Fertility Preservation and Pregnancy After Breast Cancer: A Study
VerifiedAdded on 2023/06/10
|7
|5191
|74
Case Study
AI Summary
This case study investigates fertility preservation and pregnancy outcomes in young breast cancer patients diagnosed between 2000 and 2016 at a large cancer center. The study assesses time-dependent variations in fertility preservation discussions and techniques, comparing cohorts from 2004-2006 and 2014-2016. Results indicate a significant increase in documented fertility discussions in the later cohort. The study also examines pregnancy outcomes in 26 patients who became pregnant after breast cancer, finding no safety concerns for women or newborns, with only two patients experiencing breast cancer relapse. The research highlights the growing importance of addressing fertility issues in young breast cancer patients and suggests that pregnancy after breast cancer is safe, warranting careful management and comprehensive discussion with patients.

Patterns of Fertility Preservation and Pregnancy
Outcome After Breast Cancer at a Large
Comprehensive Cancer Center
Maria Vittoria Dieci, MD,1,2 Cristina Ghiotto, MD,2 Caterina Barbieri, MD,1 Gaia Griguolo, MD,1,2
Carlo Saccardi, PhD, MD,3 Michele Gangemi, MD,3 Alfonso Pluchinotta, MD,4
Elisabetta DiLiso, MD,2 Carlo Alberto Giorgi, MD,2 Tommaso Giarratano, MD,2
Giulia Tasca, MD,1,2 Grazia Vernaci, MD,1,2 GiovanniFaggioni, MD,2
Pierfranco Conte, MD,1,2 and Valentina Guarneri, PhD, MD1,2
Abstract
Background: In the last decades,long-term outcomes of breast cancer (BC) patients have improved,raising
new survivorship issues, including fertility preservation and safety of pregnancy after BC. This study
evolution in patterns of fertility discussion/preservation over time and reports pregnancy outcomes in
of young BC patients.
Methods: A retrospective cohort of 590 BC patients aged £40 diagnosed between 2000 and 2016 ata large
cancer center was identified. Fertility counseling and preservation patterns for patients receiving che
were analyzed and compared fortwo cohorts:2004–2006 and 2014–2016 (totaln = 161).Outcomes were
reported for patients with documented pregnancy after BC.
Results: Significantly, more patients diagnosed in 2014–2016 had evidence of discussion on fertility i
or application of fertility preservation techniques versus patients diagnosed in 2004–2006 (82.9% vs.66.0%,
p = 0.017).In particular,there was a significantdifference in rate of documented fertility issues discussion
(67.6% vs.34.0%,p < 0.001).Age >35 and parity were associated with lower rates of fertility discussion/
preservation.However,rates significantly improved over time (77.6% in 2014–2016 vs.58.1% in 2004–2006
for patients aged >35,p = 0.046;80.7% in 2014–2016 vs.57.6% in 2004–2006 for patients with children at
diagnosis, p = 0.018). Twenty-six patients with pregnancy after BC were identified; eight delivered a
>40.No complications for women or newborns were reported.Only two patients experienced BC relapse.
Conclusions: In this small retrospective cohort, no safety concerns were identified for pregnancy afte
importance attributed by clinicians to address fertility issues has increased over time.
Keywords: cancer survivorship, fertility counseling, breast cancer, fertility preservation, pregnancy af
Introduction
Breast cancer (BC) is the mostcommon malignancy
in women, accounting for 1.67 million new cancer cases
diagnosed in 2012 worldwide,1 with more than 10% of new
cases diagnosed in women younger than the age of 40 years
(morethan 190,000 new casesestimated worldwidein
2012).2 BC is also the most common malignancy in women in
Italy,with more than 50,000 estimated new cases in 2017,
and accounts for almost half (41%) of the malignancies di-
agnosed in women younger than the age of 50 years.3
In the lastdecades,BC mortality hasconsistently de-
creased,1thanks to the extensive use of screening and advance
in adjuvant systemic treatments. However, both chemotherap
1Department of Surgery,Oncology and Gastroenterology,University of Padova,Padova,Italy.
2Medical Oncology 2,Istituto Oncologico Veneto IRCCS,Padova,Italy.
3Department of Woman and Child Health,University of Padova,Padova,Italy.
4Department of Surgery-Breast Surgery,Policlinico of Abano Terme,Padova,Italy.
ª Maria Vittoria Dieci et al. 2018; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms
Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and rep
any medium,provided the original work is properly cited.
JOURNAL OF WOMEN’S HEALTH
Volume 00, Number 00, 2018
Mary Ann Liebert, Inc.
DOI: 10.1089/jwh.2018.6986
1
Outcome After Breast Cancer at a Large
Comprehensive Cancer Center
Maria Vittoria Dieci, MD,1,2 Cristina Ghiotto, MD,2 Caterina Barbieri, MD,1 Gaia Griguolo, MD,1,2
Carlo Saccardi, PhD, MD,3 Michele Gangemi, MD,3 Alfonso Pluchinotta, MD,4
Elisabetta DiLiso, MD,2 Carlo Alberto Giorgi, MD,2 Tommaso Giarratano, MD,2
Giulia Tasca, MD,1,2 Grazia Vernaci, MD,1,2 GiovanniFaggioni, MD,2
Pierfranco Conte, MD,1,2 and Valentina Guarneri, PhD, MD1,2
Abstract
Background: In the last decades,long-term outcomes of breast cancer (BC) patients have improved,raising
new survivorship issues, including fertility preservation and safety of pregnancy after BC. This study
evolution in patterns of fertility discussion/preservation over time and reports pregnancy outcomes in
of young BC patients.
Methods: A retrospective cohort of 590 BC patients aged £40 diagnosed between 2000 and 2016 ata large
cancer center was identified. Fertility counseling and preservation patterns for patients receiving che
were analyzed and compared fortwo cohorts:2004–2006 and 2014–2016 (totaln = 161).Outcomes were
reported for patients with documented pregnancy after BC.
Results: Significantly, more patients diagnosed in 2014–2016 had evidence of discussion on fertility i
or application of fertility preservation techniques versus patients diagnosed in 2004–2006 (82.9% vs.66.0%,
p = 0.017).In particular,there was a significantdifference in rate of documented fertility issues discussion
(67.6% vs.34.0%,p < 0.001).Age >35 and parity were associated with lower rates of fertility discussion/
preservation.However,rates significantly improved over time (77.6% in 2014–2016 vs.58.1% in 2004–2006
for patients aged >35,p = 0.046;80.7% in 2014–2016 vs.57.6% in 2004–2006 for patients with children at
diagnosis, p = 0.018). Twenty-six patients with pregnancy after BC were identified; eight delivered a
>40.No complications for women or newborns were reported.Only two patients experienced BC relapse.
Conclusions: In this small retrospective cohort, no safety concerns were identified for pregnancy afte
importance attributed by clinicians to address fertility issues has increased over time.
Keywords: cancer survivorship, fertility counseling, breast cancer, fertility preservation, pregnancy af
Introduction
Breast cancer (BC) is the mostcommon malignancy
in women, accounting for 1.67 million new cancer cases
diagnosed in 2012 worldwide,1 with more than 10% of new
cases diagnosed in women younger than the age of 40 years
(morethan 190,000 new casesestimated worldwidein
2012).2 BC is also the most common malignancy in women in
Italy,with more than 50,000 estimated new cases in 2017,
and accounts for almost half (41%) of the malignancies di-
agnosed in women younger than the age of 50 years.3
In the lastdecades,BC mortality hasconsistently de-
creased,1thanks to the extensive use of screening and advance
in adjuvant systemic treatments. However, both chemotherap
1Department of Surgery,Oncology and Gastroenterology,University of Padova,Padova,Italy.
2Medical Oncology 2,Istituto Oncologico Veneto IRCCS,Padova,Italy.
3Department of Woman and Child Health,University of Padova,Padova,Italy.
4Department of Surgery-Breast Surgery,Policlinico of Abano Terme,Padova,Italy.
ª Maria Vittoria Dieci et al. 2018; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms
Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and rep
any medium,provided the original work is properly cited.
JOURNAL OF WOMEN’S HEALTH
Volume 00, Number 00, 2018
Mary Ann Liebert, Inc.
DOI: 10.1089/jwh.2018.6986
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

and endocrine treatmentmay affectthe reproductive func-
tion of young BC patients.As the number of BC survivors
increases, the need to preserve fertility in young BC patients
and their potentialdesire to build or complete their family
beyond the diagnosis of BC is becoming a major issue.
