In-Depth Analysis: Fe-Fe3C Phase Diagram, Heat Treatment, Properties
VerifiedAdded on 2023/06/12
|12
|602
|224
Homework Assignment
AI Summary
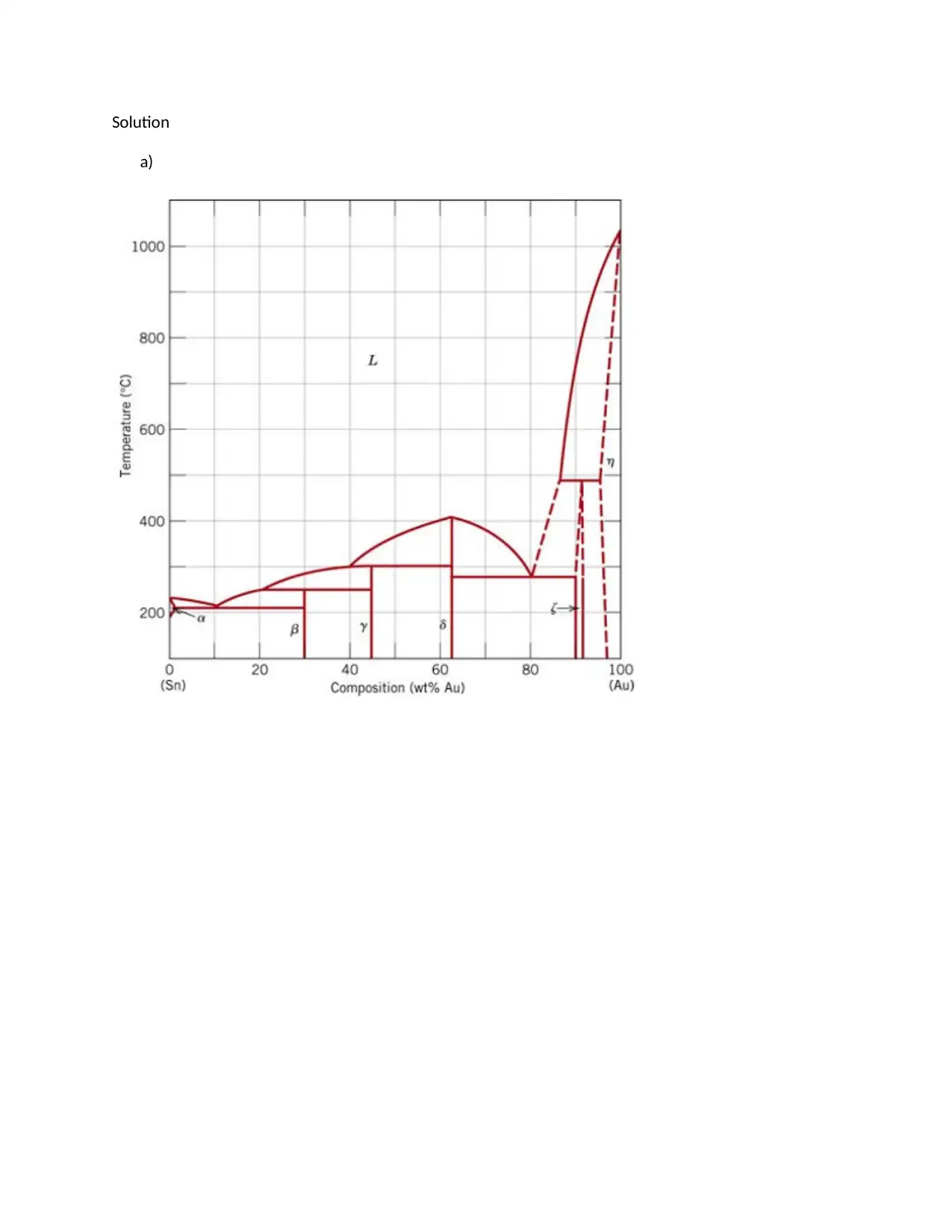
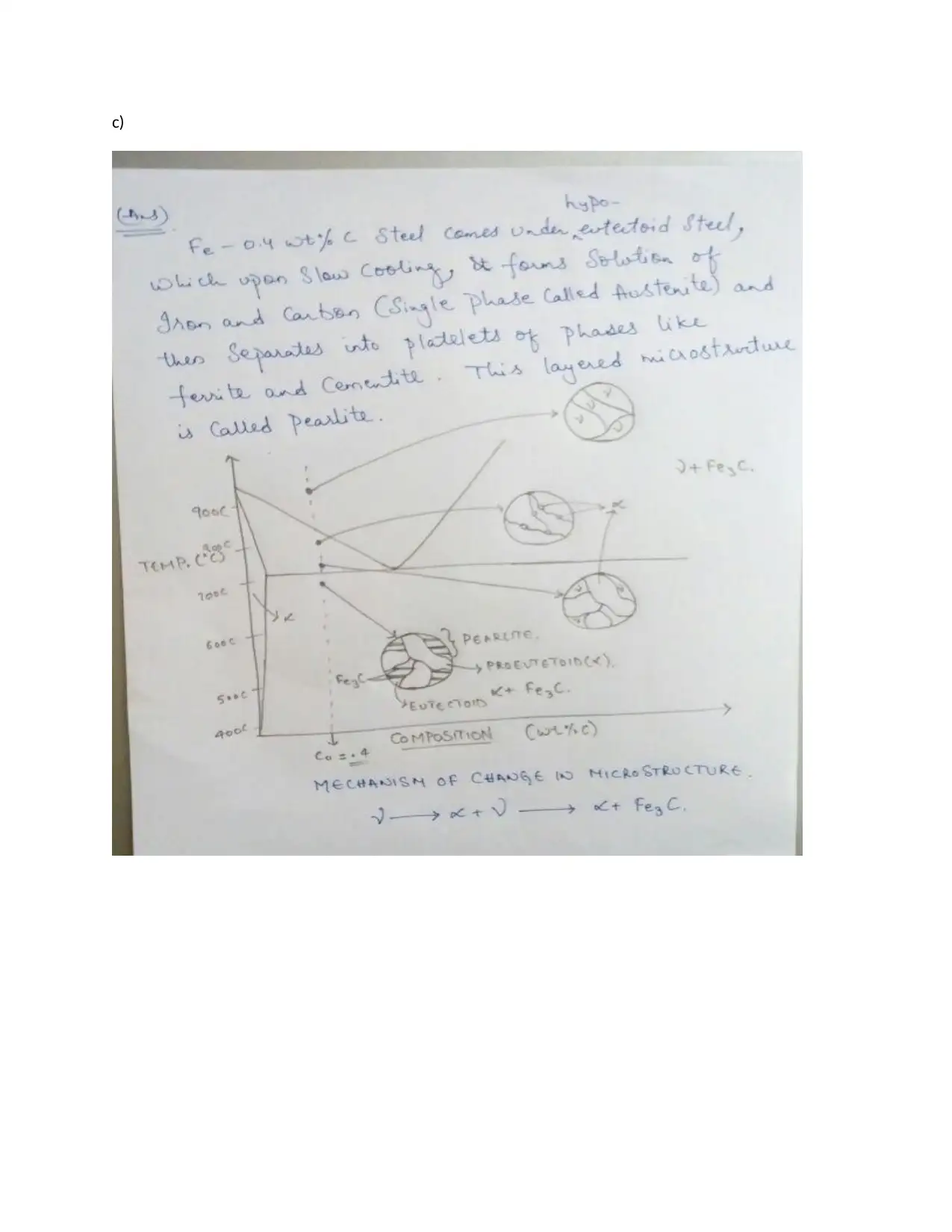
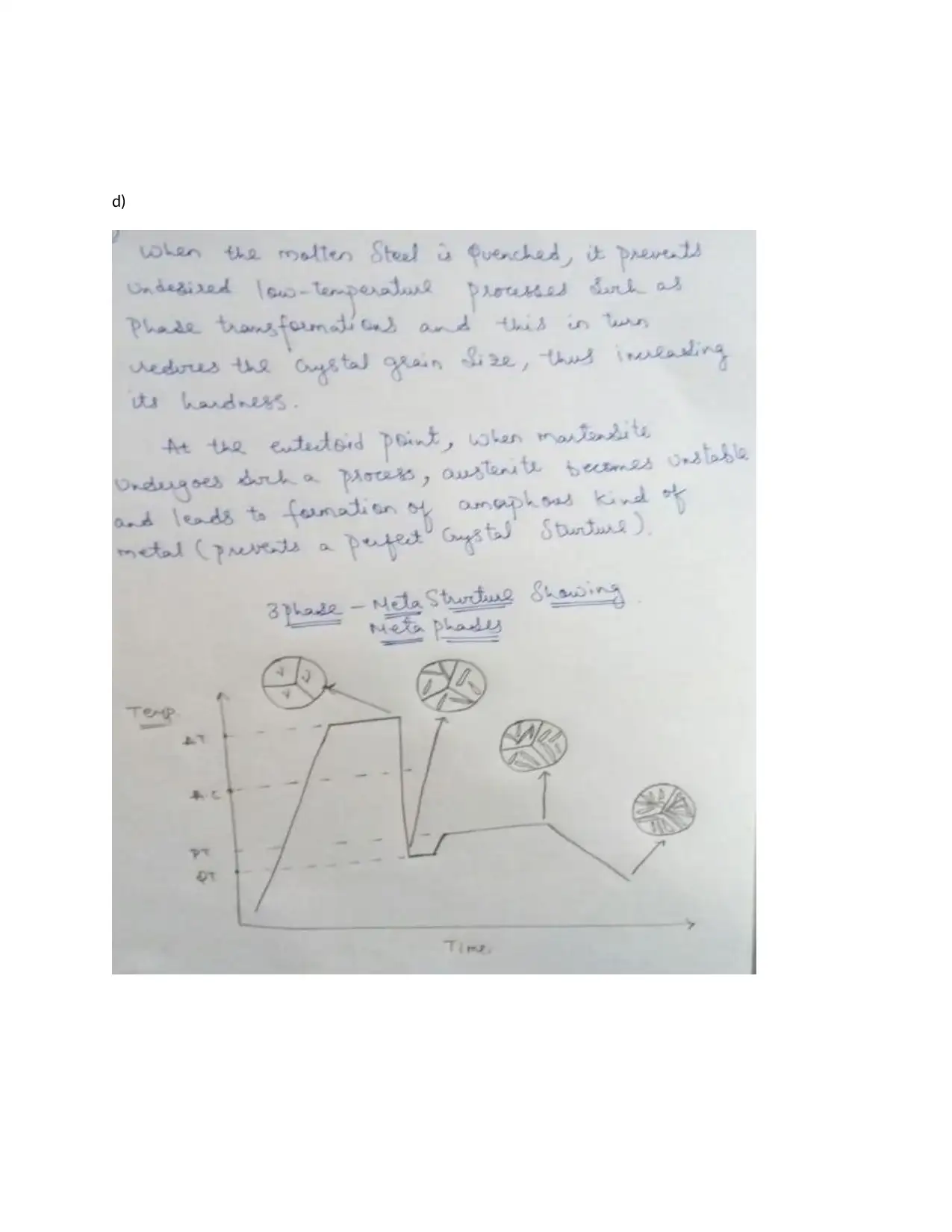
