Development Support Services and the
Home Office Website before planning any work involving animals.
Insurance: Please consult as early as possible with the Insurance Officer, where, for
example: application is made to an external ethics committee/organisation, e.g. an NHS
Ethics Committee; where a project is likely to fall outside or require an extension to the
University existing insurance cover, (full details are available at:
http://www.dur.ac.uk/procurement.office/), where there is some significant Risk involved, or
where a funder/sponsor requires a particular insurance policy to be in place.
SECTION I: DESIGN AND CONDUCT OF THE STUDY
1.1 TITLE OF PROJECT:
Different ways through which finance activities can be aligned to the corporate
strategies for achieving long-term goals
1.2 TYPE OF PROJECT: whether research, teaching, learning, reach-out or consultancy:
Research
1.3 PRINCIPAL APPLICANT’S NAME, qualifications, post held, student/staff/other:
1.4 APPLICANT’S CONTACT INFORMATION: email address, department, contact
address, telephone number:
1.5 NAME of the SUPERVISOR or ACADEMIC-IN-CHARGE, with his/her e-mail address,
university department, contact address and telephone number:
1.6 LIST ALL CO-WORKERS, including collaborators/co-holders of funding/grants to be
used in/for this project, their: status, employer (and department) and research/relevant
experience:
1.7 ETHICAL REVIEW FOR CO-WORKERS: where co-workers are from another
department/institution, please indicate whether each has also applied for ethical
review, with details:
committee/organisation, including the NHS Research Ethics Committee, should be lodged
with the secretary to the Ethics Advisory Sub-Committee and the Insurance Officer.
Please consult with the Research and Economic Development Support Services and the
Home Office Website before planning any work involving animals.
Insurance: Please consult as early as possible with the Insurance Officer, where, for
example: application is made to an external ethics committee/organisation, e.g. an NHS
Ethics Committee; where a project is likely to fall outside or require an extension to the
University existing insurance cover, (full details are available at:
http://www.dur.ac.uk/procurement.office/), where there is some significant Risk involved, or
where a funder/sponsor requires a particular insurance policy to be in place.
SECTION I: DESIGN AND CONDUCT OF THE STUDY
1.1 TITLE OF PROJECT:
Different ways through which finance activities can be aligned to the corporate
strategies for achieving long-term goals
1.2 TYPE OF PROJECT: whether research, teaching, learning, reach-out or consultancy:
Research
1.3 PRINCIPAL APPLICANT’S NAME, qualifications, post held, student/staff/other:
1.4 APPLICANT’S CONTACT INFORMATION: email address, department, contact
address, telephone number:
1.5 NAME of the SUPERVISOR or ACADEMIC-IN-CHARGE, with his/her e-mail address,
university department, contact address and telephone number:
1.6 LIST ALL CO-WORKERS, including collaborators/co-holders of funding/grants to be
used in/for this project, their: status, employer (and department) and research/relevant
experience:
1.7 ETHICAL REVIEW FOR CO-WORKERS: where co-workers are from another
department/institution, please indicate whether each has also applied for ethical
review, with details:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

1.8 FUNDING: Please state the source and amount of funding for the work, for the
project applicant, and whether the study will result in financial payment or payment in
kind to the department, school or faculty.
1.9 SPONSOR: Please identify the Sponsor for the project, if applicable
NO
1.10 ABSTRACT OF THE PROJECT, including intended start and end dates:
The main aim of the current dissertation is to recognize different ways through which
financial activities can aligned to the corporate strategies with a particular aim of the
long term objectives. The study has been conducted by splitting the aim into four
distinct objectives with application of qualitative research. In this regard both primary
and secondary data have been collected in order to derive valid conclusion from
collected information. It has been found from the analysis chapter that integration of
both aspect proves to be effective in achieving organizational objectives. However,
department of finance should have inclusion of competent workforce who can
effectively coordinate with management in order to formulate effective strategy. It
enables company to create long run growth increased customer base. Furthermore,
suggestions given on the dissertation depict that corporation should have long term
vision with measurement of performance indicator. It will help to ensure proper
alignment of financial function with corporate strategies.
Start date: 6th October 2016 Expected End date: 20th October 2016
1.11 AIMS and OBJECTIVES: Please state the Project aims/objectives, any potential
value added for the participant group and/or society in general and, where applicable,
the Research Question, including, where appropriate, the hypothesis to be tested.