Pregnancy after BC has been discouraged for a long time in
the belief that increased estrogen levels during gestation may
promote the growth of micrometastatic disease, favoring BC
relapse. However, recent studies have shown that pregnancy
after BC diagnosis is safe, contradicting the initial position.
In the large retrospective study by Azim et al., no difference
in disease-free survival was described between BC patients
who became pregnant(n = 333)and a matched cohortof
controls (n = 874); results were similar according to estrogen
receptor status of primary BC.4,5
On these bases,currentguidelinesdo not recommend
abortion in case of pregnancy after BC diagnosis.6–8
The increased awareness of the safety of pregnancy after
BC should influence clinician’s sensibility toward fertility
issues, calling for a careful management and comprehensive
discussion with young BC patients.
This work was conducted retrospectively at a large cancer
Institution (Istituto Oncologico Veneto, Padova, Italy), with
two main aims:
to assess time-dependentvariations in fertility preser-
vation for young BC patients
to describe characteristics, BC outcome and pregnancy
outcome of consecutive patients who became pregnant
after BC diagnosis.
Methods
An Institutional Review Board-approved chart review was
performed on all patients diagnosed with primary nonmeta-
static BC at the age of 40 years or younger who were referred
at the Istituto Oncologico Veneto o Padova (Italy) between
2000 and 2016. Due to the limited possibilities of the success
of fertility preservation techniques in women older than 40
years of age,9 we focused our attention only on women aged
40 years or younger.
To assesstime-dependentchangesin patternsof fertil-
ity preservation,young patients who received adjuvantor
neoadjuvantchemotherapy were identified and two cohorts
were considered: patients diagnosed in the years 2004–2006
and patients diagnosed in the years 2014–2016. Collected data
included age at diagnosis, clinicopathological tumor features,
type of systemic treatment, parity at diagnosis, documentation
in medical records of fertility issues discussion,and fertility
preservation method.All data were retrospectively extracted
from medicalrecords,including multidisciplinary meeting
reports, and were collected in a dedicated database.
Next, we focused on the cohort of patients diagnosed with
BC at the age of 40 or younger between 2000 and 2016 who
had documentation in clinical records of pregnancy thereaf-
ter. For these patients, the following data were collected: age
at diagnosis, tumor stage, hormone receptor (HR) and human
epidermalgrowth factor receptor 2 (HER2) status,type of
surgery, type and duration of systemic treatment, radiother-
apy,fertility preservation,delivery date,age atdelivery,
pregnancy outcome, breastfeeding, subsequent pregnancies,
abortions, and oncologic follow-up.
Statisticalanalysis
Statistical analysis was performed with SPSS Version 24.
All the analyses,unlessotherwise specified,were de-
scriptive in nature. Descriptive statistics included, as appro-
priate,frequency countsand percentagesin contingency
tables,medians and ranges,concordance percentages,and
graphical displays such as bar charts. The chi-square test wa
used to evaluate the associations between variables.
Disease-free survivalfrom BC diagnosis was defined as
the time interval from BC diagnosis to locoregional/distant
relapse,second primary invasive BC,and death from any
cause (whichever occurred first).
Disease-free survival from delivery was calculated as the
time interval from the date of delivery to locoregional/distant
relapse,second primary invasive BC,and death from any
cause (whichever occurred first).
Survival curves were estimated according to the Kaplan–
Maier method. All hypothesis tests were conducted at a two-
sided alpha level of 0.05.
Results
Overall, medical records of more than 9,000 patients with a
diagnosis of primary BC between 2000 and 2016 were re-
viewed, and n = 590 patients (around 6% of the total) aged 4
years or younger at diagnosis were identified (Supplementar
Fig. S1; Supplementary Data are available online atwww
.liebertpub.com/jwh).
Patterns of fertility issues discussion and fertility
preservation among young BC patients
To assess variations in fertility preservation patterns over
time, we considered young patients who received adjuvant o
neoadjuvant chemotherapy and who were diagnosed in year
2004–2006 (n = 50,6% of totalpatients diagnosed in that
period) or in years 2014–2016 (n = 111, 6% of total patients
diagnosed in thatperiod) (Supplementary Fig.S1).Patient
characteristics are reported in Table 1.
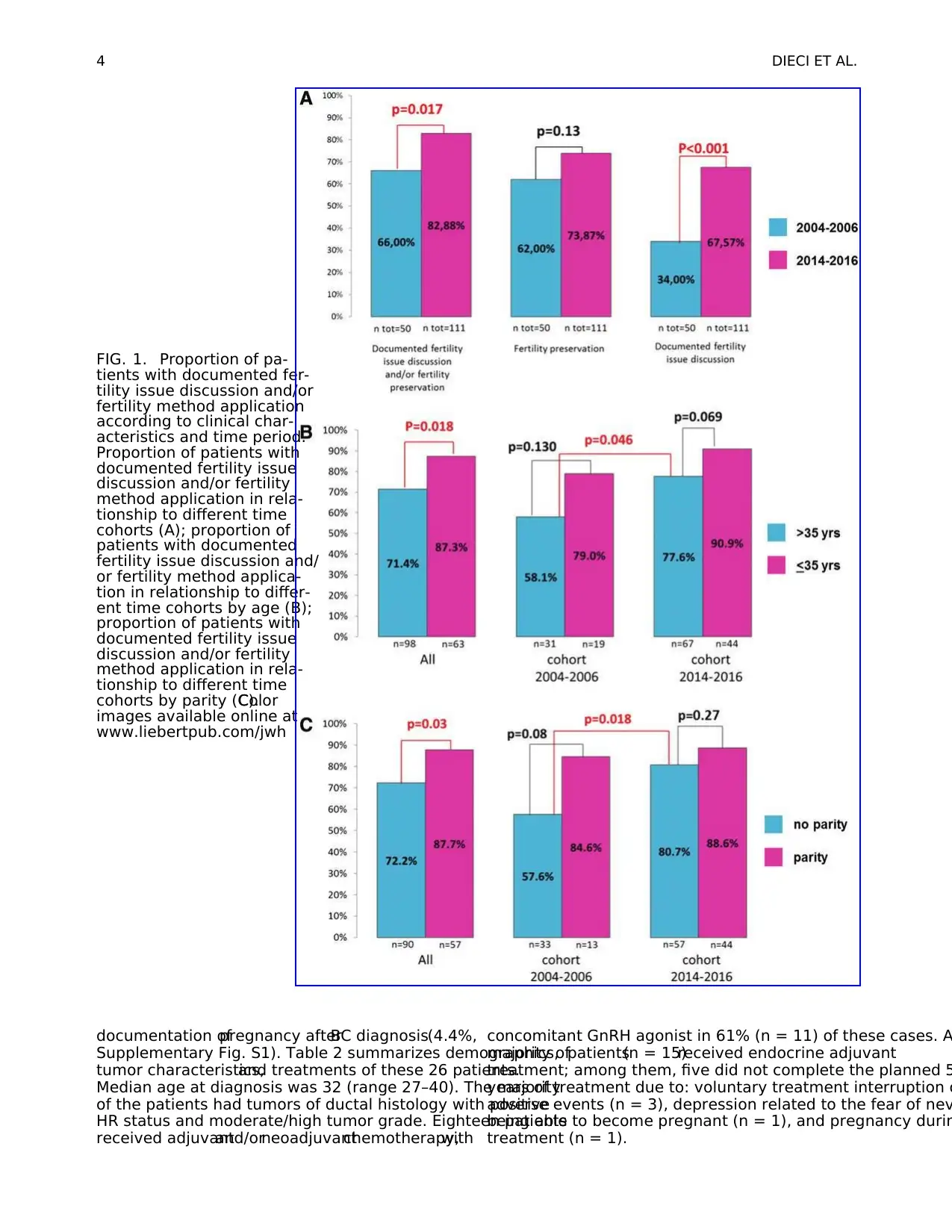
Overall,for 77.6% outof 161 totalpatients,there was
evidence in medical records of discussion on fertility issues
related to chemotherapy and/or application of fertility pres-
ervation methods, with significant difference when compar-
ing the two time cohorts (82.9% of patients diagnosed in
years 2014–2016 vs.66.0% of patients diagnosed in 2004–
2006, p = 0.017), as shown in Figure 1A. When considering
fertility preservation rate alone,no significantdifference
between the two time cohorts was observed, although it was
numerically higherin the more recentcohort(73.9% vs.
