Flow Cytometry Analysis Report: University Biology Assignment Report
VerifiedAdded on 2023/04/22
|6
|1194
|178
Report
AI Summary
This report analyzes a technical cytometry publication (Crawford et al., 2014) to provide an overview of how flow cytometry can be used to analyze different cell populations and describe the effects of PMA+Ionomycin induced triggering on both ERK1/2 phosphorylation and prolonged IFN-gamma production in human T cells. Flow cytometry, a powerful technique, is used to detect cellular function and identify responses. The study uses phospho-flow to detect intracellular kinase signaling intermediates and combines it with surface markers to observe changes in kinase pathways by cellular subset. The report details experiments where T cells were stimulated with PMA+Ionomycin, and the phosphorylation of ERK1/2 and production of IFN-gamma were assessed. The results reveal that ERK1/2 phosphorylation is rapid and sustained, while IFN-gamma production is slower and requires a longer stimulation period. The report supports its findings with data from the publication, including figures, and correctly cites the references used.

Running head: ANALYSE USING FLOW CYTOMETRY
Analyse using Flow Cytometry
Name of the Student
Name of the University
Author’s Note:
Analyse using Flow Cytometry
Name of the Student
Name of the University
Author’s Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1ANALYSE USING FLOW CYTOMETRY
Answer to the question number 1:
Flow cytometry is one of the modern technology which can be used for the detection of
responses from the cellular function. Various cellular function can be detected like the use of
ICS or intracellular cytokine staining for the detection of cytokines inflammatory production
(Freer and Rindi 2013; Lovelace and Maecker 2018) or the use of phosphor specific
antibodies for the detection of the kinase signalling intermediates of intracellular
phosphorylation (Schulz et al. 2012). These two different cellular function can be detected in
flow cytometry in a single experiment thus allowing to examine the associations between
effector functions and signalling pathway activity. This experimental method can be very
much beneficial in case of viral infections, autoimmune conditions, or cancers as these kind
of disease’s cellular kinase activity have an effect on cell function immunity and can lead to
the understanding of disease pathogenesis (Gorelik and Richardson 2010; Steelman et al.
2011)). It has been reported in the recent study that the sub populations of activated CD8+ T
cells in adults is signal regulated by extracellular kinase during early HIV infection. The
changes in the signalling pathway of CD8+ T cells may have an effect on the immune
function of the CD8+ T cells. Recently, Crawford et al. (2014) have developed a combined
protocol of Phospho- Flow- ICS to present the CD8+ T cells impairments on the HIV
infected humans. This protocol was also able to display the ERK1/2 (p-ERK1/2-refractory)
phosphorylation, cytotoxic capacity, and cytokine production (IFN-c) deficits. This ICS and
phosphor- flow assay utilizes permeabilization and fixation agents which allow antibody
facilitated identification of intra- cellular epitopes. Various T cell functions like
differentiation and proliferation can also be detected by flow cytometry method (Crawford et
al. 2014).
Answer to the question number 1:
Flow cytometry is one of the modern technology which can be used for the detection of
responses from the cellular function. Various cellular function can be detected like the use of
ICS or intracellular cytokine staining for the detection of cytokines inflammatory production
(Freer and Rindi 2013; Lovelace and Maecker 2018) or the use of phosphor specific
antibodies for the detection of the kinase signalling intermediates of intracellular
phosphorylation (Schulz et al. 2012). These two different cellular function can be detected in
flow cytometry in a single experiment thus allowing to examine the associations between
effector functions and signalling pathway activity. This experimental method can be very
much beneficial in case of viral infections, autoimmune conditions, or cancers as these kind
of disease’s cellular kinase activity have an effect on cell function immunity and can lead to
the understanding of disease pathogenesis (Gorelik and Richardson 2010; Steelman et al.
2011)). It has been reported in the recent study that the sub populations of activated CD8+ T
cells in adults is signal regulated by extracellular kinase during early HIV infection. The
changes in the signalling pathway of CD8+ T cells may have an effect on the immune
function of the CD8+ T cells. Recently, Crawford et al. (2014) have developed a combined
protocol of Phospho- Flow- ICS to present the CD8+ T cells impairments on the HIV
infected humans. This protocol was also able to display the ERK1/2 (p-ERK1/2-refractory)
phosphorylation, cytotoxic capacity, and cytokine production (IFN-c) deficits. This ICS and
phosphor- flow assay utilizes permeabilization and fixation agents which allow antibody
facilitated identification of intra- cellular epitopes. Various T cell functions like
differentiation and proliferation can also be detected by flow cytometry method (Crawford et
al. 2014).
