Foetal Respiratory Distress Syndrome: A Comprehensive Analysis
VerifiedAdded on 2023/03/29
|8
|1576
|220
Essay
AI Summary
This essay provides a detailed analysis of Fetal Respiratory Distress Syndrome (RDS), a common respiratory complication in preterm infants, also known as hyaline membrane disease. It explores the etiology and pathophysiology of RDS, emphasizing the role of surfactant deficiency and atelectasis. The essay outlines the diagnostic methods, including chest X-rays and blood gas tests, and details the signs and symptoms such as tachypnea, retractions, and grunting. Furthermore, it examines the embryological development of the respiratory system, highlighting the crucial stages of surfactant production. The paper concludes with a summary of the key points, emphasizing the importance of understanding RDS for effective management and future research. The essay references various studies and provides a comprehensive overview of the condition.

1
Respiratory Distress Syndrome (RDS)
Name:
Institution:
Tutor:
Date:
Respiratory Distress Syndrome (RDS)
Name:
Institution:
Tutor:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Introduction
Fetal Respiratory Distress Syndrome (RDS), otherwise known as hyaline membrane disease, is
one of the most common respiratory complications among preterm infants. Preterm infants have
structurally immature lungs with low surfactant thus they are prone to atelectasis which is the
cause of the condition. It is diagnosed among any preterm infants with breathing difficulties,
tachypnea, retractions, and grunting. RDS is a serious condition with statistics showing that
40,000 infants in Australia are affected per year. Furthermore, it also contributes to 20% of
deaths in Australia (Tagami, Sakka, & Monnet, 2018, p. 305). Within the framework of this
essay, there will be an analysis of the etiology and pathophysiology of RDS, diagnosis, signs and
symptoms and the embryological development of the disease. There will also be a conclusion to
summarize the main points in the essay
The etiology and pathophysiology of the disease
Several studies agree that the major cause of respiratory Distress syndrome in children is an
inadequate pulmonary surfactant. Pulmonary surfactant, according to Thompson, Chambers, &
Liu, is defined or rather is a composition of lipids and proteins produced by epithelial cells in the
alveolar spaces (Thompson, Chambers, & Liu, 2017, p. 568). The main function is to lower the
surface tension at the air and liquid interface of the lungs. Since the lungs in infants and
structurally immature and low in surfactant, they have a low tendency to both compliance and
the tendency to atelectasis. Other studies also note that low alveolar radius and a feeble chest
wall contribute to atelectasis (Sweet et al., 2016, p. 122). Atelectasis then leads to
hypoventilation in alveolar space and V/Q mismatch in the well perfused but impartially
ventilated sections of the lungs. V/Q mismatch is defined as the imbalance in exchange of air
between the lungs and the environment and the flow of blood in the lungs. This two conditions
Introduction
Fetal Respiratory Distress Syndrome (RDS), otherwise known as hyaline membrane disease, is
one of the most common respiratory complications among preterm infants. Preterm infants have
structurally immature lungs with low surfactant thus they are prone to atelectasis which is the
cause of the condition. It is diagnosed among any preterm infants with breathing difficulties,
tachypnea, retractions, and grunting. RDS is a serious condition with statistics showing that
40,000 infants in Australia are affected per year. Furthermore, it also contributes to 20% of
deaths in Australia (Tagami, Sakka, & Monnet, 2018, p. 305). Within the framework of this
essay, there will be an analysis of the etiology and pathophysiology of RDS, diagnosis, signs and
symptoms and the embryological development of the disease. There will also be a conclusion to
summarize the main points in the essay
The etiology and pathophysiology of the disease
Several studies agree that the major cause of respiratory Distress syndrome in children is an
inadequate pulmonary surfactant. Pulmonary surfactant, according to Thompson, Chambers, &
Liu, is defined or rather is a composition of lipids and proteins produced by epithelial cells in the
alveolar spaces (Thompson, Chambers, & Liu, 2017, p. 568). The main function is to lower the
surface tension at the air and liquid interface of the lungs. Since the lungs in infants and
structurally immature and low in surfactant, they have a low tendency to both compliance and
the tendency to atelectasis. Other studies also note that low alveolar radius and a feeble chest
wall contribute to atelectasis (Sweet et al., 2016, p. 122). Atelectasis then leads to
hypoventilation in alveolar space and V/Q mismatch in the well perfused but impartially
ventilated sections of the lungs. V/Q mismatch is defined as the imbalance in exchange of air
between the lungs and the environment and the flow of blood in the lungs. This two conditions
3
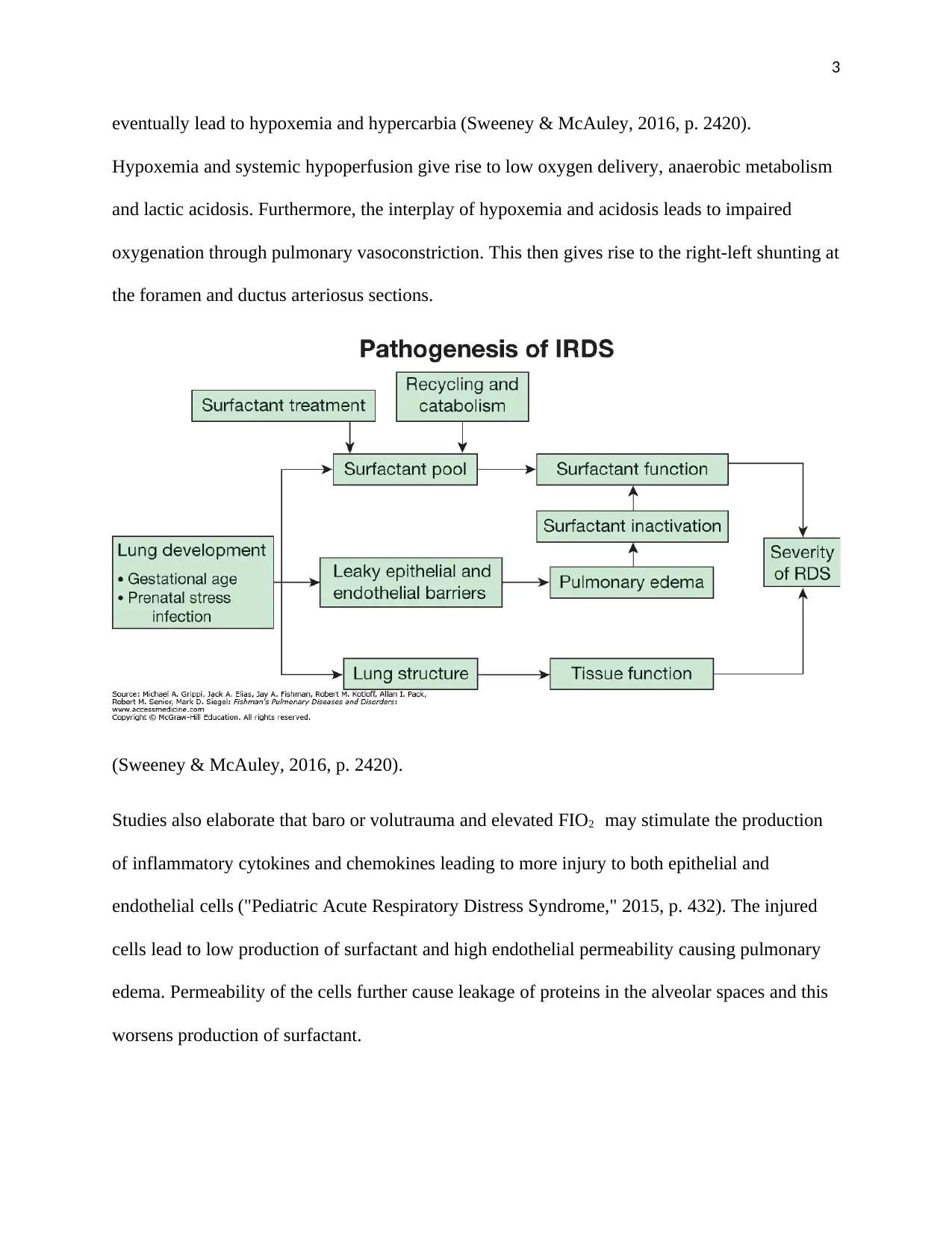
eventually lead to hypoxemia and hypercarbia (Sweeney & McAuley, 2016, p. 2420).