62.0%, p = 0.13, Fig. 1A). However,there was a significant
difference in the frequency of documentation of fertility is-
sues discussion according to time cohort:75 outof 111
(67.6%) patients diagnosed in years 2014–2016 had docu-
mentation in medical records of discussion of fertility issues
versus 17 (34.0%) of the 50 patients diagnosed in years 200
2006 ( p < 0.001). Overall, the most frequent fertility method
was gonadotropin-releasing hormone (GnRH)analog ad-
ministration concomitantto chemotherapy (n = 103 of 113
totalpatients),while oocyte cryopreservation (followed by
GnRH analog administration) was used in 10 patients (all of
which in the 2014–2016 cohort).
2 DIECI ET AL.
tion of young BC patients.As the number of BC survivors
increases, the need to preserve fertility in young BC patients
and their potentialdesire to build or complete their family
beyond the diagnosis of BC is becoming a major issue.
Pregnancy after BC has been discouraged for a long time in
the belief that increased estrogen levels during gestation may
promote the growth of micrometastatic disease, favoring BC
relapse. However, recent studies have shown that pregnancy
after BC diagnosis is safe, contradicting the initial position.
In the large retrospective study by Azim et al., no difference
in disease-free survival was described between BC patients
who became pregnant(n = 333)and a matched cohortof
controls (n = 874); results were similar according to estrogen
receptor status of primary BC.4,5
On these bases,currentguidelinesdo not recommend
abortion in case of pregnancy after BC diagnosis.6–8
The increased awareness of the safety of pregnancy after
BC should influence clinician’s sensibility toward fertility
issues, calling for a careful management and comprehensive
discussion with young BC patients.
This work was conducted retrospectively at a large cancer
Institution (Istituto Oncologico Veneto, Padova, Italy), with
two main aims:
to assess time-dependentvariations in fertility preser-
vation for young BC patients
to describe characteristics, BC outcome and pregnancy
outcome of consecutive patients who became pregnant
after BC diagnosis.
Methods
An Institutional Review Board-approved chart review was
performed on all patients diagnosed with primary nonmeta-
static BC at the age of 40 years or younger who were referred
at the Istituto Oncologico Veneto o Padova (Italy) between
2000 and 2016. Due to the limited possibilities of the success
of fertility preservation techniques in women older than 40
years of age,9 we focused our attention only on women aged
40 years or younger.
To assesstime-dependentchangesin patternsof fertil-
ity preservation,young patients who received adjuvantor
neoadjuvantchemotherapy were identified and two cohorts
were considered: patients diagnosed in the years 2004–2006
and patients diagnosed in the years 2014–2016. Collected data
included age at diagnosis, clinicopathological tumor features,
type of systemic treatment, parity at diagnosis, documentation
in medical records of fertility issues discussion,and fertility
preservation method.All data were retrospectively extracted
from medicalrecords,including multidisciplinary meeting
reports, and were collected in a dedicated database.
Next, we focused on the cohort of patients diagnosed with
BC at the age of 40 or younger between 2000 and 2016 who
had documentation in clinical records of pregnancy thereaf-
ter. For these patients, the following data were collected: age
at diagnosis, tumor stage, hormone receptor (HR) and human
epidermalgrowth factor receptor 2 (HER2) status,type of
surgery, type and duration of systemic treatment, radiother-
apy,fertility preservation,delivery date,age atdelivery,
pregnancy outcome, breastfeeding, subsequent pregnancies,
abortions, and oncologic follow-up.
Statisticalanalysis
Statistical analysis was performed with SPSS Version 24.
All the analyses,unlessotherwise specified,were de-
scriptive in nature. Descriptive statistics included, as appro-
priate,frequency countsand percentagesin contingency
tables,medians and ranges,concordance percentages,and
graphical displays such as bar charts. The chi-square test wa
used to evaluate the associations between variables.
Disease-free survivalfrom BC diagnosis was defined as
the time interval from BC diagnosis to locoregional/distant
relapse,second primary invasive BC,and death from any
cause (whichever occurred first).
Disease-free survival from delivery was calculated as the
time interval from the date of delivery to locoregional/distant
relapse,second primary invasive BC,and death from any
cause (whichever occurred first).
Survival curves were estimated according to the Kaplan–
Maier method. All hypothesis tests were conducted at a two-
sided alpha level of 0.05.
Results
Overall, medical records of more than 9,000 patients with a
diagnosis of primary BC between 2000 and 2016 were re-
viewed, and n = 590 patients (around 6% of the total) aged 4
years or younger at diagnosis were identified (Supplementar
Fig. S1; Supplementary Data are available online atwww
.liebertpub.com/jwh).
Patterns of fertility issues discussion and fertility
preservation among young BC patients
To assess variations in fertility preservation patterns over
time, we considered young patients who received adjuvant o
neoadjuvant chemotherapy and who were diagnosed in year
2004–2006 (n = 50,6% of totalpatients diagnosed in that
period) or in years 2014–2016 (n = 111, 6% of total patients
diagnosed in thatperiod) (Supplementary Fig.S1).Patient
characteristics are reported in Table 1.
Overall,for 77.6% outof 161 totalpatients,there was
evidence in medical records of discussion on fertility issues
related to chemotherapy and/or application of fertility pres-
ervation methods, with significant difference when compar-
ing the two time cohorts (82.9% of patients diagnosed in
years 2014–2016 vs.66.0% of patients diagnosed in 2004–
2006, p = 0.017), as shown in Figure 1A. When considering
fertility preservation rate alone,no significantdifference
between the two time cohorts was observed, although it was
numerically higherin the more recentcohort(73.9% vs.
62.0%, p = 0.13, Fig. 1A). However,there was a significant
difference in the frequency of documentation of fertility is-
sues discussion according to time cohort:75 outof 111
(67.6%) patients diagnosed in years 2014–2016 had docu-
mentation in medical records of discussion of fertility issues
versus 17 (34.0%) of the 50 patients diagnosed in years 200
2006 ( p < 0.001). Overall, the most frequent fertility method
was gonadotropin-releasing hormone (GnRH)analog ad-
ministration concomitantto chemotherapy (n = 103 of 113
totalpatients),while oocyte cryopreservation (followed by
GnRH analog administration) was used in 10 patients (all of
which in the 2014–2016 cohort).
2 DIECI ET AL.
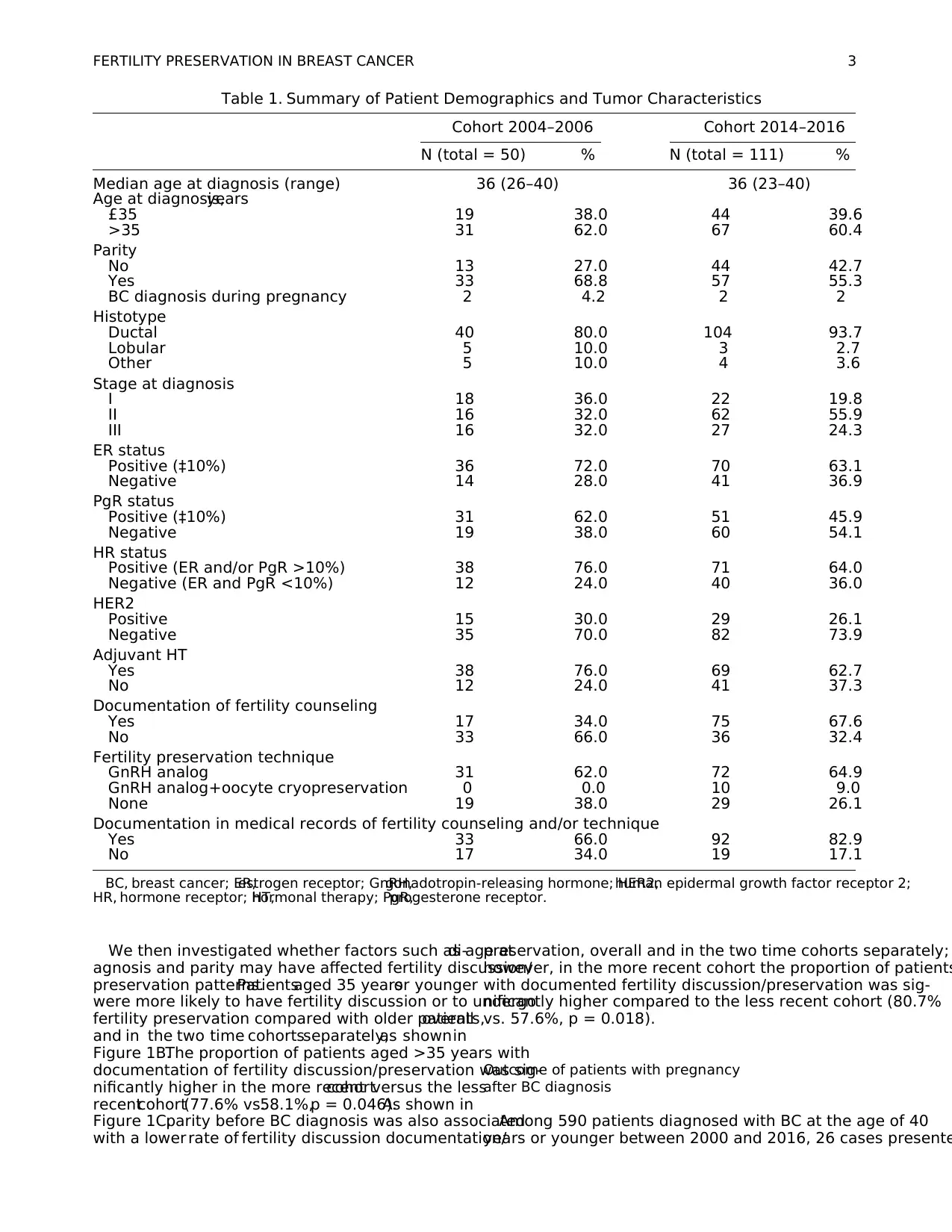
We then investigated whether factors such as age atdi-
agnosis and parity may have affected fertility discussion/
preservation patterns.Patientsaged 35 yearsor younger
were more likely to have fertility discussion or to undergo
fertility preservation compared with older patients,overall
and in the two time cohortsseparately,as shownin
Figure 1B.The proportion of patients aged >35 years with
documentation of fertility discussion/preservation was sig-
nificantly higher in the more recentcohortversus the less
recentcohort(77.6% vs.58.1%,p = 0.046).As shown in
Figure 1C,parity before BC diagnosis was also associated
with a lower rate of fertility discussion documentation/
preservation, overall and in the two time cohorts separately;
however, in the more recent cohort the proportion of patients
with documented fertility discussion/preservation was sig-
nificantly higher compared to the less recent cohort (80.7%
vs. 57.6%, p = 0.018).
Outcome of patients with pregnancy
after BC diagnosis
Among 590 patients diagnosed with BC at the age of 40
years or younger between 2000 and 2016, 26 cases presente
Table 1. Summary of Patient Demographics and Tumor Characteristics
Cohort 2004–2006 Cohort 2014–2016
N (total = 50) % N (total = 111) %
Median age at diagnosis (range) 36 (26–40) 36 (23–40)
Age at diagnosis,years
£35 19 38.0 44 39.6
>35 31 62.0 67 60.4
Parity
No 13 27.0 44 42.7
Yes 33 68.8 57 55.3
BC diagnosis during pregnancy 2 4.2 2 2
Histotype
Ductal 40 80.0 104 93.7
Lobular 5 10.0 3 2.7
Other 5 10.0 4 3.6
Stage at diagnosis
I 18 36.0 22 19.8
II 16 32.0 62 55.9
III 16 32.0 27 24.3
ER status
Positive (‡10%) 36 72.0 70 63.1
Negative 14 28.0 41 36.9
PgR status
Positive (‡10%) 31 62.0 51 45.9
Negative 19 38.0 60 54.1
HR status
Positive (ER and/or PgR >10%) 38 76.0 71 64.0
Negative (ER and PgR <10%) 12 24.0 40 36.0
HER2
Positive 15 30.0 29 26.1
Negative 35 70.0 82 73.9
Adjuvant HT
Yes 38 76.0 69 62.7
No 12 24.0 41 37.3
Documentation of fertility counseling
Yes 17 34.0 75 67.6
No 33 66.0 36 32.4
Fertility preservation technique
GnRH analog 31 62.0 72 64.9
GnRH analog+oocyte cryopreservation 0 0.0 10 9.0
None 19 38.0 29 26.1
Documentation in medical records of fertility counseling and/or technique
Yes 33 66.0 92 82.9
No 17 34.0 19 17.1
BC, breast cancer; ER,estrogen receptor; GnRH,gonadotropin-releasing hormone; HER2,human epidermal growth factor receptor 2;
HR, hormone receptor; HT,hormonal therapy; PgR,progesterone receptor.
FERTILITY PRESERVATION IN BREAST CANCER 3
agnosis and parity may have affected fertility discussion/
preservation patterns.Patientsaged 35 yearsor younger
were more likely to have fertility discussion or to undergo
fertility preservation compared with older patients,overall
and in the two time cohortsseparately,as shownin
Figure 1B.The proportion of patients aged >35 years with
documentation of fertility discussion/preservation was sig-
nificantly higher in the more recentcohortversus the less
recentcohort(77.6% vs.58.1%,p = 0.046).As shown in
Figure 1C,parity before BC diagnosis was also associated
with a lower rate of fertility discussion documentation/
preservation, overall and in the two time cohorts separately;
however, in the more recent cohort the proportion of patients
with documented fertility discussion/preservation was sig-
nificantly higher compared to the less recent cohort (80.7%
vs. 57.6%, p = 0.018).
Outcome of patients with pregnancy
after BC diagnosis
Among 590 patients diagnosed with BC at the age of 40
years or younger between 2000 and 2016, 26 cases presente
Table 1. Summary of Patient Demographics and Tumor Characteristics
Cohort 2004–2006 Cohort 2014–2016
N (total = 50) % N (total = 111) %
Median age at diagnosis (range) 36 (26–40) 36 (23–40)
Age at diagnosis,years
£35 19 38.0 44 39.6
>35 31 62.0 67 60.4
Parity
No 13 27.0 44 42.7
Yes 33 68.8 57 55.3
BC diagnosis during pregnancy 2 4.2 2 2
Histotype
Ductal 40 80.0 104 93.7
Lobular 5 10.0 3 2.7
Other 5 10.0 4 3.6
Stage at diagnosis
I 18 36.0 22 19.8
II 16 32.0 62 55.9
III 16 32.0 27 24.3
ER status
Positive (‡10%) 36 72.0 70 63.1
Negative 14 28.0 41 36.9
PgR status
Positive (‡10%) 31 62.0 51 45.9
Negative 19 38.0 60 54.1
HR status
Positive (ER and/or PgR >10%) 38 76.0 71 64.0
Negative (ER and PgR <10%) 12 24.0 40 36.0
HER2
Positive 15 30.0 29 26.1
Negative 35 70.0 82 73.9
Adjuvant HT
Yes 38 76.0 69 62.7
No 12 24.0 41 37.3
Documentation of fertility counseling
Yes 17 34.0 75 67.6
No 33 66.0 36 32.4
Fertility preservation technique
GnRH analog 31 62.0 72 64.9
GnRH analog+oocyte cryopreservation 0 0.0 10 9.0
None 19 38.0 29 26.1
Documentation in medical records of fertility counseling and/or technique
Yes 33 66.0 92 82.9
No 17 34.0 19 17.1
BC, breast cancer; ER,estrogen receptor; GnRH,gonadotropin-releasing hormone; HER2,human epidermal growth factor receptor 2;
HR, hormone receptor; HT,hormonal therapy; PgR,progesterone receptor.
FERTILITY PRESERVATION IN BREAST CANCER 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
documentation ofpregnancy afterBC diagnosis(4.4%,
Supplementary Fig. S1). Table 2 summarizes demographics,
tumor characteristics,and treatments of these 26 patients.
Median age at diagnosis was 32 (range 27–40). The majority
of the patients had tumors of ductal histology with positive
HR status and moderate/high tumor grade. Eighteen patients
received adjuvantand/orneoadjuvantchemotherapy,with
concomitant GnRH agonist in 61% (n = 11) of these cases. A
majority ofpatients(n = 15)received endocrine adjuvant
treatment; among them, five did not complete the planned 5
years of treatment due to: voluntary treatment interruption o
adverse events (n = 3), depression related to the fear of nev
being able to become pregnant (n = 1), and pregnancy durin
treatment (n = 1).
FIG. 1. Proportion of pa-
tients with documented fer-
tility issue discussion and/or
fertility method application
according to clinical char-
acteristics and time period.
Proportion of patients with
documented fertility issue
discussion and/or fertility
method application in rela-
tionship to different time
cohorts (A); proportion of
patients with documented
fertility issue discussion and/
or fertility method applica-
tion in relationship to differ-
ent time cohorts by age (B);
proportion of patients with
documented fertility issue
discussion and/or fertility
method application in rela-
tionship to different time
cohorts by parity (C).Color
images available online at
www.liebertpub.com/jwh
4 DIECI ET AL.
Supplementary Fig. S1). Table 2 summarizes demographics,
tumor characteristics,and treatments of these 26 patients.