1.12 DESIGN OF STUDY and METHODOLOGY, in brief:
Type of investigation-Qualitative
Research design-Descriptive research design
Research approach- Inductive approach
Research philosophy-Interpretivism philosophy
Data collection-Primary (Questionnaire) and secondary (Journals, books and online
material)
Data analysis - Thematic analysis of qualitative technique
1.13 INTENDED LOCATION/S FOR THE STUDY, [particularly where the study is to
be conducted outside University Premises]:
Retail organizations of UK such as Tesco, Morrison’s, Asda and Waitrose as well as Sainsbury are
considered
1.14 STATISTICS
Has statistical advice been sought on study design?
YES NO NOT APPLICABLE
project applicant, and whether the study will result in financial payment or payment in
kind to the department, school or faculty.
1.9 SPONSOR: Please identify the Sponsor for the project, if applicable
NO
1.10 ABSTRACT OF THE PROJECT, including intended start and end dates:
The main aim of the current dissertation is to recognize different ways through which
financial activities can aligned to the corporate strategies with a particular aim of the
long term objectives. The study has been conducted by splitting the aim into four
distinct objectives with application of qualitative research. In this regard both primary
and secondary data have been collected in order to derive valid conclusion from
collected information. It has been found from the analysis chapter that integration of
both aspect proves to be effective in achieving organizational objectives. However,
department of finance should have inclusion of competent workforce who can
effectively coordinate with management in order to formulate effective strategy. It
enables company to create long run growth increased customer base. Furthermore,
suggestions given on the dissertation depict that corporation should have long term
vision with measurement of performance indicator. It will help to ensure proper
alignment of financial function with corporate strategies.
Start date: 6th October 2016 Expected End date: 20th October 2016
1.11 AIMS and OBJECTIVES: Please state the Project aims/objectives, any potential
value added for the participant group and/or society in general and, where applicable,
the Research Question, including, where appropriate, the hypothesis to be tested.
1.12 DESIGN OF STUDY and METHODOLOGY, in brief:
Type of investigation-Qualitative
Research design-Descriptive research design
Research approach- Inductive approach
Research philosophy-Interpretivism philosophy
Data collection-Primary (Questionnaire) and secondary (Journals, books and online
material)
Data analysis - Thematic analysis of qualitative technique
1.13 INTENDED LOCATION/S FOR THE STUDY, [particularly where the study is to
be conducted outside University Premises]:
Retail organizations of UK such as Tesco, Morrison’s, Asda and Waitrose as well as Sainsbury are
considered
1.14 STATISTICS
Has statistical advice been sought on study design?
YES NO NOT APPLICABLE
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

If YES, from whom? If NO, give reasons
▪ The requirement of statistical advice was not necessary in the study as it was
totally based on theoretical aspect.
1.15 RISKS AND HAZARDS: for the principal applicant/investigator:
Has a full risk assessment been carried out and agreed by the applicant’s
school/department relating to the principal applicant’s involvement? See Health and
Safety Manual, Section R2 and, where work is off-site, Section F1
YES/NO
1.16 EXPERT INDEPENDENT REVIEW: Please state who has conducted an expert
independent review of your proposed project, and his/her verdict. (For a student, this
will be your supervisor; for staff, the review may be by another member of your
department.)
Supervisor
1.17 CONSENT: Please give details of any other consents applied for and/or obtained
from: NHS Local Research Committees in this country, or their equivalent overseas,
for medical/clinical projects etc. and from other relevant organisations, and attach
copies of any relevant application forms submitted and decision letter/s
received.
No
1.18 INSURANCE: please confirm that, where necessary, a copy of the application form
has been lodged with the University’s Insurance Officer: [where, for example:
application is made to an external ethics committee/organisation, including an NHS
Ethics Committee; where a project is likely to fall outside or require an extension to
the University existing insurance cover, (full details are available at:
http://www.dur.ac.uk/procurement.office/), where there is some significant Risk
involved, or where a funder/sponsor requires a particular insurance policy to be in
place.]
YES/NO
1.19 MONITORING: are arrangements in place for monitoring the conduct of the project?
Yes
1.20 PROPOSED ROUTES OF PUBLICATION – where applicable/relevant (for students,
this may be by dissertation; for staff: an indication of the type of publication
envisaged)
▪ The requirement of statistical advice was not necessary in the study as it was
totally based on theoretical aspect.
1.15 RISKS AND HAZARDS: for the principal applicant/investigator:
Has a full risk assessment been carried out and agreed by the applicant’s
school/department relating to the principal applicant’s involvement? See Health and
Safety Manual, Section R2 and, where work is off-site, Section F1
YES/NO
1.16 EXPERT INDEPENDENT REVIEW: Please state who has conducted an expert
independent review of your proposed project, and his/her verdict. (For a student, this
will be your supervisor; for staff, the review may be by another member of your
department.)