Hypoxemia and systemic hypoperfusion give rise to low oxygen delivery, anaerobic metabolism
and lactic acidosis. Furthermore, the interplay of hypoxemia and acidosis leads to impaired
oxygenation through pulmonary vasoconstriction. This then gives rise to the right-left shunting at
the foramen and ductus arteriosus sections.
(Sweeney & McAuley, 2016, p. 2420).
Studies also elaborate that baro or volutrauma and elevated FIO2 may stimulate the production
of inflammatory cytokines and chemokines leading to more injury to both epithelial and
endothelial cells ("Pediatric Acute Respiratory Distress Syndrome," 2015, p. 432). The injured
cells lead to low production of surfactant and high endothelial permeability causing pulmonary
edema. Permeability of the cells further cause leakage of proteins in the alveolar spaces and this
worsens production of surfactant.
eventually lead to hypoxemia and hypercarbia (Sweeney & McAuley, 2016, p. 2420).
Hypoxemia and systemic hypoperfusion give rise to low oxygen delivery, anaerobic metabolism
and lactic acidosis. Furthermore, the interplay of hypoxemia and acidosis leads to impaired
oxygenation through pulmonary vasoconstriction. This then gives rise to the right-left shunting at
the foramen and ductus arteriosus sections.
(Sweeney & McAuley, 2016, p. 2420).
Studies also elaborate that baro or volutrauma and elevated FIO2 may stimulate the production
of inflammatory cytokines and chemokines leading to more injury to both epithelial and
endothelial cells ("Pediatric Acute Respiratory Distress Syndrome," 2015, p. 432). The injured
cells lead to low production of surfactant and high endothelial permeability causing pulmonary
edema. Permeability of the cells further cause leakage of proteins in the alveolar spaces and this
worsens production of surfactant.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
The likely tests that would be used to diagnose the disease
There are different tests that can be used to diagnose Respiratory Distress Syndrome. The first
test is a chest X-ray of the lungs. It is used to check images of lungs for any abnormalities. Other
tests include blood gas tests (Han & Mallampalli, 2015, p. 857). This test investigates the level of
oxygen, carbon dioxide and acids in arterial blood. Low oxygen and high carbon dioxide indicate
RDS. Echocardiography is a test that looks at the structure of the heart to rule out the possibility
of heart problems. Physical assessment is also done to look out for the appearance, color and
breathing efforts of the infant.
The symptoms and test results likely found in the infant
The signs and symptoms of respiratory distress syndrome normally appear immediately after the
infant is born. Some of the common signs and symptoms that have been recorded included
tachypnea. This is a condition where the infant is breathing at a very high speed or rate. It is
characterized by more than 65 breaths per minute. Other significant signs and symptoms include
nasal flaring, intercostal and subcostal retractions, grunting and even cyanosis at room air.
Studies by Fuller, Mohr, & Kollef elaborate that tachypnea is due to increased minute ventilation
as the body tries to compensate for low tidal volume and elevated dead space (Fuller, Mohr, &
Kollef, 2018). Retractions occur since the baby is forced to produce high intrathoracic pressure
so as to expand the already poor compliant lungs. As the body attempts to maintain FRC through
forced expiration, the glottis is closed and this leads to grunting. At this stage, studies
recommend resuscitation and stabilization. If the case is complicated, it will progress for 48 to 72
hours (Grissom et al., 2015, p. 290). Other studies note that recovery often coincides with
diuresis after oliguria. Other signs and test results are hypotension, acidosis, and hyperkalemia.
