Advanced Biotechnology (BIO80001): Fluorescence Imaging Project
VerifiedAdded on 2022/09/26
|11
|2499
|28
Report
AI Summary
This report presents a research proposal focused on utilizing fluorescence live cell imaging to study protein diffusion within mitochondria and the endoplasmic reticulum (ER) in mammalian cells. The study aims to understand the movement of proteins, particularly calcium-binding proteins, and how they interact within these organelles. The background section provides a literature review on calcium-binding proteins and their role in cellular processes, including the interaction between ER and mitochondria. The core problem revolves around identifying the mechanisms of protein transportation, specifically focusing on the 70S transportation device. The proposed solution involves tagging DNA and RNA with fluorescent markers to visualize protein movement using microscopy. The report details sample preparation, imaging techniques, and the significance of the research, highlighting the potential for future advancements in understanding cellular metabolism and disease processes. The study emphasizes the use of fluorescent proteins (FPs) for molecular imaging and discusses the importance of brighter probes for low-copy expression and easy identification of the matrix material that are released by transportation device and gets categorised as 70S. The research also highlights the importance of the photo physical possessions of FPs in addition to ways such things effect imaging requests.

Running head: IMAGING TECHNIQUE
IMAGING PROJECT: FLUORESCENCE TECHNIQUE
Name of the Student
Nam of the University
Author note
IMAGING PROJECT: FLUORESCENCE TECHNIQUE
Name of the Student
Nam of the University
Author note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1IMAGING TECHNIQUE
Abstract
This research proposal discusses about the fluorescent live cell imaging done that is
used to measure or to track how fast the protein diffuse through mitochondria in a live cell or
mammalian cells. This technique would define who the DNA and RNA are tagged so that
protein identification can be done easily. The background discusses about the calcium
proteins present in ER and mitochondria and movement of these proteins. The main issue in
this research is to identify the ways the transportation is done through transportation device
that is categorised as 70S. The images that would be obtained would be marked green or blue
depending on the fluorescent tags that are being used. The tagging of DNA and RNA would
help to differentiate between nucleic acids and other elements in the cell. The tagged
elements would have strong significant on the results The images obtained would help in the
interpretation of the continuous movement of cells. The microscope view or observance
would show the clear movement of the cell in and out of mitochondria. This method is
chosen as it is highly scientific and make the research work easy for identification and
diagnosis. This method also is essential for future prospects as it can make a distinct visual
significance for the experimental material. