Cancer Biology: Experimental Design Report on BKLY Protein's Role
VerifiedAdded on 2023/06/15
|8
|1621
|317
Report
AI Summary
This report outlines experimental designs to investigate the apoptotic properties of a novel protein, BKLY, identified with structural similarity to Bcl-2 family proteins. The experiments aim to confirm plasmid uptake in cells, demonstrate protein expression, and determine whether BKLY possesses pro- or anti-apoptotic properties. Three methods, including MTT assay for transformed cell identification, Annexin V staining assay for protein expression, and flow cytometry for apoptotic property identification, are proposed to provide comprehensive evidence. Controls are incorporated in each experiment to ensure accurate data analysis and conclusions regarding BKLY's role in apoptosis. This resource is available on Desklib, a platform offering study tools and solved assignments for students.

Cancer Biology Experimental Design Report
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Contents
Introduction:...............................................................................................................................2
Experiments:..............................................................................................................................3
Identification of transformed cells: MTT assay.....................................................................3
Identification of cells showing protein expression: Annexin V staining assay......................3
Identification of nature of apoptotic properties: Flow Cytometry..........................................4
References:.................................................................................................................................5
Introduction:...............................................................................................................................2
Experiments:..............................................................................................................................3
Identification of transformed cells: MTT assay.....................................................................3
Identification of cells showing protein expression: Annexin V staining assay......................3
Identification of nature of apoptotic properties: Flow Cytometry..........................................4
References:.................................................................................................................................5

Introduction:
The appearance of characteristic morphological features, and the occurrence of specific
energy dependant biochemical mechanisms which indicate towards the process of
programmed cell death, are referred to as apoptosis. It is responsible for a number of
biological functions such as normal cell turnover, appropriate development of the immune
system, development of embryo, hormonal atrophy, and chemically induced cell death
(Elmore 2007). This process also helps in maintaining tissue homeostasis, and forms normal
part of growth and development events. Also, appropriate apoptosis involves differential and
appropriate response of the different types of cells to physiological and pathological stimuli.
However, any disturbance in the homeostatic equilibrium could either result in unwarranted
cellular proliferation or atrophy characterized with faster cellular death (Hejmadi 2009).
Therefore, the dysfunction of the homeostatic pathways, often leads to terminal proliferation
and differentiation of cells, resulting in cancer. The development and progression of cancers
have often been attributed to the suppressed apoptotic mechanisms during carcinogenesis.
The tumour cells either acquire resistance to apoptotic pathways by the means of anti-
apoptotic proteins, or evade the immune surveillance (Fulda 2009). For example, the
uncontrolled expression of Bcl-2 gene results in failure of cell death, contributing to cancer.
However, apoptosis also presents therapeutic opportunities for cancer. The comprehension
and analysis of the cell cycle signalling pathways could help identify the possible mechanism
of cell cycle which could be exploited for arresting or controlling the cell cycle and apoptotic
mechanisms (Gerl & Vaux 2005). The table below shows the features of cells undergoing
apoptosis.
Morphological features Biochemical features Physiological significance
Blebbing of membranes
Chromatin aggregation
Tightly regulated stepwise
activation of enzymatic
Absent inflammatory
The appearance of characteristic morphological features, and the occurrence of specific
energy dependant biochemical mechanisms which indicate towards the process of
programmed cell death, are referred to as apoptosis. It is responsible for a number of
biological functions such as normal cell turnover, appropriate development of the immune
system, development of embryo, hormonal atrophy, and chemically induced cell death
(Elmore 2007). This process also helps in maintaining tissue homeostasis, and forms normal
part of growth and development events. Also, appropriate apoptosis involves differential and
appropriate response of the different types of cells to physiological and pathological stimuli.
However, any disturbance in the homeostatic equilibrium could either result in unwarranted
cellular proliferation or atrophy characterized with faster cellular death (Hejmadi 2009).
Therefore, the dysfunction of the homeostatic pathways, often leads to terminal proliferation
and differentiation of cells, resulting in cancer. The development and progression of cancers
have often been attributed to the suppressed apoptotic mechanisms during carcinogenesis.
The tumour cells either acquire resistance to apoptotic pathways by the means of anti-
apoptotic proteins, or evade the immune surveillance (Fulda 2009). For example, the
uncontrolled expression of Bcl-2 gene results in failure of cell death, contributing to cancer.