Supervisor
1.17 CONSENT: Please give details of any other consents applied for and/or obtained
from: NHS Local Research Committees in this country, or their equivalent overseas,
for medical/clinical projects etc. and from other relevant organisations, and attach
copies of any relevant application forms submitted and decision letter/s
received.
No
1.18 INSURANCE: please confirm that, where necessary, a copy of the application form
has been lodged with the University’s Insurance Officer: [where, for example:
application is made to an external ethics committee/organisation, including an NHS
Ethics Committee; where a project is likely to fall outside or require an extension to
the University existing insurance cover, (full details are available at:
http://www.dur.ac.uk/procurement.office/), where there is some significant Risk
involved, or where a funder/sponsor requires a particular insurance policy to be in
place.]
YES/NO
1.19 MONITORING: are arrangements in place for monitoring the conduct of the project?
Yes
1.20 PROPOSED ROUTES OF PUBLICATION – where applicable/relevant (for students,
this may be by dissertation; for staff: an indication of the type of publication
envisaged)

SECTION II: RECRUITMENT OF PROJECT PARTICIPANTS
2.1 Who are the Participants?
Please tick/give further details as appropriate:
Y/N/further details
Male
Female
Children under 18
Children in care
Individuals with a learning disability
Individuals suffering from dementia
Prisoners
Young offenders (16-21 years old)
Individuals in Care Homes
Elderly persons
Individuals without legal capacity to consent
Other Vulnerable Groups
Specific Ethnic Groups
Undergraduate Students
Postgraduate Students
Staff Yes
2.2 If students: course, year, size of groups, and % of students involved
(Names of students may be required subsequently)
2.3 How many participants are to be recruited – please give either an exact figure or a
maximum
Total 10 respondents have been selected.
2.4 Selection Criteria e.g. age, male/female/using the categories at (a) above?
There was no such criteria.
2.5 SAMPLE SIZE: Please describe the statistical/other rationale for the sample
size/number of participants to be used in this study and how the study size will yield
meaningful results.
10 respondents have been selected. These nare selected as per the purposive sampling
where purpose of research is already communicated to them. It facilitates in deriving
relevant data in less time and fulfils the purpose of the study in right way.
2.6 How are the participants to be recruited – by letter/invitation? (Please provide a copy)
Invitation through phone call
2.7 INTERNET: For projects conducted over the internet, please advise on precautions
taken to comply with relevant jurisdictions, ISP regulations and to protect participants’
well-being.
2.8 Is there any link with the investigator (student, friend, etc.)?
2.1 Who are the Participants?
Please tick/give further details as appropriate:
Y/N/further details
Male
Female
Children under 18
Children in care
Individuals with a learning disability
Individuals suffering from dementia
Prisoners
Young offenders (16-21 years old)
Individuals in Care Homes
Elderly persons
Individuals without legal capacity to consent
Other Vulnerable Groups
Specific Ethnic Groups
Undergraduate Students
Postgraduate Students
Staff Yes
2.2 If students: course, year, size of groups, and % of students involved
(Names of students may be required subsequently)
2.3 How many participants are to be recruited – please give either an exact figure or a
maximum
Total 10 respondents have been selected.
2.4 Selection Criteria e.g. age, male/female/using the categories at (a) above?
There was no such criteria.
2.5 SAMPLE SIZE: Please describe the statistical/other rationale for the sample
size/number of participants to be used in this study and how the study size will yield
meaningful results.
10 respondents have been selected. These nare selected as per the purposive sampling
where purpose of research is already communicated to them. It facilitates in deriving
relevant data in less time and fulfils the purpose of the study in right way.
2.6 How are the participants to be recruited – by letter/invitation? (Please provide a copy)
Invitation through phone call
2.7 INTERNET: For projects conducted over the internet, please advise on precautions
taken to comply with relevant jurisdictions, ISP regulations and to protect participants’
well-being.
2.8 Is there any link with the investigator (student, friend, etc.)?
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

No
2.9 If there is a link, what safeguards to preserve objectivity and transparency and to
prevent conflicts of interest are in place?