Median age at diagnosis was 32 (range 27–40). The majority
of the patients had tumors of ductal histology with positive
HR status and moderate/high tumor grade. Eighteen patients
received adjuvantand/orneoadjuvantchemotherapy,with
concomitant GnRH agonist in 61% (n = 11) of these cases. A
majority ofpatients(n = 15)received endocrine adjuvant
treatment; among them, five did not complete the planned 5
years of treatment due to: voluntary treatment interruption o
adverse events (n = 3), depression related to the fear of nev
being able to become pregnant (n = 1), and pregnancy durin
treatment (n = 1).
FIG. 1. Proportion of pa-
tients with documented fer-
tility issue discussion and/or
fertility method application
according to clinical char-
acteristics and time period.
Proportion of patients with
documented fertility issue
discussion and/or fertility
method application in rela-
tionship to different time
cohorts (A); proportion of
patients with documented
fertility issue discussion and/
or fertility method applica-
tion in relationship to differ-
ent time cohorts by age (B);
proportion of patients with
documented fertility issue
discussion and/or fertility
method application in rela-
tionship to different time
cohorts by parity (C).Color
images available online at
www.liebertpub.com/jwh
4 DIECI ET AL.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
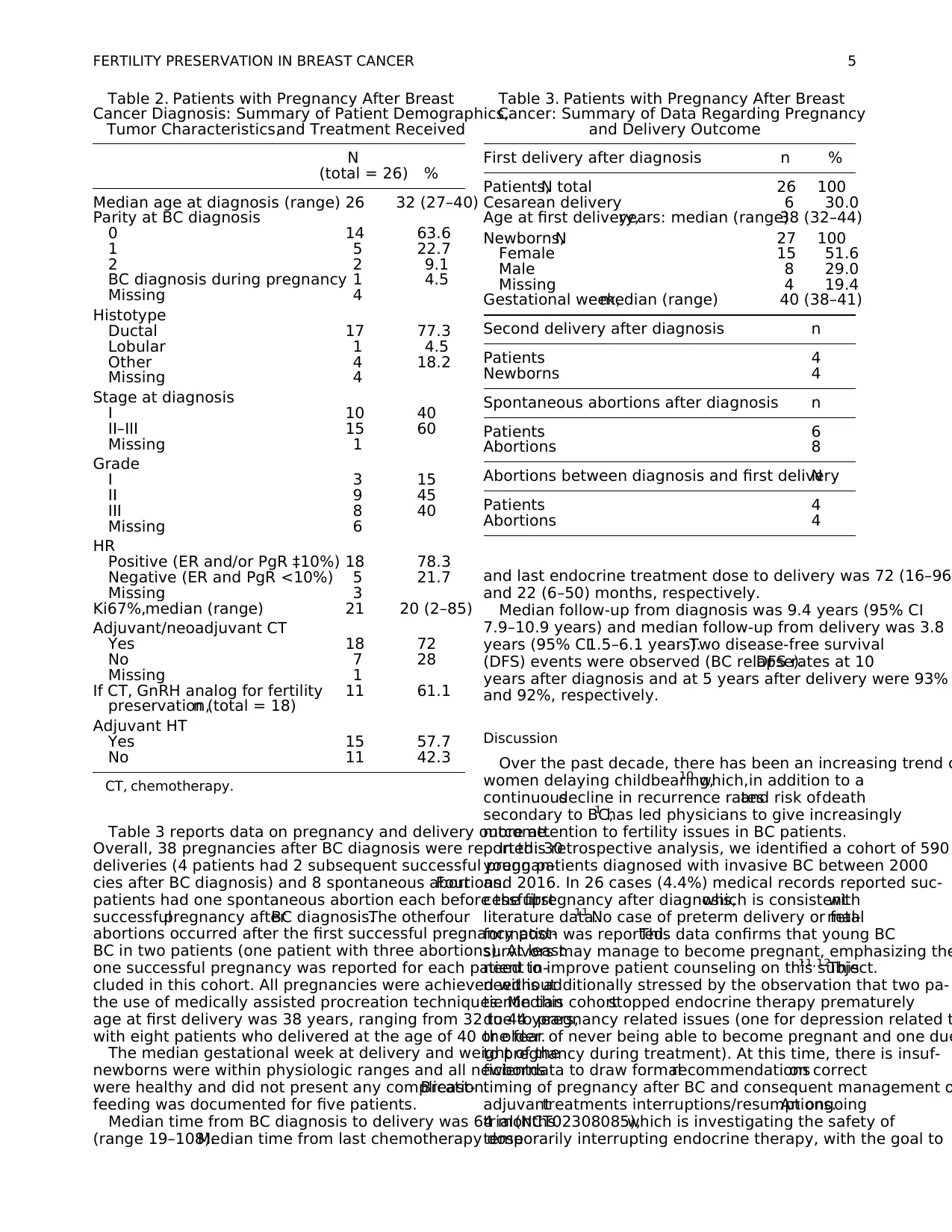
Table 3 reports data on pregnancy and delivery outcome.
Overall, 38 pregnancies after BC diagnosis were reported: 30
deliveries (4 patients had 2 subsequent successful pregnan-
cies after BC diagnosis) and 8 spontaneous abortions.Four
patients had one spontaneous abortion each before the first
successfulpregnancy afterBC diagnosis.The otherfour
abortions occurred after the first successful pregnancy post-
BC in two patients (one patient with three abortions). At least
one successful pregnancy was reported for each patient in-
cluded in this cohort. All pregnancies were achieved without
the use of medically assisted procreation techniques. Median
age at first delivery was 38 years, ranging from 32 to 44 years,
with eight patients who delivered at the age of 40 or older.
The median gestational week at delivery and weight of the
newborns were within physiologic ranges and all newborns
were healthy and did not present any complication.Breast-
feeding was documented for five patients.
Median time from BC diagnosis to delivery was 64 months
(range 19–108).Median time from last chemotherapy dose
and last endocrine treatment dose to delivery was 72 (16–96)
and 22 (6–50) months, respectively.
Median follow-up from diagnosis was 9.4 years (95% CI
7.9–10.9 years) and median follow-up from delivery was 3.8
years (95% CI1.5–6.1 years).Two disease-free survival
(DFS) events were observed (BC relapse).DFS rates at 10
years after diagnosis and at 5 years after delivery were 93%
and 92%, respectively.
Discussion
Over the past decade, there has been an increasing trend o
women delaying childbearing,10 which,in addition to a
continuousdecline in recurrence ratesand risk ofdeath
secondary to BC,1 has led physicians to give increasingly
more attention to fertility issues in BC patients.
In this retrospective analysis, we identified a cohort of 590
young patients diagnosed with invasive BC between 2000
and 2016. In 26 cases (4.4%) medical records reported suc-
cessfulpregnancy after diagnosis,which is consistentwith
literature data.11 No case of preterm delivery or fetalmal-
formation was reported.This data confirms that young BC
survivors may manage to become pregnant, emphasizing the
need to improve patient counseling on this subject.11,12
This
need is additionally stressed by the observation that two pa-
tientin this cohortstopped endocrine therapy prematurely
due to pregnancy related issues (one for depression related t
the fear of never being able to become pregnant and one due
to pregnancy during treatment). At this time, there is insuf-
ficientdata to draw formalrecommendationson correct
timing of pregnancy after BC and consequent management o
adjuvanttreatments interruptions/resumptions.An ongoing
trial(NCT02308085),which is investigating the safety of
temporarily interrupting endocrine therapy, with the goal to
Table 2. Patients with Pregnancy After Breast
Cancer Diagnosis: Summary of Patient Demographics,
Tumor Characteristics,and Treatment Received
N
(total = 26) %
Median age at diagnosis (range) 26 32 (27–40)
Parity at BC diagnosis
0 14 63.6
1 5 22.7
2 2 9.1
BC diagnosis during pregnancy 1 4.5
Missing 4
Histotype
Ductal 17 77.3
Lobular 1 4.5
Other 4 18.2
Missing 4
Stage at diagnosis
I 10 40
II–III 15 60
Missing 1
Grade
I 3 15
II 9 45
III 8 40
Missing 6
HR
Positive (ER and/or PgR ‡10%) 18 78.3
Negative (ER and PgR <10%) 5 21.7
Missing 3
Ki67%,median (range) 21 20 (2–85)
Adjuvant/neoadjuvant CT
Yes 18 72
No 7 28
Missing 1
If CT, GnRH analog for fertility
preservation,n (total = 18)
11 61.1
Adjuvant HT
Yes 15 57.7
No 11 42.3
CT, chemotherapy.