2ANALYSE USING FLOW CYTOMETRY
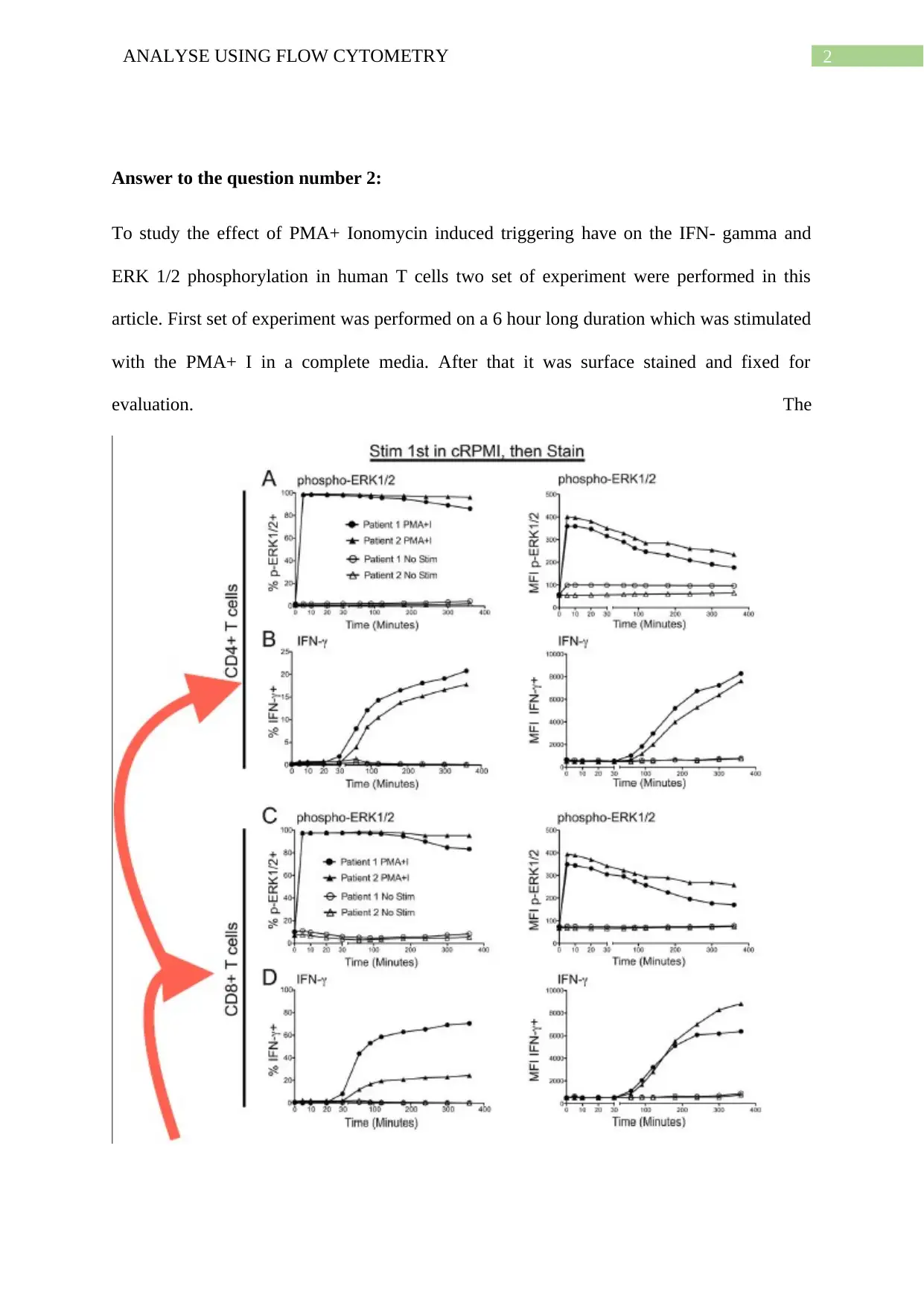
Answer to the question number 2:
To study the effect of PMA+ Ionomycin induced triggering have on the IFN- gamma and
ERK 1/2 phosphorylation in human T cells two set of experiment were performed in this
article. First set of experiment was performed on a 6 hour long duration which was stimulated
with the PMA+ I in a complete media. After that it was surface stained and fixed for
evaluation. The
Answer to the question number 2:
To study the effect of PMA+ Ionomycin induced triggering have on the IFN- gamma and
ERK 1/2 phosphorylation in human T cells two set of experiment were performed in this
article. First set of experiment was performed on a 6 hour long duration which was stimulated
with the PMA+ I in a complete media. After that it was surface stained and fixed for
evaluation. The
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3ANALYSE USING FLOW CYTOMETRY
Figure 1: The effect of PMA+ Ionomycin induced triggering have on the IFN- gamma and
ERK 1/2 phosphorylation in human T cells during the 6 hour long experiment (Source:
Crawford et al. 2014)
second set of experiment was conducted for 30 min long duration. However, unlike the first
set of experiment it was first surface stained and after that it was stimulated with PMA+ I in
PBS media which was stopped by fixation. The effect of PMA+ Ionomycin induced
triggering have on the IFN- gamma and ERK 1/2 phosphorylation in human T cells is
presented on the Figure 1. From the data presented in the Figure 1A and 1C, it can be inferred
that the ERK 1/2 phosphorylation was induced within the initial 5 minutes and almost all of T
cells were induced by it. This induced stimulation effect lasted through all the 6 hour long
duration of the experiment. Nevertheless, the intensity of the mean fluorescence seems to be
decreasing slightly from 90 minutes time point after the initial maximum stimulation. From
this findings it can be inferred that the percentage frequency and feedback inhibition of
induced p- ERK ½ cells might be declining slowing after the first 90 minutes (Crawford et al.