A chest radiograph would indicate lung volumes as well as the bilateral, reticular granular
The likely tests that would be used to diagnose the disease
There are different tests that can be used to diagnose Respiratory Distress Syndrome. The first
test is a chest X-ray of the lungs. It is used to check images of lungs for any abnormalities. Other
tests include blood gas tests (Han & Mallampalli, 2015, p. 857). This test investigates the level of
oxygen, carbon dioxide and acids in arterial blood. Low oxygen and high carbon dioxide indicate
RDS. Echocardiography is a test that looks at the structure of the heart to rule out the possibility
of heart problems. Physical assessment is also done to look out for the appearance, color and
breathing efforts of the infant.
The symptoms and test results likely found in the infant
The signs and symptoms of respiratory distress syndrome normally appear immediately after the
infant is born. Some of the common signs and symptoms that have been recorded included
tachypnea. This is a condition where the infant is breathing at a very high speed or rate. It is
characterized by more than 65 breaths per minute. Other significant signs and symptoms include
nasal flaring, intercostal and subcostal retractions, grunting and even cyanosis at room air.
Studies by Fuller, Mohr, & Kollef elaborate that tachypnea is due to increased minute ventilation
as the body tries to compensate for low tidal volume and elevated dead space (Fuller, Mohr, &
Kollef, 2018). Retractions occur since the baby is forced to produce high intrathoracic pressure
so as to expand the already poor compliant lungs. As the body attempts to maintain FRC through
forced expiration, the glottis is closed and this leads to grunting. At this stage, studies
recommend resuscitation and stabilization. If the case is complicated, it will progress for 48 to 72
hours (Grissom et al., 2015, p. 290). Other studies note that recovery often coincides with
diuresis after oliguria. Other signs and test results are hypotension, acidosis, and hyperkalemia.
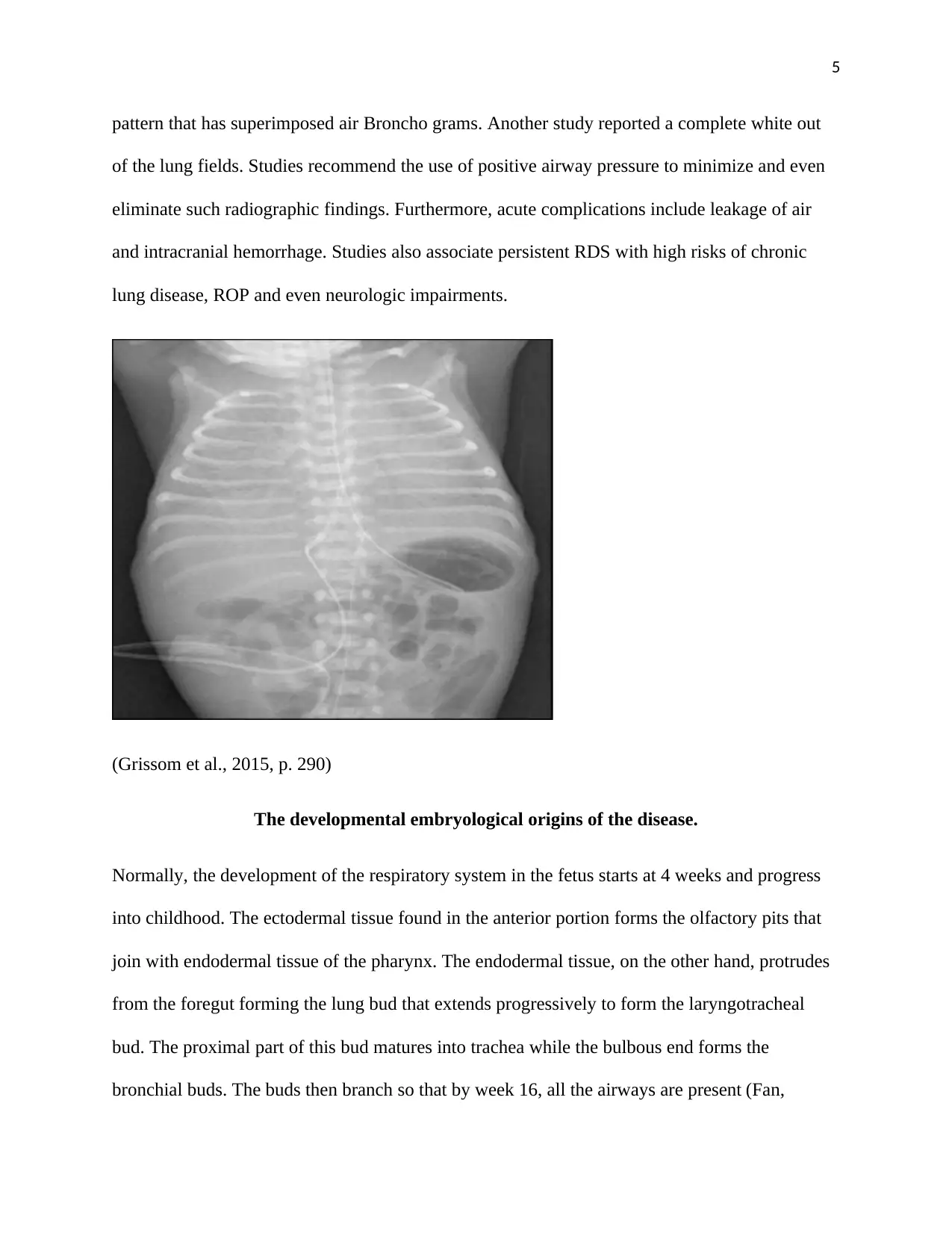
A chest radiograph would indicate lung volumes as well as the bilateral, reticular granular
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
5
pattern that has superimposed air Broncho grams. Another study reported a complete white out
of the lung fields. Studies recommend the use of positive airway pressure to minimize and even
eliminate such radiographic findings. Furthermore, acute complications include leakage of air
and intracranial hemorrhage. Studies also associate persistent RDS with high risks of chronic