However, apoptosis also presents therapeutic opportunities for cancer. The comprehension
and analysis of the cell cycle signalling pathways could help identify the possible mechanism
of cell cycle which could be exploited for arresting or controlling the cell cycle and apoptotic
mechanisms (Gerl & Vaux 2005). The table below shows the features of cells undergoing
apoptosis.
Morphological features Biochemical features Physiological significance
Blebbing of membranes
Chromatin aggregation
Tightly regulated stepwise
activation of enzymatic
Absent inflammatory
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
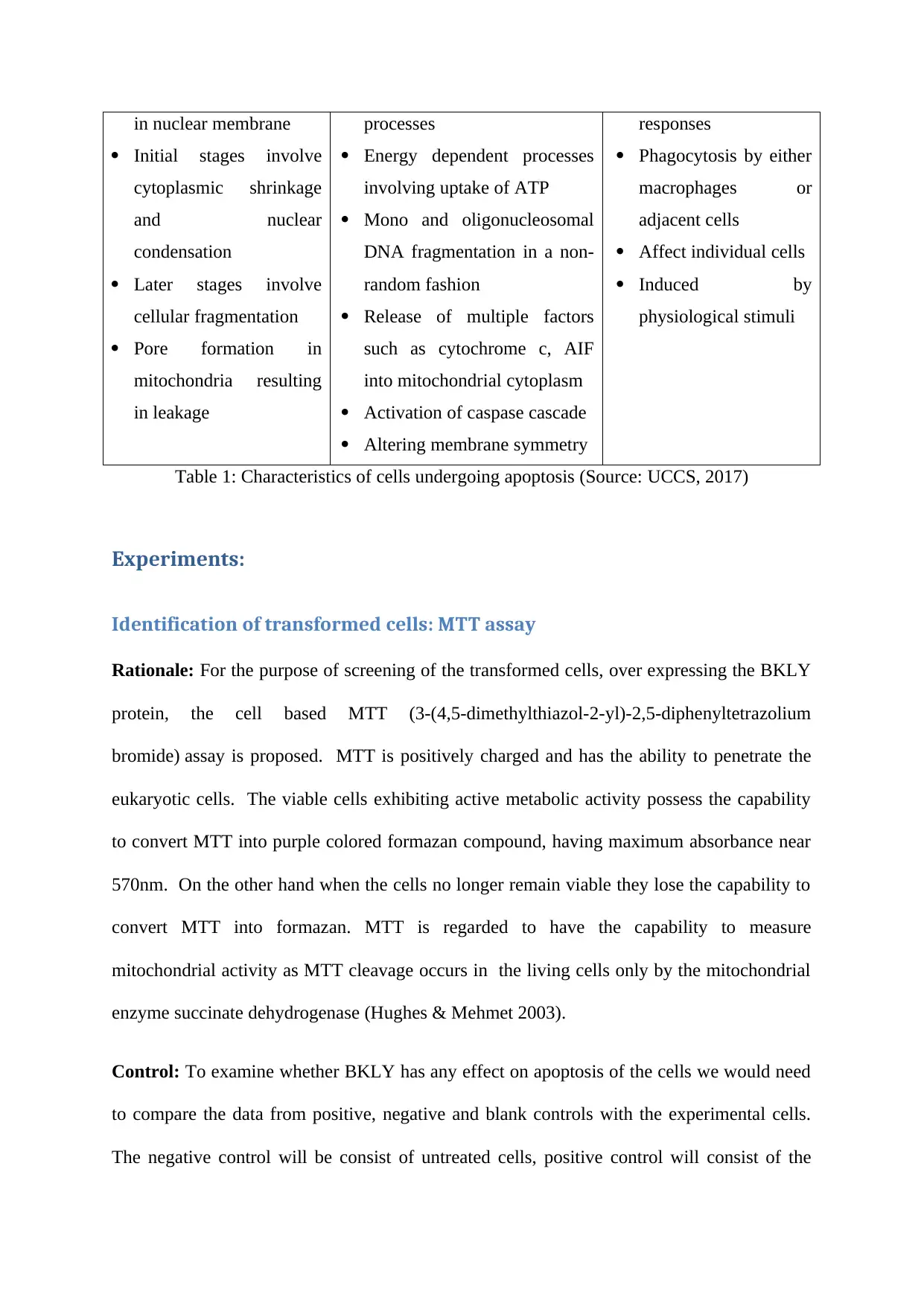
in nuclear membrane
Initial stages involve
cytoplasmic shrinkage
and nuclear
condensation
Later stages involve
cellular fragmentation
Pore formation in
mitochondria resulting
in leakage
processes
Energy dependent processes
involving uptake of ATP
Mono and oligonucleosomal
DNA fragmentation in a non-
random fashion
Release of multiple factors
such as cytochrome c, AIF
into mitochondrial cytoplasm
Activation of caspase cascade
Altering membrane symmetry
responses
Phagocytosis by either
macrophages or
adjacent cells
Affect individual cells
Induced by
physiological stimuli
Table 1: Characteristics of cells undergoing apoptosis (Source: UCCS, 2017)
Experiments:
Identification of transformed cells: MTT assay
Rationale: For the purpose of screening of the transformed cells, over expressing the BKLY
protein, the cell based MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide) assay is proposed. MTT is positively charged and has the ability to penetrate the
eukaryotic cells. The viable cells exhibiting active metabolic activity possess the capability
to convert MTT into purple colored formazan compound, having maximum absorbance near
570nm. On the other hand when the cells no longer remain viable they lose the capability to
convert MTT into formazan. MTT is regarded to have the capability to measure
mitochondrial activity as MTT cleavage occurs in the living cells only by the mitochondrial
enzyme succinate dehydrogenase (Hughes & Mehmet 2003).
Control: To examine whether BKLY has any effect on apoptosis of the cells we would need
to compare the data from positive, negative and blank controls with the experimental cells.
The negative control will be consist of untreated cells, positive control will consist of the
Initial stages involve
cytoplasmic shrinkage
and nuclear
condensation
Later stages involve
cellular fragmentation
Pore formation in
mitochondria resulting
in leakage
processes
Energy dependent processes
involving uptake of ATP
Mono and oligonucleosomal
DNA fragmentation in a non-
random fashion
Release of multiple factors
such as cytochrome c, AIF
into mitochondrial cytoplasm
Activation of caspase cascade
Altering membrane symmetry
responses
Phagocytosis by either
macrophages or
adjacent cells
Affect individual cells
Induced by
physiological stimuli