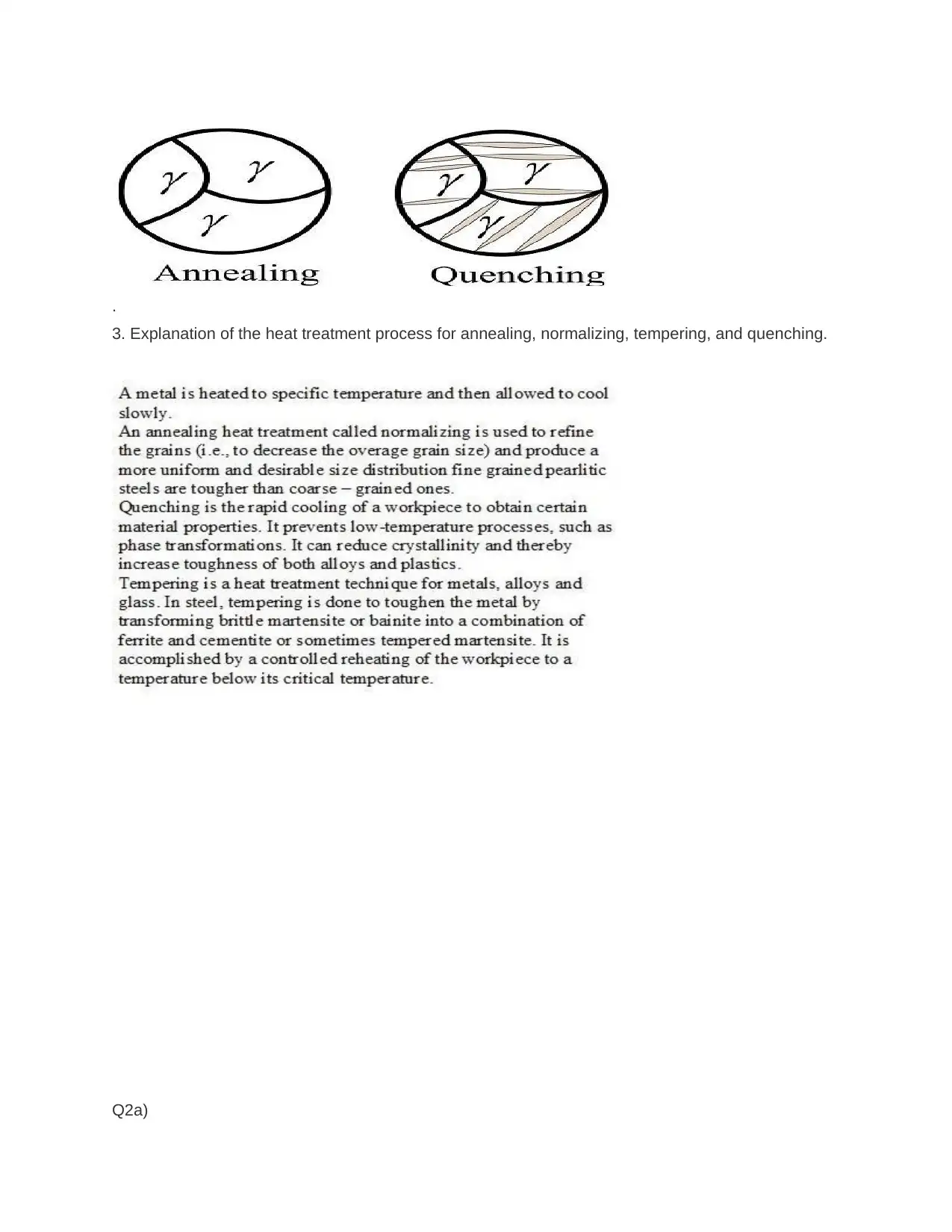
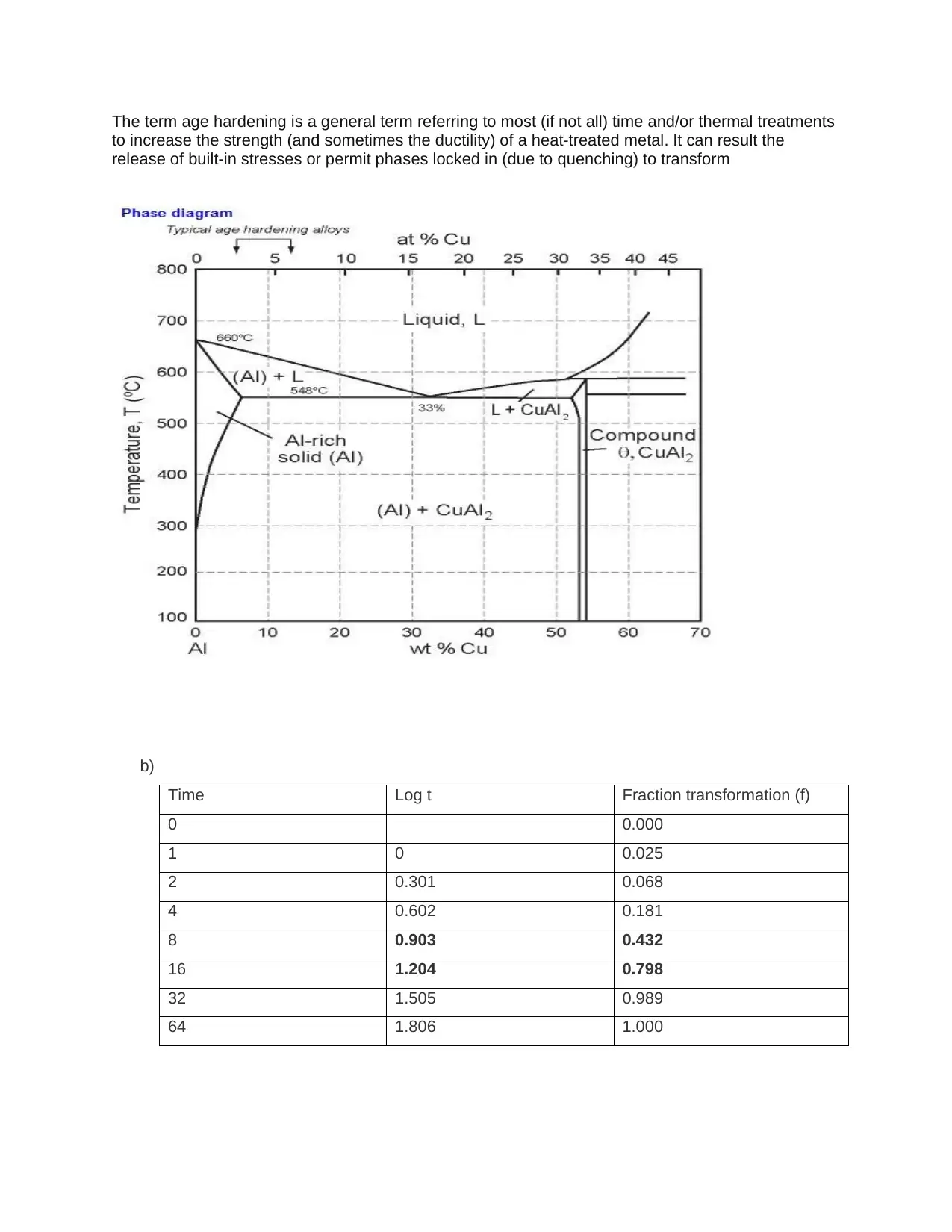
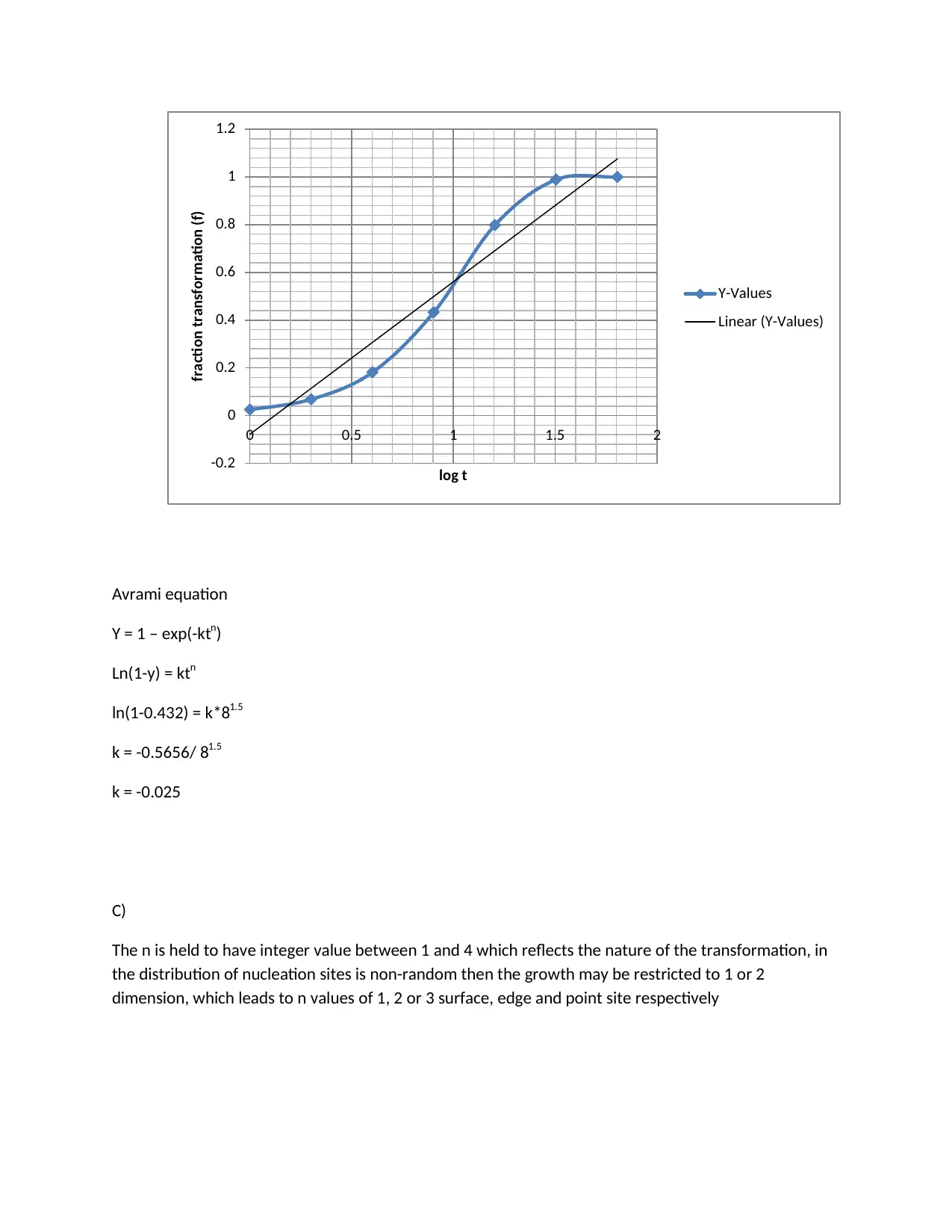
This assignment provides detailed solutions related to the Fe-Fe3C phase diagram and various heat treatment processes. It covers topics such as the characteristics of ferrite, cementite, austenite, and pearlite, including their composition, crystal structure, and mechanical properties. The solution also addresses the causes of peak broadening in materials analysis, such as instrumental factors, crystallite size, strain, and defects, along with the relationship between microstrain and peak broadening. Furthermore, it includes calculations related to crystallite size determination using the Scherrer equation. The assignment contrasts quenching and annealing procedures and their effects on microscopic, material, and macroscopic properties. It also provides sketches of expected microstructures for quenched and annealed specimens, explanations of heat treatment processes like annealing, normalizing, tempering, and quenching, and discusses age hardening and phase transformations using the Avrami equation to analyze transformation kinetics. The role of nucleation sites in transformation processes is also examined.
1 out of 12







![[object Object]](/_next/static/media/star-bottom.7253800d.svg)