Table 3. Patients with Pregnancy After Breast
Cancer: Summary of Data Regarding Pregnancy
and Delivery Outcome
First delivery after diagnosis n %
Patients,N total 26 100
Cesarean delivery 6 30.0
Age at first delivery,years: median (range)38 (32–44)
Newborns,N 27 100
Female 15 51.6
Male 8 29.0
Missing 4 19.4
Gestational week,median (range) 40 (38–41)
Second delivery after diagnosis n
Patients 4
Newborns 4
Spontaneous abortions after diagnosis n
Patients 6
Abortions 8
Abortions between diagnosis and first deliveryN
Patients 4
Abortions 4
FERTILITY PRESERVATION IN BREAST CANCER 5
Overall, 38 pregnancies after BC diagnosis were reported: 30
deliveries (4 patients had 2 subsequent successful pregnan-
cies after BC diagnosis) and 8 spontaneous abortions.Four
patients had one spontaneous abortion each before the first
successfulpregnancy afterBC diagnosis.The otherfour
abortions occurred after the first successful pregnancy post-
BC in two patients (one patient with three abortions). At least
one successful pregnancy was reported for each patient in-
cluded in this cohort. All pregnancies were achieved without
the use of medically assisted procreation techniques. Median
age at first delivery was 38 years, ranging from 32 to 44 years,
with eight patients who delivered at the age of 40 or older.
The median gestational week at delivery and weight of the
newborns were within physiologic ranges and all newborns
were healthy and did not present any complication.Breast-
feeding was documented for five patients.
Median time from BC diagnosis to delivery was 64 months
(range 19–108).Median time from last chemotherapy dose
and last endocrine treatment dose to delivery was 72 (16–96)
and 22 (6–50) months, respectively.
Median follow-up from diagnosis was 9.4 years (95% CI
7.9–10.9 years) and median follow-up from delivery was 3.8
years (95% CI1.5–6.1 years).Two disease-free survival
(DFS) events were observed (BC relapse).DFS rates at 10
years after diagnosis and at 5 years after delivery were 93%
and 92%, respectively.
Discussion
Over the past decade, there has been an increasing trend o
women delaying childbearing,10 which,in addition to a
continuousdecline in recurrence ratesand risk ofdeath
secondary to BC,1 has led physicians to give increasingly
more attention to fertility issues in BC patients.
In this retrospective analysis, we identified a cohort of 590
young patients diagnosed with invasive BC between 2000
and 2016. In 26 cases (4.4%) medical records reported suc-
cessfulpregnancy after diagnosis,which is consistentwith
literature data.11 No case of preterm delivery or fetalmal-
formation was reported.This data confirms that young BC
survivors may manage to become pregnant, emphasizing the
need to improve patient counseling on this subject.11,12
This
need is additionally stressed by the observation that two pa-
tientin this cohortstopped endocrine therapy prematurely
due to pregnancy related issues (one for depression related t
the fear of never being able to become pregnant and one due
to pregnancy during treatment). At this time, there is insuf-
ficientdata to draw formalrecommendationson correct
timing of pregnancy after BC and consequent management o
adjuvanttreatments interruptions/resumptions.An ongoing
trial(NCT02308085),which is investigating the safety of
temporarily interrupting endocrine therapy, with the goal to
Table 2. Patients with Pregnancy After Breast
Cancer Diagnosis: Summary of Patient Demographics,
Tumor Characteristics,and Treatment Received
N
(total = 26) %
Median age at diagnosis (range) 26 32 (27–40)
Parity at BC diagnosis
0 14 63.6
1 5 22.7
2 2 9.1
BC diagnosis during pregnancy 1 4.5
Missing 4
Histotype
Ductal 17 77.3
Lobular 1 4.5
Other 4 18.2
Missing 4
Stage at diagnosis
I 10 40
II–III 15 60
Missing 1
Grade
I 3 15
II 9 45
III 8 40
Missing 6
HR
Positive (ER and/or PgR ‡10%) 18 78.3
Negative (ER and PgR <10%) 5 21.7
Missing 3
Ki67%,median (range) 21 20 (2–85)
Adjuvant/neoadjuvant CT
Yes 18 72
No 7 28
Missing 1
If CT, GnRH analog for fertility
preservation,n (total = 18)
11 61.1
Adjuvant HT
Yes 15 57.7
No 11 42.3
CT, chemotherapy.
Table 3. Patients with Pregnancy After Breast
Cancer: Summary of Data Regarding Pregnancy
and Delivery Outcome
First delivery after diagnosis n %
Patients,N total 26 100
Cesarean delivery 6 30.0
Age at first delivery,years: median (range)38 (32–44)
Newborns,N 27 100
Female 15 51.6
Male 8 29.0
Missing 4 19.4
Gestational week,median (range) 40 (38–41)
Second delivery after diagnosis n
Patients 4
Newborns 4
Spontaneous abortions after diagnosis n
Patients 6
Abortions 8
Abortions between diagnosis and first deliveryN
Patients 4
Abortions 4
FERTILITY PRESERVATION IN BREAST CANCER 5

permitting pregnancy, will help physicians collect evidence
to adequately respond to patients on these issues. Due to the
limited sample size of our cohortand to the absence of a
controlgroup,data on pregnancy after BC are exploratory
and descriptive in nature.Indeed,the risk of selection bias
called ‘‘health mother effect’’ could not be controlled in our
study.Even so,it is reassuring thatour experience ap-
pears somehow consistent with more recent evidence from
large cohorts reporting thatpregnancy does notincrease
risk of BC relapse,even in HR+ BC patients,4,5 and that
newborns are not exposed to higher risk of prenatal/perinatal
complications.13
Interestingly, observing the characteristics of patients who
successfully became pregnant after BC may also help dispel
some presumptions regarding which patients should undergo
fertility preservation and fertility preservation counseling. In
fact, both age and parity are well-known barriers to fertility
preservation counseling as clinicians may be induced to as-
sume that patients have completed child-bearing in the first
case,or have fully satisfied theirdesire forfamily in the
second case.14–16In our cohort of patients who successfully
mothered children after BC, we observed 7 patients who al-
ready had children at time of diagnosis, and patients as old as
40 years at diagnosis, thus pointing out that motherhood after
cancer mightnotbe a prerogative of extremely young pa-
tients nor a desire reserved to childless women.
This leads to the other aim of the study,which was to
evaluate physician awareness regarding fertility issues by
assessing time-dependentchanges in a 10-yearperiod in
fertility preservation counseling and techniques in young BC
patients undergoing neo/adjuvant chemotherapy.
In both time cohorts (2004–2006 and 2014–2016),the
majority ofBC patients aged £40 years had evidence in
medicalrecords of discussion on fertility issues and/or ap-
plication offertility preservation methods.This compares
favorably with published data reporting a counseling rate of
around 26% in BC patients.14,16Nevertheless,the most re-
centcohortshowed a significantly higherrate (82.9% vs.
66.0%, p = 0.017) of fertility counseling documentation and/
or application of fertility preservation methods. This differ-
ence is mostly due to an increase in the documentation of
fertility counseling (67.6% vs. 34.0%, p = 0.06).
Even if counseling does not finally translate in the appli-
cation of fertility preservation techniques, its documentation
in medicalrecordsis not irrelevant.Actually,it reflects
physicians’ awareness and the importance attributed to ad-
dressing fertility issues before initiation of treatment,inde-
pendently from the decision taken by the patient.Clinical
data show thatfemale survivors who previously received
fertility counseling have less regret and improved quality of
life, independently from the final decision on pursuing or not
the fertility preservation.17
In our study, the proportion of patients aged >35 years and/
or who already had children receiving counseling was sig-
nificantly higher in the more recent cohort versus the older
cohort,highlighting progressive increase in clinician’s per-
ceived importance of extending counseling to all young BC
patients. These modifications in clinical practice adequately
mirror the evolution of guidelines,as depicted by the 2013
ASCO recommendation that no patient should be excluded
from fertility preservation discussion forany reason,in-
cluding age, prognosis, socioeconomic status, or parity.18,19
In the most recent cohort (2014–2016),9.0% of patients
finally underwentoocyte cryopreservation procedures,an
acceptance rate similar to the one reported by Ruddy et al. in
2014.20 However,in both the 2004–2006 cohortand the
2014–2016 cohort,more than 60% of patients received an
GnRH analog during chemotherapy. This is in keeping with
the general attitude of Italian oncologists: in a recent survey,
aimed to investigate the approach of Italian oncologists and
breast surgeons dealing with BC to fertility issues,65% of
panelistsdeclared to useconcomitantadministration of
GnRH analogs and chemotherapy regularly in their common
clinical practice.21 This attitude might be influenced by the
fact that the PROMISE trial,a large randomized trial con-
cerning the use of GnRH analogs in this setting,was con-
ducted in Italy.22Moreover, this option is also recommended
by national oncological guidelines.23
Conclusions
Physicians’ attention to fertility issues and counseling has
increased over time, also regarding women who already hav
children and older patients.