2014). However, stimulation of IFN- Ɣ presented a different kind of trend in comparison with
induced p- ERK ½ cells. From the Figure 1B and 1D, it can be seen that the longer
stimulation period (more than 30 minute) is needed for the detection of the IFN- Ɣ cells. In
case of both the figure, kind of sigmoid curve has been observed. The IFN- Ɣ cells were not
detected for the initial 30 minutes, then it’s increased rapidly from 30 minutes to till 2 hour
time point. After that, increment was very slowly up to the 6 hour long and a slow increment
was noticed. Another set of experiment was conducted for 30 minute long where the cells
were stained first and after that stimulation with PMA+ I and fixation were performed. In this
scenario, similar kind of trend has been observed for both the p- ERK ½ and IFN- Ɣ cells. In
this set of experiment, rapid detection of p- ERK ½ cells were noticed within the initial 2
minutes instead of first 5 minutes in comparison with the first set of experiment (6 hour
Figure 1: The effect of PMA+ Ionomycin induced triggering have on the IFN- gamma and
ERK 1/2 phosphorylation in human T cells during the 6 hour long experiment (Source:
Crawford et al. 2014)
second set of experiment was conducted for 30 min long duration. However, unlike the first
set of experiment it was first surface stained and after that it was stimulated with PMA+ I in
PBS media which was stopped by fixation. The effect of PMA+ Ionomycin induced
triggering have on the IFN- gamma and ERK 1/2 phosphorylation in human T cells is
presented on the Figure 1. From the data presented in the Figure 1A and 1C, it can be inferred
that the ERK 1/2 phosphorylation was induced within the initial 5 minutes and almost all of T
cells were induced by it. This induced stimulation effect lasted through all the 6 hour long
duration of the experiment. Nevertheless, the intensity of the mean fluorescence seems to be
decreasing slightly from 90 minutes time point after the initial maximum stimulation. From
this findings it can be inferred that the percentage frequency and feedback inhibition of
induced p- ERK ½ cells might be declining slowing after the first 90 minutes (Crawford et al.