No
2.10 Are any participants likely to be pregnant, or would pregnant women be excluded?
No
SECTION III: CARE AND PROTECTION OF PARTICIPANTS
3.1 Please describe briefly what will happen to the participants – interviews, questionnaires,
the anticipated duration of each, number of sessions, intervals between sessions.
questionnaires,
3.2 Will any financial or other payment be offered or given to participants? [Please note:
usually no payment must serve as an inducement to participate. Travelling, out of
pocket expenses and loss of earnings may be reimbursed. In addition to this, in
experiments in economics and psychology in particular it is common to pay
participants. Provided such payments are within the normal parameters of the
discipline, some inducement to participate is assumed and is acceptable]
No and it is the reason that questionnaire method has been selected so that
employees do not require to devote much of their time.
3.3 ARE SUBSTANCES TO BE GIVEN TO PARTICIPANTS?
YES/NO
If YES -- complete Appendix A
3.4 ARE SAMPLES TO BE TAKEN FROM PARTICIPANTS?
YES/NO
If YES -- complete Appendix A
3.5 ARE OTHER PROCEDURES TO BE APPLIED i.e. A QUESTIONNAIRE, PAPER OR
OTHER TOOL?
YES/NO
If YES -- complete Appendix A, including a copy of your questionnaire
3.6 DETAILS OF DRUGS AND MATERIALS TO BE USED (name of compound and dosage
where appropriate - full details to be given in Appendix (A) with details of NHS
LREC/equivalent consent sought and obtained)
NO drug material used
2.9 If there is a link, what safeguards to preserve objectivity and transparency and to
prevent conflicts of interest are in place?
No
2.10 Are any participants likely to be pregnant, or would pregnant women be excluded?
No
SECTION III: CARE AND PROTECTION OF PARTICIPANTS
3.1 Please describe briefly what will happen to the participants – interviews, questionnaires,
the anticipated duration of each, number of sessions, intervals between sessions.
questionnaires,
3.2 Will any financial or other payment be offered or given to participants? [Please note:
usually no payment must serve as an inducement to participate. Travelling, out of
pocket expenses and loss of earnings may be reimbursed. In addition to this, in
experiments in economics and psychology in particular it is common to pay
participants. Provided such payments are within the normal parameters of the
discipline, some inducement to participate is assumed and is acceptable]
No and it is the reason that questionnaire method has been selected so that
employees do not require to devote much of their time.
3.3 ARE SUBSTANCES TO BE GIVEN TO PARTICIPANTS?
YES/NO
If YES -- complete Appendix A
3.4 ARE SAMPLES TO BE TAKEN FROM PARTICIPANTS?
YES/NO
If YES -- complete Appendix A
3.5 ARE OTHER PROCEDURES TO BE APPLIED i.e. A QUESTIONNAIRE, PAPER OR
OTHER TOOL?
YES/NO
If YES -- complete Appendix A, including a copy of your questionnaire
