CHEM101 Lab: Experiment 8 - Heat Capacity and Enthalpy Changes
VerifiedAdded on 2021/12/15
|6
|501
|59
Practical Assignment
AI Summary
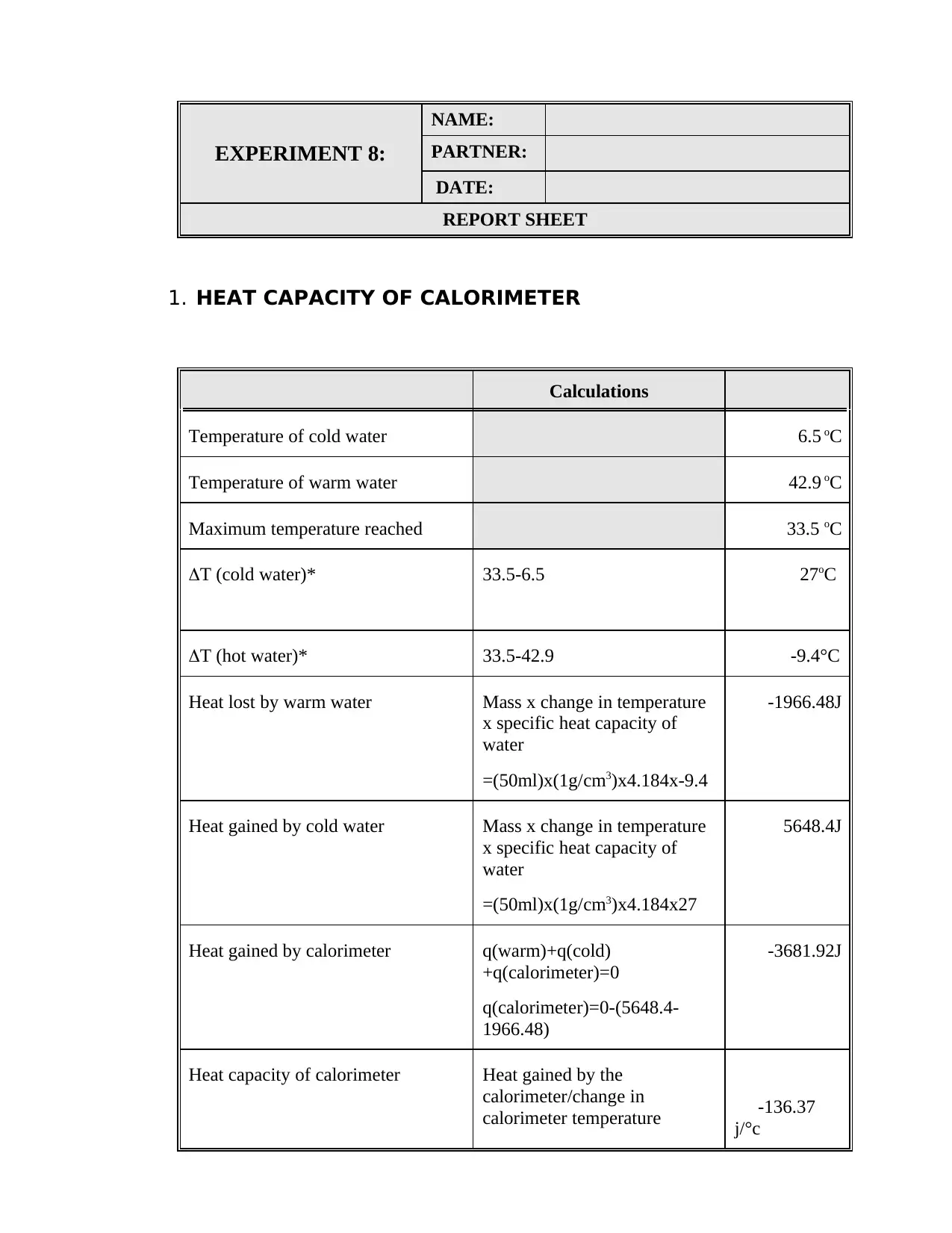
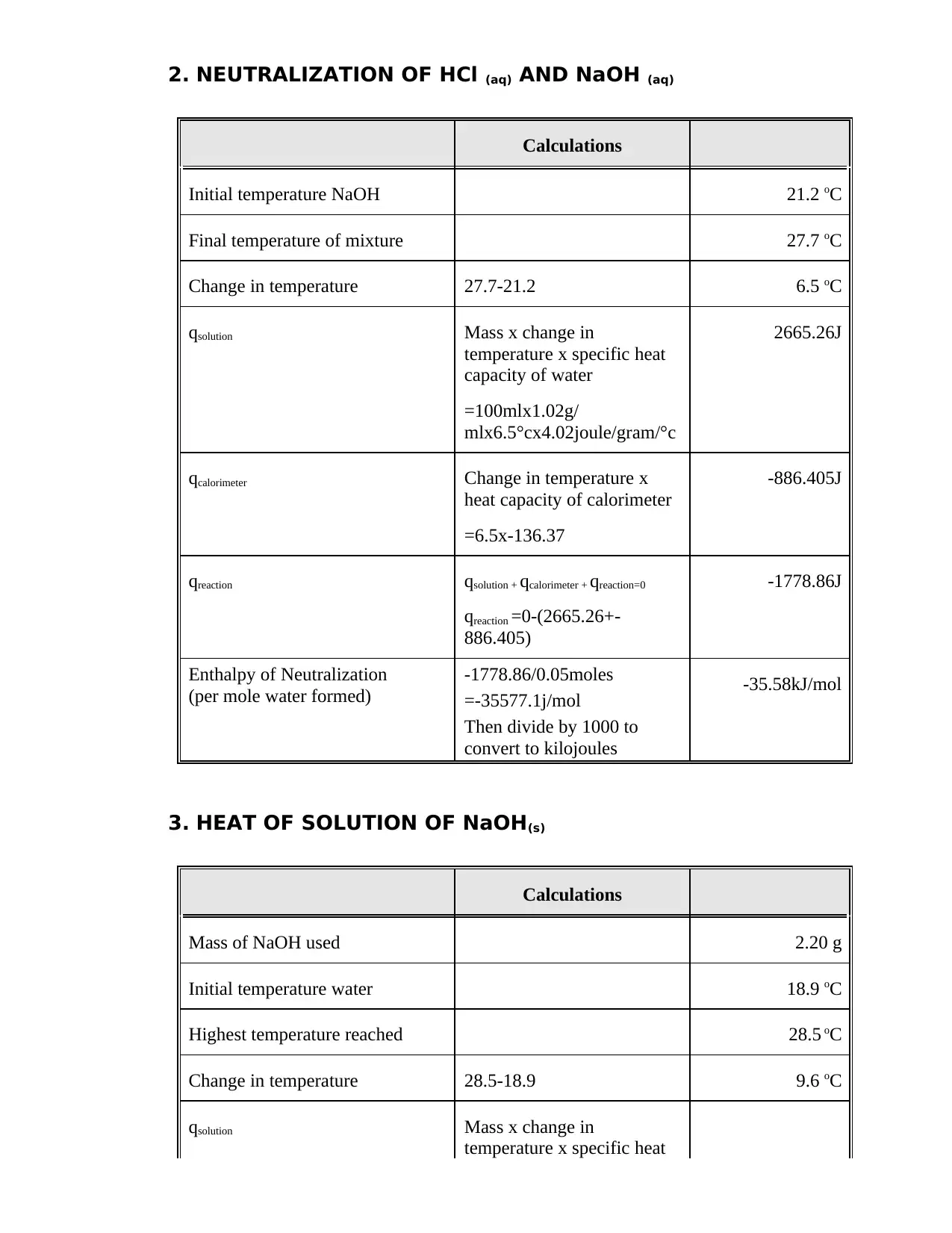
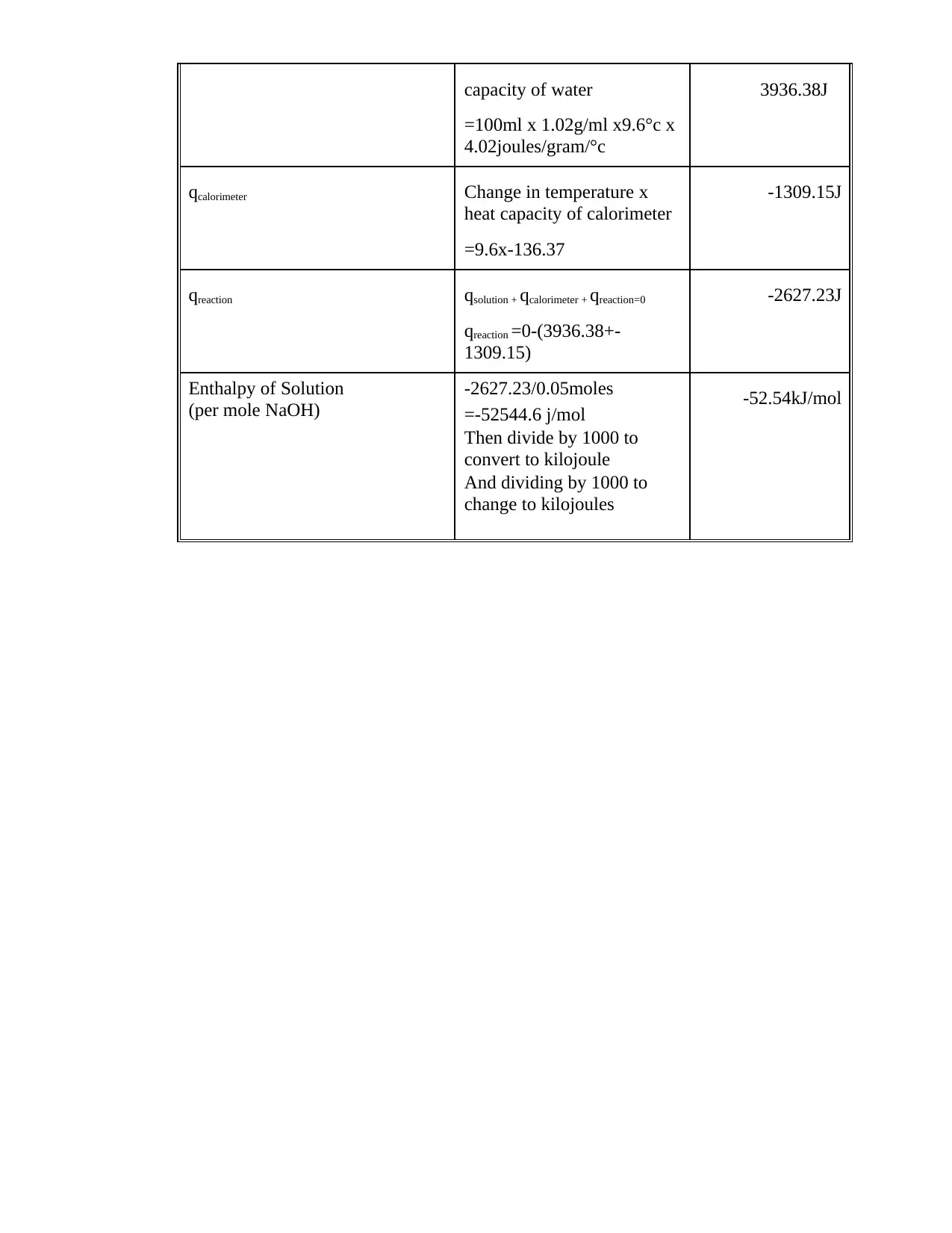
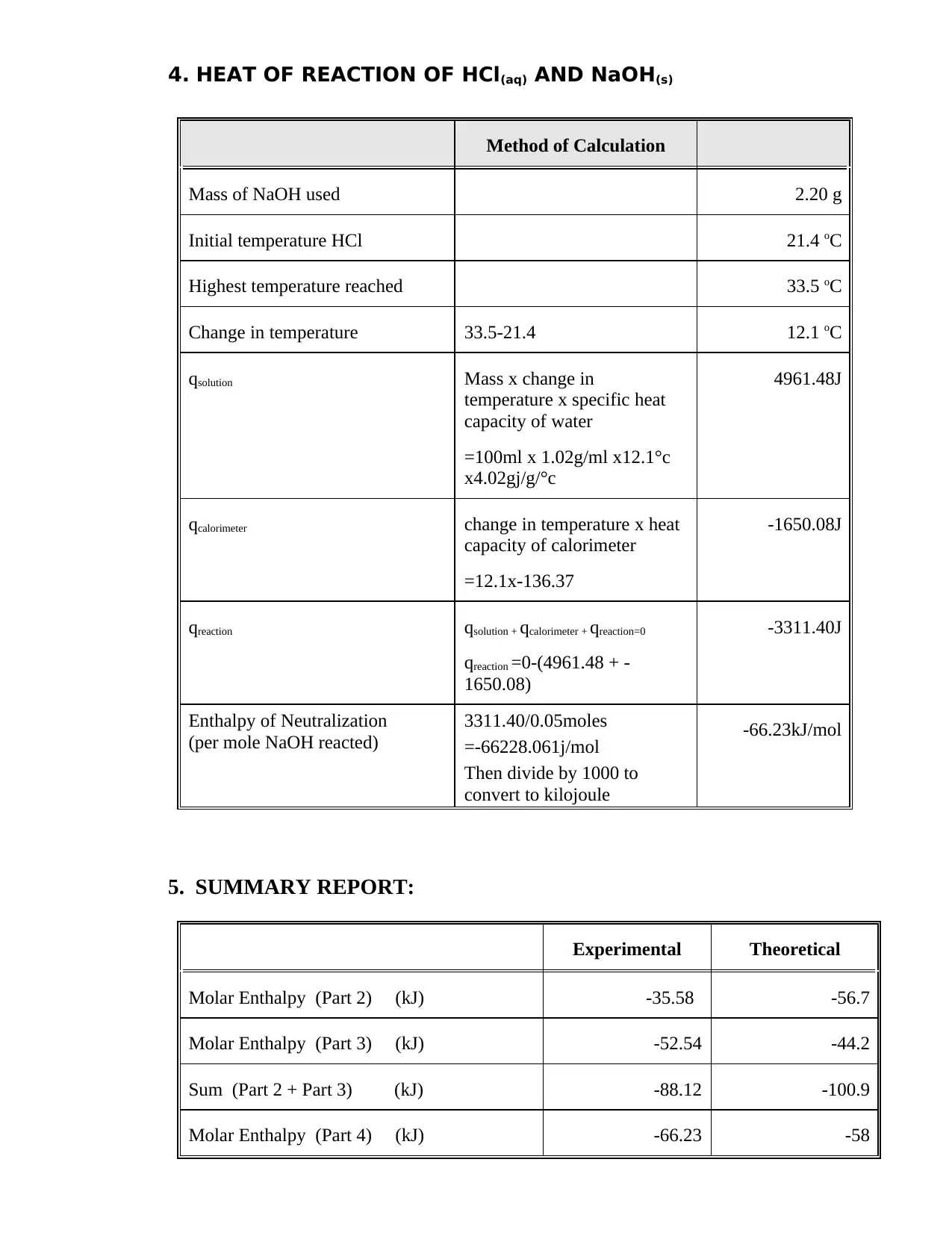
This document is a student's lab report detailing Experiment 8, which focuses on calorimetric measurements and thermochemical calculations. The report includes calculations for the heat capacity of a calorimeter, the enthalpy of neutralization of HCl and NaOH, and the heat of solution of NaOH. The student performed experiments, recorded data, and computed the heat changes (q) for each process, as well as the enthalpy changes (ΔH) per mole of water formed or NaOH reacted. The report includes a summary table comparing the experimental and theoretical molar enthalpies for the reactions. Calculations involve the use of the formula: q = m x c x ΔT, where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature. The final results are presented in kJ/mol.
1 out of 6






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)