2014). However, stimulation of IFN- Ɣ presented a different kind of trend in comparison with
induced p- ERK ½ cells. From the Figure 1B and 1D, it can be seen that the longer
stimulation period (more than 30 minute) is needed for the detection of the IFN- Ɣ cells. In
case of both the figure, kind of sigmoid curve has been observed. The IFN- Ɣ cells were not
detected for the initial 30 minutes, then it’s increased rapidly from 30 minutes to till 2 hour
time point. After that, increment was very slowly up to the 6 hour long and a slow increment
was noticed. Another set of experiment was conducted for 30 minute long where the cells
were stained first and after that stimulation with PMA+ I and fixation were performed. In this
scenario, similar kind of trend has been observed for both the p- ERK ½ and IFN- Ɣ cells. In
this set of experiment, rapid detection of p- ERK ½ cells were noticed within the initial 2
minutes instead of first 5 minutes in comparison with the first set of experiment (6 hour
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4ANALYSE USING FLOW CYTOMETRY
duration) and the stimulation remained elevated for 30 minutes instead of 90 minutes.
However, no frequency loss of p- ERK ½ cells were noticed and mean fluorescence intensity
was higher when comparing with the first set of experiment. No inductions of p- ERK ½ cells
phosphorylation were noticed in case of only antibody staining (Crawford et al. 2014).
duration) and the stimulation remained elevated for 30 minutes instead of 90 minutes.
However, no frequency loss of p- ERK ½ cells were noticed and mean fluorescence intensity
was higher when comparing with the first set of experiment. No inductions of p- ERK ½ cells
phosphorylation were noticed in case of only antibody staining (Crawford et al. 2014).

5ANALYSE USING FLOW CYTOMETRY
References:
Crawford, T.Q., Jalbert, E., Ndhlovu, L.C. and Barbour, J.D., 2014. Concomitant evaluation
of PMA+ ionomycin‐induced kinase phosphorylation and cytokine production in T cell
subsets by flow cytometry. Cytometry Part A, 85(3), pp.268-276.
Freer, G. and Rindi, L., 2013. Intracellular cytokine detection by fluorescence-activated flow
cytometry: basic principles and recent advances. Methods, 61(1), pp.30-38.
Gorelik, G. and Richardson, B., 2010. Key role of ERK pathway signaling in
lupus. Autoimmunity, 43(1), pp.17-22.
Lovelace, P. and Maecker, H.T., 2018. Multiparameter intracellular cytokine staining.
In Flow cytometry protocols (pp. 151-166). Humana Press, New York, NY.
Schulz, K.R., Danna, E.A., Krutzik, P.O. and Nolan, G.P., 2012. Single‐cell phospho‐protein
analysis by flow cytometry. Current Protocols in Immunology, 96(1), pp.8-17.
Steelman, L.S., Franklin, R.A., Abrams, S.L., Chappell, W., Kempf, C.R., Bäsecke, J.,
Stivala, F., Donia, M., Fagone, P., Nicoletti, F. and Libra, M., 2011. Roles of the
Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia, 25(7), p.1080.
References:
Crawford, T.Q., Jalbert, E., Ndhlovu, L.C. and Barbour, J.D., 2014. Concomitant evaluation
of PMA+ ionomycin‐induced kinase phosphorylation and cytokine production in T cell
subsets by flow cytometry. Cytometry Part A, 85(3), pp.268-276.
Freer, G. and Rindi, L., 2013. Intracellular cytokine detection by fluorescence-activated flow
cytometry: basic principles and recent advances. Methods, 61(1), pp.30-38.
Gorelik, G. and Richardson, B., 2010. Key role of ERK pathway signaling in
lupus. Autoimmunity, 43(1), pp.17-22.
Lovelace, P. and Maecker, H.T., 2018. Multiparameter intracellular cytokine staining.
In Flow cytometry protocols (pp. 151-166). Humana Press, New York, NY.
Schulz, K.R., Danna, E.A., Krutzik, P.O. and Nolan, G.P., 2012. Single‐cell phospho‐protein
analysis by flow cytometry. Current Protocols in Immunology, 96(1), pp.8-17.
Steelman, L.S., Franklin, R.A., Abrams, S.L., Chappell, W., Kempf, C.R., Bäsecke, J.,
Stivala, F., Donia, M., Fagone, P., Nicoletti, F. and Libra, M., 2011. Roles of the
Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia, 25(7), p.1080.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.