3.6 DETAILS OF DRUGS AND MATERIALS TO BE USED (name of compound and dosage
where appropriate - full details to be given in Appendix (A) with details of NHS
LREC/equivalent consent sought and obtained)
NO drug material used
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
3.7 CONTROLS [In quantitative research: a group of participants who receive a different
comparator treatment/experience – or if none is available, no treatment/] (if needed?).
If so, how many, who are they, how recruited/selected?
It was qualitative study
3.8 RISKS AND HAZARDS
Consider the ways in which the participant/s might be harmed (the hazards). Assess
the risks (likelihood of the harm being realised and the severity of the outcome) using
the principles described in the Health and Safety Manual Section R2 Complete the
following table; or append a completed general risk assessment form (Appendix 5 to
Section R2 of the Health and Safety Manual)
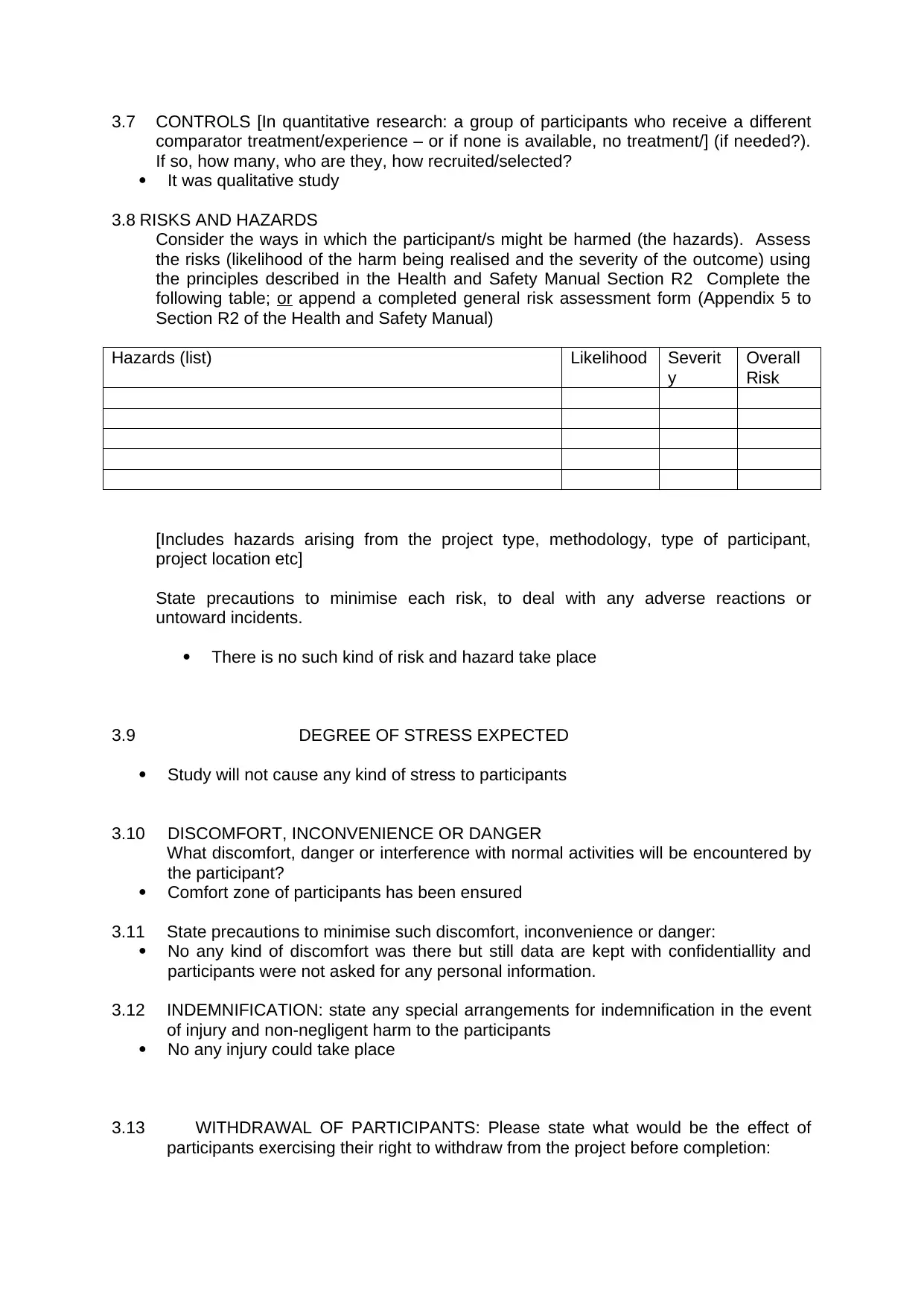
Hazards (list) Likelihood Severit
y
Overall
Risk
[Includes hazards arising from the project type, methodology, type of participant,
project location etc]
State precautions to minimise each risk, to deal with any adverse reactions or
untoward incidents.
There is no such kind of risk and hazard take place
3.9 DEGREE OF STRESS EXPECTED
Study will not cause any kind of stress to participants
3.10 DISCOMFORT, INCONVENIENCE OR DANGER
What discomfort, danger or interference with normal activities will be encountered by
the participant?
Comfort zone of participants has been ensured
3.11 State precautions to minimise such discomfort, inconvenience or danger:
No any kind of discomfort was there but still data are kept with confidentiallity and
participants were not asked for any personal information.
3.12 INDEMNIFICATION: state any special arrangements for indemnification in the event
of injury and non-negligent harm to the participants
No any injury could take place
3.13 WITHDRAWAL OF PARTICIPANTS: Please state what would be the effect of
participants exercising their right to withdraw from the project before completion:
comparator treatment/experience – or if none is available, no treatment/] (if needed?).
If so, how many, who are they, how recruited/selected?
It was qualitative study
3.8 RISKS AND HAZARDS
Consider the ways in which the participant/s might be harmed (the hazards). Assess
the risks (likelihood of the harm being realised and the severity of the outcome) using
the principles described in the Health and Safety Manual Section R2 Complete the
following table; or append a completed general risk assessment form (Appendix 5 to
Section R2 of the Health and Safety Manual)
Hazards (list) Likelihood Severit
y
Overall
Risk
[Includes hazards arising from the project type, methodology, type of participant,
project location etc]
State precautions to minimise each risk, to deal with any adverse reactions or
untoward incidents.