lung disease, ROP and even neurologic impairments.
(Grissom et al., 2015, p. 290)
The developmental embryological origins of the disease.
Normally, the development of the respiratory system in the fetus starts at 4 weeks and progress
into childhood. The ectodermal tissue found in the anterior portion forms the olfactory pits that
join with endodermal tissue of the pharynx. The endodermal tissue, on the other hand, protrudes
from the foregut forming the lung bud that extends progressively to form the laryngotracheal
bud. The proximal part of this bud matures into trachea while the bulbous end forms the
bronchial buds. The buds then branch so that by week 16, all the airways are present (Fan,
pattern that has superimposed air Broncho grams. Another study reported a complete white out
of the lung fields. Studies recommend the use of positive airway pressure to minimize and even
eliminate such radiographic findings. Furthermore, acute complications include leakage of air
and intracranial hemorrhage. Studies also associate persistent RDS with high risks of chronic
lung disease, ROP and even neurologic impairments.
(Grissom et al., 2015, p. 290)
The developmental embryological origins of the disease.
Normally, the development of the respiratory system in the fetus starts at 4 weeks and progress
into childhood. The ectodermal tissue found in the anterior portion forms the olfactory pits that
join with endodermal tissue of the pharynx. The endodermal tissue, on the other hand, protrudes
from the foregut forming the lung bud that extends progressively to form the laryngotracheal
bud. The proximal part of this bud matures into trachea while the bulbous end forms the
bronchial buds. The buds then branch so that by week 16, all the airways are present (Fan,

6
Brodie, & Slutsky, 2018, p. 306). After 16 weeks, respiratory bronchioles and alveolar ducts are
also formed. It is also at this stage that alveolar type 1 cells are produced. Type II pulmonary
cells also develop and begin synthesizing of surfactant. As the fetus grows, the respiratory
system also continues to expand and more of alveoli and surfactant are produced. At week 36,
the alveolar precursors are mature and fully functional (Bellani et al., 2016, p. 788). During birth,
the thoracic cavity is compressed and this expels most of the fluids from the lungs. During the
initial inhalation of the infant, the lungs become inflated. Breathing movements start between
weeks 20 or 21 (Bellani et al., 2016, p. 788). This only happens with contractions of respiratory
muscles that cause the fetus to inhale and exhale amniotic fluid. These developments occur
progressively until birth.