Table 1: Characteristics of cells undergoing apoptosis (Source: UCCS, 2017)
Experiments:
Identification of transformed cells: MTT assay
Rationale: For the purpose of screening of the transformed cells, over expressing the BKLY
protein, the cell based MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide) assay is proposed. MTT is positively charged and has the ability to penetrate the
eukaryotic cells. The viable cells exhibiting active metabolic activity possess the capability
to convert MTT into purple colored formazan compound, having maximum absorbance near
570nm. On the other hand when the cells no longer remain viable they lose the capability to
convert MTT into formazan. MTT is regarded to have the capability to measure
mitochondrial activity as MTT cleavage occurs in the living cells only by the mitochondrial
enzyme succinate dehydrogenase (Hughes & Mehmet 2003).
Control: To examine whether BKLY has any effect on apoptosis of the cells we would need
to compare the data from positive, negative and blank controls with the experimental cells.
The negative control will be consist of untreated cells, positive control will consist of the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

cells treated with cytotoxic chemical, and blank will contain no cells. The control and
experimental cell lines will be maintained under similar conditions of temperature and
incubation time periods.
Conclusion: If the BKLY protein is involved in the apoptotic process then differences in
metabolic activity of the apoptotic cells which have taken up the plasmids will help in
identification of the transfected cells. The change in colour from yellow water soluble
tetrazolium MTT to purpled colored insoluble formazan will help in identifying the non-
viable cells, indicating towards transfection with BKLY gene.
Identification of cells showing protein expression: Annexin V staining assay
Rationale: In order to detect the cells expressing the protein, the immunohistochemical
technique using Annexin V is suggested. Annexin V is a calcium dependent, phospholipid
binding protein, which has the capability to serve as an excellent tool for detecting the
ongoing process of apoptosis in cells. The process of apoptosis involves translocation of
phosphatidylserine from inner leaflet to outer leaflet of the plasma membrane. The integrity
of the phosphatidylserine exposed membrane is thus breached. The calcium dependant
binding of Annexin V with the breached membrane is further tested using vital dyes such as
Propidium Iodide. These dyes can penetrate the membrane only when the membrane integrity
is breached. However, it must be noted that Annexin V shows preferable binding tendencies
with apoptotic cells even in condition of excess necrosis. Hence, it is crucial to use
combination of Annexin V assay with Propidium Idodie, a DNA marker. This further allows
monitoring the progression of different stages of apoptosis. The staining procedures follow
fluorescence microscopy (Petrovsky et al. 2003).