There is no such kind of risk and hazard take place
3.9 DEGREE OF STRESS EXPECTED
Study will not cause any kind of stress to participants
3.10 DISCOMFORT, INCONVENIENCE OR DANGER
What discomfort, danger or interference with normal activities will be encountered by
the participant?
Comfort zone of participants has been ensured
3.11 State precautions to minimise such discomfort, inconvenience or danger:
No any kind of discomfort was there but still data are kept with confidentiallity and
participants were not asked for any personal information.
3.12 INDEMNIFICATION: state any special arrangements for indemnification in the event
of injury and non-negligent harm to the participants
No any injury could take place
3.13 WITHDRAWAL OF PARTICIPANTS: Please state what would be the effect of
participants exercising their right to withdraw from the project before completion:

▪ Participants are selected with prior information and their involvement is based
on their consent. Hence situation of withdrawal cannot take place.
SECTION IV: INFORMED CONSENT PROCESS
4.1 Who will explain the investigation to the participant?
Researcher
4.2 Will a full written information sheet written in layman's language which the participant will
understand be given to the participant before consent is requested, and therefore
before the project starts, to read and retain?
Yes
4.3 Will written consent be obtained? This is the normal expectation, therefore if your
response is that you do not intend to obtain written consent, please explain in detail
Yes
4.4 How and where will consent be recorded? Where schoolchildren/minors/persons with a
mental incapacity are involved, there should be full details of your procedures for
ensuring their and, as appropriate, their teachers’ and/or parent’s, and where
applicable, carer’s informed written consent would be given before participation
commences. [Please note that at present there may not be a person with legal
authority to consent on behalf of an adult who lacks legal capacity to consent].
Please attach copies of written consent.
Oral consent will be taken through phone call
4.5 In the case of longitudinal studies, how will participants be reminded of the need to
consent/how will that consent be renegotiated?
on their consent. Hence situation of withdrawal cannot take place.
SECTION IV: INFORMED CONSENT PROCESS
4.1 Who will explain the investigation to the participant?
Researcher
4.2 Will a full written information sheet written in layman's language which the participant will
understand be given to the participant before consent is requested, and therefore
before the project starts, to read and retain?
Yes
4.3 Will written consent be obtained? This is the normal expectation, therefore if your
response is that you do not intend to obtain written consent, please explain in detail
Yes
4.4 How and where will consent be recorded? Where schoolchildren/minors/persons with a
mental incapacity are involved, there should be full details of your procedures for
ensuring their and, as appropriate, their teachers’ and/or parent’s, and where
applicable, carer’s informed written consent would be given before participation
commences. [Please note that at present there may not be a person with legal
authority to consent on behalf of an adult who lacks legal capacity to consent].
Please attach copies of written consent.
Oral consent will be taken through phone call
4.5 In the case of longitudinal studies, how will participants be reminded of the need to
consent/how will that consent be renegotiated?
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
SECTION V: PARTICIPANT INFORMATION SHEET
5.1 Please attach a copy of the information sheet to your form, or advise on why one is not
to be used. Where schoolchildren/minors/vulnerable people are involved, there
should also be an information sheet directed at the teachers,
parents/guardians/carers. The information sheet should be written in simple
language, which the participants will understand, in translation and/or with
illustrations where necessary, on appropriately headed paper.