In this small retrospective cohort, no safety concerns were
identified for pregnancy after BC. However, due to the lim-
ited sample size of our cohort and to the absence of a contro
group,data on pregnancy after BC are only descriptive,as
risk of selection bias could not be controlled.Even so,this
evidence appears consistentwith data from large cohorts
reporting that pregnancy does not increase risk of BC relapse
even in HR+ BC patients.4,5Nevertheless, there are insuffi-
cient data to draw formal recommendations on correct timing
of pregnancy after BC,and ongoing trials are investigating
the best management of adjuvant treatments’ interruptions/
resumptions.
This analysis contributes in stressing the importance of
adequate fertility issue discussion and counseling for young
BC patients and the need to keep reducing barriers in the
field.
Ethical Approval
All proceduresperformed in studiesinvolving human
participants were in accordance with the ethical standards of
the institutional and/or national research committee and with
the Declaration of Helsinki 1964 and its later amendments or
comparable ethical standards. The study was approved by th
local Ethics Committee. For this type of study, formal con-
sent is not required.
Author Disclosure Statement
No competing financial interests exist.
References
1. Torre LA,Bray F,SiegelRL, Ferlay J,Lortet-TieulentJ,
Jemal A.Global cancer statistics,2012.CA Cancer J Clin
2015;65:87–108.
2. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-
Foucher E, Bray F. Cancer incidence and mortality among
young adultsaged 20–39 yearsworldwide in 2012:A
population-based study. Lancet Oncol 2017;18:1579–1589.
3. AIRTUM-AIOM. The numbers ofcancerin Italy 2017.
Il Pensiero Scientifico Editore,2017.
6 DIECI ET AL.
to adequately respond to patients on these issues. Due to the
limited sample size of our cohortand to the absence of a
controlgroup,data on pregnancy after BC are exploratory
and descriptive in nature.Indeed,the risk of selection bias
called ‘‘health mother effect’’ could not be controlled in our
study.Even so,it is reassuring thatour experience ap-
pears somehow consistent with more recent evidence from
large cohorts reporting thatpregnancy does notincrease
risk of BC relapse,even in HR+ BC patients,4,5 and that
newborns are not exposed to higher risk of prenatal/perinatal
complications.13
Interestingly, observing the characteristics of patients who
successfully became pregnant after BC may also help dispel
some presumptions regarding which patients should undergo
fertility preservation and fertility preservation counseling. In
fact, both age and parity are well-known barriers to fertility
preservation counseling as clinicians may be induced to as-
sume that patients have completed child-bearing in the first
case,or have fully satisfied theirdesire forfamily in the
second case.14–16In our cohort of patients who successfully
mothered children after BC, we observed 7 patients who al-
ready had children at time of diagnosis, and patients as old as
40 years at diagnosis, thus pointing out that motherhood after
cancer mightnotbe a prerogative of extremely young pa-
tients nor a desire reserved to childless women.
This leads to the other aim of the study,which was to
evaluate physician awareness regarding fertility issues by
assessing time-dependentchanges in a 10-yearperiod in
fertility preservation counseling and techniques in young BC
patients undergoing neo/adjuvant chemotherapy.
In both time cohorts (2004–2006 and 2014–2016),the
majority ofBC patients aged £40 years had evidence in
medicalrecords of discussion on fertility issues and/or ap-
plication offertility preservation methods.This compares
favorably with published data reporting a counseling rate of
around 26% in BC patients.14,16Nevertheless,the most re-
centcohortshowed a significantly higherrate (82.9% vs.
66.0%, p = 0.017) of fertility counseling documentation and/
or application of fertility preservation methods. This differ-
ence is mostly due to an increase in the documentation of
fertility counseling (67.6% vs. 34.0%, p = 0.06).
Even if counseling does not finally translate in the appli-
cation of fertility preservation techniques, its documentation
in medicalrecordsis not irrelevant.Actually,it reflects
physicians’ awareness and the importance attributed to ad-
dressing fertility issues before initiation of treatment,inde-
pendently from the decision taken by the patient.Clinical
data show thatfemale survivors who previously received
fertility counseling have less regret and improved quality of
life, independently from the final decision on pursuing or not
the fertility preservation.17
In our study, the proportion of patients aged >35 years and/
or who already had children receiving counseling was sig-
nificantly higher in the more recent cohort versus the older
cohort,highlighting progressive increase in clinician’s per-
ceived importance of extending counseling to all young BC
patients. These modifications in clinical practice adequately
mirror the evolution of guidelines,as depicted by the 2013
ASCO recommendation that no patient should be excluded
from fertility preservation discussion forany reason,in-
cluding age, prognosis, socioeconomic status, or parity.18,19
In the most recent cohort (2014–2016),9.0% of patients
finally underwentoocyte cryopreservation procedures,an
acceptance rate similar to the one reported by Ruddy et al. in
2014.20 However,in both the 2004–2006 cohortand the
2014–2016 cohort,more than 60% of patients received an
GnRH analog during chemotherapy. This is in keeping with
the general attitude of Italian oncologists: in a recent survey,
aimed to investigate the approach of Italian oncologists and
breast surgeons dealing with BC to fertility issues,65% of
panelistsdeclared to useconcomitantadministration of
GnRH analogs and chemotherapy regularly in their common
clinical practice.21 This attitude might be influenced by the
fact that the PROMISE trial,a large randomized trial con-
cerning the use of GnRH analogs in this setting,was con-
ducted in Italy.22Moreover, this option is also recommended
by national oncological guidelines.23
Conclusions
Physicians’ attention to fertility issues and counseling has
increased over time, also regarding women who already hav
children and older patients.
In this small retrospective cohort, no safety concerns were
identified for pregnancy after BC. However, due to the lim-
ited sample size of our cohort and to the absence of a contro
group,data on pregnancy after BC are only descriptive,as
risk of selection bias could not be controlled.Even so,this
evidence appears consistentwith data from large cohorts
reporting that pregnancy does not increase risk of BC relapse
even in HR+ BC patients.4,5Nevertheless, there are insuffi-
cient data to draw formal recommendations on correct timing
of pregnancy after BC,and ongoing trials are investigating
the best management of adjuvant treatments’ interruptions/
resumptions.
This analysis contributes in stressing the importance of
adequate fertility issue discussion and counseling for young
BC patients and the need to keep reducing barriers in the
field.
Ethical Approval
All proceduresperformed in studiesinvolving human
participants were in accordance with the ethical standards of
the institutional and/or national research committee and with
the Declaration of Helsinki 1964 and its later amendments or
comparable ethical standards. The study was approved by th
local Ethics Committee. For this type of study, formal con-
sent is not required.
Author Disclosure Statement
No competing financial interests exist.
References
1. Torre LA,Bray F,SiegelRL, Ferlay J,Lortet-TieulentJ,
Jemal A.Global cancer statistics,2012.CA Cancer J Clin
2015;65:87–108.
2. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-
Foucher E, Bray F. Cancer incidence and mortality among
young adultsaged 20–39 yearsworldwide in 2012:A
population-based study. Lancet Oncol 2017;18:1579–1589.
3. AIRTUM-AIOM. The numbers ofcancerin Italy 2017.
Il Pensiero Scientifico Editore,2017.
6 DIECI ET AL.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4. Azim HA, Jr., Kroman N,Paesmans M,et al.Prognostic
impactof pregnancy after breastcancer according to es-
trogen receptor status: A multicenter retrospective study. J
Clin Oncol 2013;31:73–79.
5. LambertiniM, Kroman N,Ameye L, et al. Long-term
safety of pregnancy following breast cancer according to
estrogen receptorstatus.J Natl CancerInst 2018;110:
426–429.
6. Cardoso F, Loibl S, Pagani O, et al. The European Society
of BreastCancerSpecialistsrecommendationsfor the
managementof young women with breastcancer.Eur J
Cancer 2012;48:3355–3377.
7. PeccatoriFA, Azim HA, Jr., Orecchia R,et al. Cancer,
pregnancy and fertility: ESMO Clinical Practice Guidelines
for diagnosis,treatmentand follow-up.Ann Oncol2013;
24(Suppl 6):vi160–vi170.