As explained in the previous section of etiology and pathophysiology, respiratory distress
syndrome is caused by low or absent surfactant. Production of the surfactant begins at week 16
due to the presence of Type 1 and type II pulmonary cells. It goes one up to week 36 where most
of the surfactant is produced. During preterm births, an infant is borne before it attains the
recommended number of weeks, normally below 36 weeks (Amato et al., 2015, p. 751). This
implies that the process of producing surfactant has yet to reach the optimal level. Diminished
production of surfactant in such infants is the risk factor for respiratory distress syndrome.
Conclusion
Respiratory distress syndrome is a condition affecting more than 40,000 children annually and
contributing to 20% of the mortality rates. It is therefore important that physicians understand the
pathophysiology, diagnosis, signs and symptoms and management of RDS. Furthermore, more
research should be done to come up with effective management strategies
Brodie, & Slutsky, 2018, p. 306). After 16 weeks, respiratory bronchioles and alveolar ducts are
also formed. It is also at this stage that alveolar type 1 cells are produced. Type II pulmonary
cells also develop and begin synthesizing of surfactant. As the fetus grows, the respiratory
system also continues to expand and more of alveoli and surfactant are produced. At week 36,
the alveolar precursors are mature and fully functional (Bellani et al., 2016, p. 788). During birth,
the thoracic cavity is compressed and this expels most of the fluids from the lungs. During the
initial inhalation of the infant, the lungs become inflated. Breathing movements start between
weeks 20 or 21 (Bellani et al., 2016, p. 788). This only happens with contractions of respiratory
muscles that cause the fetus to inhale and exhale amniotic fluid. These developments occur
progressively until birth.
As explained in the previous section of etiology and pathophysiology, respiratory distress
syndrome is caused by low or absent surfactant. Production of the surfactant begins at week 16
due to the presence of Type 1 and type II pulmonary cells. It goes one up to week 36 where most
of the surfactant is produced. During preterm births, an infant is borne before it attains the
recommended number of weeks, normally below 36 weeks (Amato et al., 2015, p. 751). This
implies that the process of producing surfactant has yet to reach the optimal level. Diminished
production of surfactant in such infants is the risk factor for respiratory distress syndrome.
Conclusion
Respiratory distress syndrome is a condition affecting more than 40,000 children annually and
contributing to 20% of the mortality rates. It is therefore important that physicians understand the
pathophysiology, diagnosis, signs and symptoms and management of RDS. Furthermore, more
research should be done to come up with effective management strategies
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
References
1. Amato, M. B., Meade, M. O., Slutsky, A. S., Brochard, L., Costa, E. L.,
Schoenfeld, D. A., … Brower, R. G. 2015. Driving Pressure and Survival in the Acute
Respiratory Distress Syndrome. New England Journal of Medicine, 372(8), 747-755.
doi:10.1056/nejmsa1410639
2. Bellani, G., Laffey, J. G., Pham, T., Fan, E., Brochard, L., & Esteban, A. 2016.
Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory
Distress Syndrome in Intensive Care Units in 50 Countries. JAMA, 315(8), 788.
doi:10.1001/jama.2016.0291
3. Fan, E., Brodie, D., & Slutsky, A. S. 2018. Diagnosis and Treatment in Acute Respiratory
Distress Syndrome—Reply. JAMA, 320(3), 306. doi:10.1001/jama.2018.5932
4. Fuller, B. M., Mohr, N. M., & Kollef, M. H. 2018. Diagnosis and Treatment of Acute
Respiratory Distress Syndrome. JAMA, 320(3), 305. doi:10.1001/jama.2018.5928
5. Grissom, C. K., Hirshberg, E. L., Dickerson, J. B., Brown, S. M., Lanspa, M. J.,
Liu, K. D., … Morris, A. H. 2015. Fluid Management With a Simplified Conservative
Protocol for the Acute Respiratory Distress Syndrome*. Critical Care Medicine, 43(2),
288-295. doi:10.1097/ccm.0000000000000715
6. Han, S., & Mallampalli, R. K. 2015. The Acute Respiratory Distress Syndrome: From
Mechanism to Translation. The Journal of Immunology, 194(3), 855-860.