Controls: To examine whether BKLY has any effect on apoptosis of the cells we would need
to compare the data from positive, negative and blank controls with the experimental cells.
experimental cell lines will be maintained under similar conditions of temperature and
incubation time periods.
Conclusion: If the BKLY protein is involved in the apoptotic process then differences in
metabolic activity of the apoptotic cells which have taken up the plasmids will help in
identification of the transfected cells. The change in colour from yellow water soluble
tetrazolium MTT to purpled colored insoluble formazan will help in identifying the non-
viable cells, indicating towards transfection with BKLY gene.
Identification of cells showing protein expression: Annexin V staining assay
Rationale: In order to detect the cells expressing the protein, the immunohistochemical
technique using Annexin V is suggested. Annexin V is a calcium dependent, phospholipid
binding protein, which has the capability to serve as an excellent tool for detecting the
ongoing process of apoptosis in cells. The process of apoptosis involves translocation of
phosphatidylserine from inner leaflet to outer leaflet of the plasma membrane. The integrity
of the phosphatidylserine exposed membrane is thus breached. The calcium dependant
binding of Annexin V with the breached membrane is further tested using vital dyes such as
Propidium Iodide. These dyes can penetrate the membrane only when the membrane integrity
is breached. However, it must be noted that Annexin V shows preferable binding tendencies
with apoptotic cells even in condition of excess necrosis. Hence, it is crucial to use
combination of Annexin V assay with Propidium Idodie, a DNA marker. This further allows
monitoring the progression of different stages of apoptosis. The staining procedures follow
fluorescence microscopy (Petrovsky et al. 2003).
Controls: To examine whether BKLY has any effect on apoptosis of the cells we would need
to compare the data from positive, negative and blank controls with the experimental cells.

The negative control will be consist of untreated cells, positive control will consist of the
cells treated with Annexin V, and blank will contain no cells. The control and experimental
cell lines will be maintained under similar conditions of temperature and incubation time
periods.
Conclusion: The untreated and treated cell lines when observed under fluorescence
microscope help identify the cells expressing the protein. The treated cell lines will show
increase in population of cells undergoing apoptosis, identified by increasing membrane
permeability.
Identification of nature of apoptotic properties: Flow Cytometry
Rationale: For the purpose of identifying the apoptotic properties of the protein, the method
of flow cytometry is proposed. Flow cytometry helps in successful quantification of
apoptosis, besides distinguishing the apoptotic cells from non-apoptotic ones. The
microscopic particles suspended in the fluid stream are counted, examined, and sorted, by
means of DNA staining. The apoptotic cells which have been stained using a fluorescent dye,
when passes through the single wavelength light beam, scatter light up to some extent. The
forward scatter versus side scattering of the light helps distinguish between the apoptotic and
non-apoptotic cells. The identification of the immunophenotype of the cell is facilitated by
the altered morphology of the plasma membrane of apoptotic cells. The phosphatidylserine is
present on the outer membrane in the apoptotic cells, which would be detected by Annexin V.
Propidium Iodide would act as the DNA specific fluorochrome, helping differentiate between
necrotic and apoptotic cells. However, the technique could prove to be time consuming
(Archana et al. 2013).
Controls: The proposed experiment would involve three controls. Control 1 will consist of
the unstained cells, Control 2 will consist of those stained with Annexin V only, and Control
cells treated with Annexin V, and blank will contain no cells. The control and experimental
cell lines will be maintained under similar conditions of temperature and incubation time
periods.
Conclusion: The untreated and treated cell lines when observed under fluorescence
microscope help identify the cells expressing the protein. The treated cell lines will show
increase in population of cells undergoing apoptosis, identified by increasing membrane
permeability.