8. Paluch-Shimon S,PaganiO, Partridge AH,et al. ESO-
ESMO 3rd internationalconsensusguidelinesfor breast
cancer in young women (BCY3). Breast 2017;35:203–217.
9. LambertiniM, AnseriniP, LevaggiA, Poggio F, Del
Mastro L.Fertility counseling of young breastcancer pa-
tients.J Thorac Dis 2013;5(Suppl 1):S68–S80.
10. Matthews TJ,Hamilton BE.Delayed childbearing:More
women are having their first child later in life. NCHS Data
Brief 2009;21:1–8.
11. Litton JK. Breastcancer and fertility.Curr TreatOptions
Oncol 2012;13:137–145.
12. Letourneau JM,Smith JF,EbbelEE, et al. Racial,socio-
economic, and demographic disparities in access to fertility
preservationin youngwomendiagnosedwith cancer.
Cancer 2012;118:4579–4588.
13. Jacob L,Kalder M,Arabin B,Kostev K.Impactof prior
breast cancer on mode of delivery and pregnancy-associated
disorders: A retrospective analysis of subsequent pregnancy
outcomes. J Cancer Res Clin Oncol 2017;143:1069–1074.
14. Quinn GP,Block RG,Clayman ML,et al. If you did not
document it,it did not happen: Rates of documentation of
discussion of infertility risk in adolescent and young adult
oncology patients’ medical records. J Oncol Pract 2015;11:
137–144.
15. Penrose R, Beatty L, Mattiske J, Koczwara B. Fertility and
cancer—A qualitative study of Australian cancer survivors.
Support Care Cancer 2012;20:1259–1265.
16. McCray DK, Simpson AB,Flyckt R, et al. Fertility in
women of reproductive age after breast cancer treatment:
Practice patterns and outcomes. Ann Surg Oncol 2016;23:
3175–3181.
17. Letourneau JM,EbbelEE, Katz PP, et al. Pretreatment
fertility counselingand fertility preservationimprove
quality oflife in reproductive age women with cancer.
Cancer 2012;118:1710–1717.
18. Lee SJ, Schover LR, Partridge AH, et al. American Society
of Clinical Oncology recommendations on fertility preser-
vation in cancer patients. J Clin Oncol 2006;24:2917–2931.
19. Loren AW,Mangu PB,Beck LN,et al.Fertility preserva-
tion for patients with cancer: American Society of Clinical
Oncology clinicalpractice guideline update.J Clin Oncol
2013;31:2500–2510.
20. Ruddy KJ, GelberSI, TamimiRM, et al. Prospective
study of fertility concerns and preservation strategies in
young women with breastcancer.J Clin Oncol 2014;11:
1151–1156.
21. Biglia N, Torrisi R, Codacci Pisanelli G, Rota S, Peccatori
FA. Gynecologicalendocrinology attitudes on fertility is-
sues in breast cancer patients: An Italian survey attitudes on
fertility issues in breast cancer patients: An Italian survey.
Gynecol Endocrinol 2015;31:458–464.
22. Del Mastro L,Boni L, MichelottiA, et al. Effectof the
gonadotropin-releasing hormone analogue triptorelin on the
occurrence ofchemotherapy-induced early menopause in
premenopausalwomen with breastcancer.JAMA 2011;
306:269–276.
23. Lambertini M, Cinquini M, Moschetti I, et al.Temporary
ovariansuppressionduring chemotherapyto preserve
ovarian function and fertility in breast cancer patients: A
GRADE approach forevidence evaluation and recom-
mendations by the Italian Association of Medical Oncol-
ogy.Eur J Cancer 2017;71:25–33.
Address correspondence to:
Maria Vittoria Dieci,MD
Department of Surgery,Oncology and Gastroenterology
University of Padova
Via Gattamelata 64
Padova 35128
Italy
E-mail: mariavittoria.dieci@unipd.it
FERTILITY PRESERVATION IN BREAST CANCER 7
impactof pregnancy after breastcancer according to es-
trogen receptor status: A multicenter retrospective study. J
Clin Oncol 2013;31:73–79.
5. LambertiniM, Kroman N,Ameye L, et al. Long-term
safety of pregnancy following breast cancer according to
estrogen receptorstatus.J Natl CancerInst 2018;110:
426–429.
6. Cardoso F, Loibl S, Pagani O, et al. The European Society
of BreastCancerSpecialistsrecommendationsfor the
managementof young women with breastcancer.Eur J
Cancer 2012;48:3355–3377.
7. PeccatoriFA, Azim HA, Jr., Orecchia R,et al. Cancer,
pregnancy and fertility: ESMO Clinical Practice Guidelines
for diagnosis,treatmentand follow-up.Ann Oncol2013;
24(Suppl 6):vi160–vi170.
8. Paluch-Shimon S,PaganiO, Partridge AH,et al. ESO-
ESMO 3rd internationalconsensusguidelinesfor breast
cancer in young women (BCY3). Breast 2017;35:203–217.
9. LambertiniM, AnseriniP, LevaggiA, Poggio F, Del
Mastro L.Fertility counseling of young breastcancer pa-
tients.J Thorac Dis 2013;5(Suppl 1):S68–S80.
10. Matthews TJ,Hamilton BE.Delayed childbearing:More
women are having their first child later in life. NCHS Data
Brief 2009;21:1–8.
11. Litton JK. Breastcancer and fertility.Curr TreatOptions
Oncol 2012;13:137–145.
12. Letourneau JM,Smith JF,EbbelEE, et al. Racial,socio-
economic, and demographic disparities in access to fertility
preservationin youngwomendiagnosedwith cancer.
Cancer 2012;118:4579–4588.
13. Jacob L,Kalder M,Arabin B,Kostev K.Impactof prior
breast cancer on mode of delivery and pregnancy-associated
disorders: A retrospective analysis of subsequent pregnancy
outcomes. J Cancer Res Clin Oncol 2017;143:1069–1074.
14. Quinn GP,Block RG,Clayman ML,et al. If you did not
document it,it did not happen: Rates of documentation of
discussion of infertility risk in adolescent and young adult
oncology patients’ medical records. J Oncol Pract 2015;11:
137–144.
15. Penrose R, Beatty L, Mattiske J, Koczwara B. Fertility and
cancer—A qualitative study of Australian cancer survivors.
Support Care Cancer 2012;20:1259–1265.
16. McCray DK, Simpson AB,Flyckt R, et al. Fertility in
women of reproductive age after breast cancer treatment:
Practice patterns and outcomes. Ann Surg Oncol 2016;23:
3175–3181.
17. Letourneau JM,EbbelEE, Katz PP, et al. Pretreatment
fertility counselingand fertility preservationimprove
quality oflife in reproductive age women with cancer.
Cancer 2012;118:1710–1717.
18. Lee SJ, Schover LR, Partridge AH, et al. American Society
of Clinical Oncology recommendations on fertility preser-
vation in cancer patients. J Clin Oncol 2006;24:2917–2931.
19. Loren AW,Mangu PB,Beck LN,et al.Fertility preserva-
tion for patients with cancer: American Society of Clinical
Oncology clinicalpractice guideline update.J Clin Oncol
2013;31:2500–2510.
20. Ruddy KJ, GelberSI, TamimiRM, et al. Prospective
study of fertility concerns and preservation strategies in
young women with breastcancer.J Clin Oncol 2014;11:
1151–1156.
21. Biglia N, Torrisi R, Codacci Pisanelli G, Rota S, Peccatori
FA. Gynecologicalendocrinology attitudes on fertility is-
sues in breast cancer patients: An Italian survey attitudes on
fertility issues in breast cancer patients: An Italian survey.
Gynecol Endocrinol 2015;31:458–464.
22. Del Mastro L,Boni L, MichelottiA, et al. Effectof the
gonadotropin-releasing hormone analogue triptorelin on the
occurrence ofchemotherapy-induced early menopause in
premenopausalwomen with breastcancer.JAMA 2011;
306:269–276.
23. Lambertini M, Cinquini M, Moschetti I, et al.Temporary
ovariansuppressionduring chemotherapyto preserve
ovarian function and fertility in breast cancer patients: A
GRADE approach forevidence evaluation and recom-
mendations by the Italian Association of Medical Oncol-
ogy.Eur J Cancer 2017;71:25–33.
Address correspondence to:
Maria Vittoria Dieci,MD
Department of Surgery,Oncology and Gastroenterology
University of Padova
Via Gattamelata 64
Padova 35128
Italy
E-mail: mariavittoria.dieci@unipd.it
FERTILITY PRESERVATION IN BREAST CANCER 7
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