doi:10.4049/jimmunol.1402513
7. Pediatric Acute Respiratory Distress Syndrome. 2015. Pediatric Critical Care Medicine,
16(5), 428-439. doi:10.1097/pcc.0000000000000350
References
1. Amato, M. B., Meade, M. O., Slutsky, A. S., Brochard, L., Costa, E. L.,
Schoenfeld, D. A., … Brower, R. G. 2015. Driving Pressure and Survival in the Acute
Respiratory Distress Syndrome. New England Journal of Medicine, 372(8), 747-755.
doi:10.1056/nejmsa1410639
2. Bellani, G., Laffey, J. G., Pham, T., Fan, E., Brochard, L., & Esteban, A. 2016.
Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory
Distress Syndrome in Intensive Care Units in 50 Countries. JAMA, 315(8), 788.
doi:10.1001/jama.2016.0291
3. Fan, E., Brodie, D., & Slutsky, A. S. 2018. Diagnosis and Treatment in Acute Respiratory
Distress Syndrome—Reply. JAMA, 320(3), 306. doi:10.1001/jama.2018.5932
4. Fuller, B. M., Mohr, N. M., & Kollef, M. H. 2018. Diagnosis and Treatment of Acute
Respiratory Distress Syndrome. JAMA, 320(3), 305. doi:10.1001/jama.2018.5928
5. Grissom, C. K., Hirshberg, E. L., Dickerson, J. B., Brown, S. M., Lanspa, M. J.,
Liu, K. D., … Morris, A. H. 2015. Fluid Management With a Simplified Conservative
Protocol for the Acute Respiratory Distress Syndrome*. Critical Care Medicine, 43(2),
288-295. doi:10.1097/ccm.0000000000000715
6. Han, S., & Mallampalli, R. K. 2015. The Acute Respiratory Distress Syndrome: From
Mechanism to Translation. The Journal of Immunology, 194(3), 855-860.
doi:10.4049/jimmunol.1402513
7. Pediatric Acute Respiratory Distress Syndrome. 2015. Pediatric Critical Care Medicine,
16(5), 428-439. doi:10.1097/pcc.0000000000000350
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
8. Sweeney, R. M., & McAuley, D. F. 2016. Acute respiratory distress syndrome. The
Lancet, 388(10058), 2416-2430. doi:10.1016/s0140-6736(16)00578-x
9. Sweet, D. G., Carnielli, V., Greisen, G., Hallman, M., Ozek, E., Plavka, R., …
Halliday, H. L. 2016. European Consensus Guidelines on the Management of Respiratory
Distress Syndrome - 2016 Update. Neonatology, 111(2), 107-125.
doi:10.1159/000448985
10. Tagami, T., Sakka, S. G., & Monnet, X. 2018. Diagnosis and Treatment of Acute
Respiratory Distress Syndrome. JAMA, 320(3), 305. doi:10.1001/jama.2018.5924
11. Thompson, B. T., Chambers, R. C., & Liu, K. D. 2017. Acute Respiratory Distress
Syndrome. New England Journal of Medicine, 377(6), 562-572.
doi:10.1056/nejmra1608077
8. Sweeney, R. M., & McAuley, D. F. 2016. Acute respiratory distress syndrome. The
Lancet, 388(10058), 2416-2430. doi:10.1016/s0140-6736(16)00578-x
9. Sweet, D. G., Carnielli, V., Greisen, G., Hallman, M., Ozek, E., Plavka, R., …
Halliday, H. L. 2016. European Consensus Guidelines on the Management of Respiratory
Distress Syndrome - 2016 Update. Neonatology, 111(2), 107-125.
doi:10.1159/000448985
10. Tagami, T., Sakka, S. G., & Monnet, X. 2018. Diagnosis and Treatment of Acute
Respiratory Distress Syndrome. JAMA, 320(3), 305. doi:10.1001/jama.2018.5924
11. Thompson, B. T., Chambers, R. C., & Liu, K. D. 2017. Acute Respiratory Distress
Syndrome. New England Journal of Medicine, 377(6), 562-572.
doi:10.1056/nejmra1608077
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