Please complete the table below to show whether your information sheet contains the
following information:
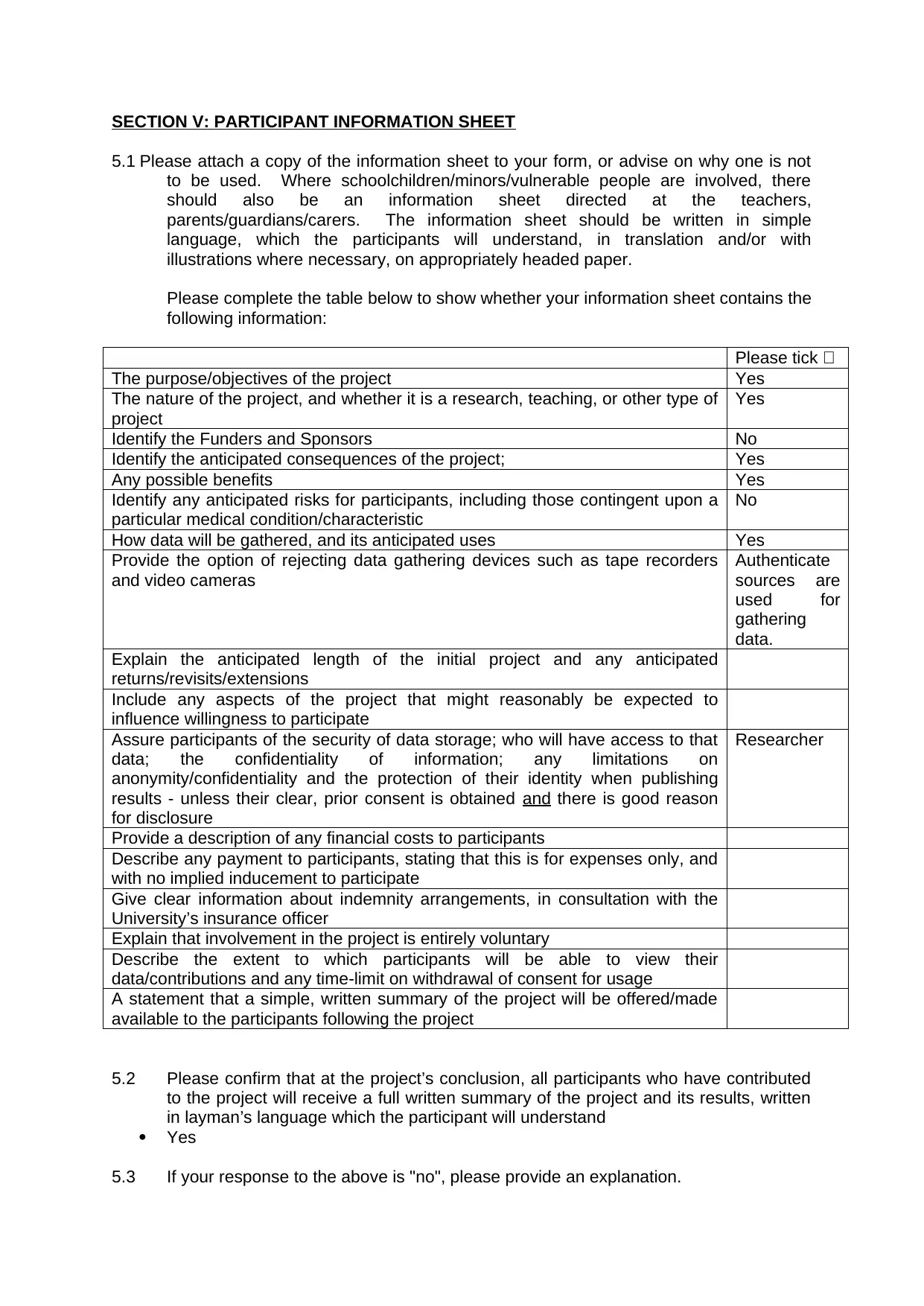
Please tick
The purpose/objectives of the project Yes
The nature of the project, and whether it is a research, teaching, or other type of
project
Yes
Identify the Funders and Sponsors No
Identify the anticipated consequences of the project; Yes
Any possible benefits Yes
Identify any anticipated risks for participants, including those contingent upon a
particular medical condition/characteristic
No
How data will be gathered, and its anticipated uses Yes
Provide the option of rejecting data gathering devices such as tape recorders
and video cameras
Authenticate
sources are
used for
gathering
data.
Explain the anticipated length of the initial project and any anticipated
returns/revisits/extensions
Include any aspects of the project that might reasonably be expected to
influence willingness to participate
Assure participants of the security of data storage; who will have access to that
data; the confidentiality of information; any limitations on
anonymity/confidentiality and the protection of their identity when publishing
results - unless their clear, prior consent is obtained and there is good reason
for disclosure
Researcher
Provide a description of any financial costs to participants
Describe any payment to participants, stating that this is for expenses only, and
with no implied inducement to participate
Give clear information about indemnity arrangements, in consultation with the
University’s insurance officer
Explain that involvement in the project is entirely voluntary
Describe the extent to which participants will be able to view their
data/contributions and any time-limit on withdrawal of consent for usage
A statement that a simple, written summary of the project will be offered/made
available to the participants following the project
5.2 Please confirm that at the project’s conclusion, all participants who have contributed
to the project will receive a full written summary of the project and its results, written
in layman’s language which the participant will understand
Yes
5.3 If your response to the above is "no", please provide an explanation.
5.1 Please attach a copy of the information sheet to your form, or advise on why one is not
to be used. Where schoolchildren/minors/vulnerable people are involved, there
should also be an information sheet directed at the teachers,
parents/guardians/carers. The information sheet should be written in simple
language, which the participants will understand, in translation and/or with
illustrations where necessary, on appropriately headed paper.