Identification of nature of apoptotic properties: Flow Cytometry
Rationale: For the purpose of identifying the apoptotic properties of the protein, the method
of flow cytometry is proposed. Flow cytometry helps in successful quantification of
apoptosis, besides distinguishing the apoptotic cells from non-apoptotic ones. The
microscopic particles suspended in the fluid stream are counted, examined, and sorted, by
means of DNA staining. The apoptotic cells which have been stained using a fluorescent dye,
when passes through the single wavelength light beam, scatter light up to some extent. The
forward scatter versus side scattering of the light helps distinguish between the apoptotic and
non-apoptotic cells. The identification of the immunophenotype of the cell is facilitated by
the altered morphology of the plasma membrane of apoptotic cells. The phosphatidylserine is
present on the outer membrane in the apoptotic cells, which would be detected by Annexin V.
Propidium Iodide would act as the DNA specific fluorochrome, helping differentiate between
necrotic and apoptotic cells. However, the technique could prove to be time consuming
(Archana et al. 2013).
Controls: The proposed experiment would involve three controls. Control 1 will consist of
the unstained cells, Control 2 will consist of those stained with Annexin V only, and Control
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3 will be stained using PI only. Thereby allowing the differentiation between healthy
(unstained), apoptotic (positive for Annexin), and necrotic cells (positive for both Annexin
and PI) respectively. This will allow the researcher to draw accurate conclusions by forming
basis of comparison and analysis of the experimental cells.
Conclusions: The apoptotic cells would appear as having hypo-diploid structure of DNA
contents, and will be represented in sub-G1 peaks on the histograms. The decreased staining
ability of fluorochrome with respect to the apoptotic DNA is attributed to the endonuclease
activity of apoptotic cells resulting in extraction of low molecular weight DNA.
References:
Archana, M., Yogesh, T.L. & Kumaraswamy, K.L., 2013. Various methods available for detection of
apoptotic cells- A review. Indian journal of cancer, 50(3), p.274.
Elmore, S., 2007. Apoptosis: A review of programmed cell death. Toxicol Pathol., 35(4), pp.495–516.
Available at: https://www.ncbi.nlm.nih.gov/pubmed/19003982.
Fulda, S., 2009. Tumor resistance to apoptosis. Int J Cancer., 124(3), pp.511–515.
Gerl, R. & Vaux, D.L., 2005. Apoptosis in the development and treatment of cancer. Carcinogenesis,
26(2), pp.263–270.
Hejmadi, M., 2009. Introduction to cancer biology, Bookboon.
Hughes, D. & Mehmet, H., 2003. Cell Proliferation and apoptosis, Garland science.
Petrovsky, A. et al., 2003. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res, 63,
pp.1936–42.
UCCS, 2017. Differences between necrosis and apoptosis. Apoptosis, Cell Death, and Cell
Proliferation Manual, p.4. Available at:
(unstained), apoptotic (positive for Annexin), and necrotic cells (positive for both Annexin
and PI) respectively. This will allow the researcher to draw accurate conclusions by forming
basis of comparison and analysis of the experimental cells.
Conclusions: The apoptotic cells would appear as having hypo-diploid structure of DNA
contents, and will be represented in sub-G1 peaks on the histograms. The decreased staining
ability of fluorochrome with respect to the apoptotic DNA is attributed to the endonuclease
activity of apoptotic cells resulting in extraction of low molecular weight DNA.
References:
Archana, M., Yogesh, T.L. & Kumaraswamy, K.L., 2013. Various methods available for detection of
apoptotic cells- A review. Indian journal of cancer, 50(3), p.274.
Elmore, S., 2007. Apoptosis: A review of programmed cell death. Toxicol Pathol., 35(4), pp.495–516.
Available at: https://www.ncbi.nlm.nih.gov/pubmed/19003982.
Fulda, S., 2009. Tumor resistance to apoptosis. Int J Cancer., 124(3), pp.511–515.
Gerl, R. & Vaux, D.L., 2005. Apoptosis in the development and treatment of cancer. Carcinogenesis,
26(2), pp.263–270.
Hejmadi, M., 2009. Introduction to cancer biology, Bookboon.
Hughes, D. & Mehmet, H., 2003. Cell Proliferation and apoptosis, Garland science.
Petrovsky, A. et al., 2003. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res, 63,
pp.1936–42.
UCCS, 2017. Differences between necrosis and apoptosis. Apoptosis, Cell Death, and Cell
Proliferation Manual, p.4. Available at:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

https://www.uccs.edu/Documents/rmelamed/apoptosis_003_004.pdf [Accessed February 11,
2018].
2018].
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.