Please complete the table below to show whether your information sheet contains the
following information:
Please tick
The purpose/objectives of the project Yes
The nature of the project, and whether it is a research, teaching, or other type of
project
Yes
Identify the Funders and Sponsors No
Identify the anticipated consequences of the project; Yes
Any possible benefits Yes
Identify any anticipated risks for participants, including those contingent upon a
particular medical condition/characteristic
No
How data will be gathered, and its anticipated uses Yes
Provide the option of rejecting data gathering devices such as tape recorders
and video cameras
Authenticate
sources are
used for
gathering
data.
Explain the anticipated length of the initial project and any anticipated
returns/revisits/extensions
Include any aspects of the project that might reasonably be expected to
influence willingness to participate
Assure participants of the security of data storage; who will have access to that
data; the confidentiality of information; any limitations on
anonymity/confidentiality and the protection of their identity when publishing
results - unless their clear, prior consent is obtained and there is good reason
for disclosure
Researcher
Provide a description of any financial costs to participants
Describe any payment to participants, stating that this is for expenses only, and
with no implied inducement to participate
Give clear information about indemnity arrangements, in consultation with the
University’s insurance officer
Explain that involvement in the project is entirely voluntary
Describe the extent to which participants will be able to view their
data/contributions and any time-limit on withdrawal of consent for usage
A statement that a simple, written summary of the project will be offered/made
available to the participants following the project
5.2 Please confirm that at the project’s conclusion, all participants who have contributed
to the project will receive a full written summary of the project and its results, written
in layman’s language which the participant will understand
Yes
5.3 If your response to the above is "no", please provide an explanation.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser


SECTION VI: CONFIDENTIALITY AND DATA PROTECTION
6.1 Please indicate what steps will be taken to safeguard the anonymity and
confidentiality of the participant’s records [whether the records are of paper, tape
recordings, video recordings...], and confirm that the requirements of the Data
Protection Acts will be complied with.
Collected data are stored in excel sheet which is locked. It is accessed only by
researcher.
6.2 Will tape or video recordings and any written transcriptions from these be destroyed at
the end of the project?
YES / NO
6.3 If NOT to be destroyed: what further use do you intend to make of the recordings and
what arrangements will be made for their secure storage?
6.4 Who will have access to the stored data, to whom would it be publicised?
Researcher
6.5 Will consent be specifically requested for the present and anticipated future use?
[Essential if the data identifies any participant]
YES/NO
If your response is "no", please give reasons:
6.6 For projects other than those conducted using patient records, will participants be
informed that, particular configurations of data may still, despite all measures, identify
an individual? And that, save for a very few exceptional circumstances, research
data given in confidence may not enjoy legal privilege, and may be liable to sub
poena by a court?
SECTION VII: COMMUNITY CONSIDERATIONS
7.1 COMMUNITY CONSIDERATIONS: please describe the suitability of the location, of the
applicant for work in that location; the anticipated impact on the local community;
consultations with the community; how the community will be informed of the project
6.1 Please indicate what steps will be taken to safeguard the anonymity and
confidentiality of the participant’s records [whether the records are of paper, tape
recordings, video recordings...], and confirm that the requirements of the Data
Protection Acts will be complied with.
Collected data are stored in excel sheet which is locked. It is accessed only by
researcher.
6.2 Will tape or video recordings and any written transcriptions from these be destroyed at
the end of the project?
YES / NO
6.3 If NOT to be destroyed: what further use do you intend to make of the recordings and
what arrangements will be made for their secure storage?
6.4 Who will have access to the stored data, to whom would it be publicised?
Researcher
6.5 Will consent be specifically requested for the present and anticipated future use?
[Essential if the data identifies any participant]
YES/NO
If your response is "no", please give reasons:
6.6 For projects other than those conducted using patient records, will participants be
informed that, particular configurations of data may still, despite all measures, identify
an individual? And that, save for a very few exceptional circumstances, research
data given in confidence may not enjoy legal privilege, and may be liable to sub
poena by a court?
SECTION VII: COMMUNITY CONSIDERATIONS
7.1 COMMUNITY CONSIDERATIONS: please describe the suitability of the location, of the
applicant for work in that location; the anticipated impact on the local community;
consultations with the community; how the community will be informed of the project
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 19
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





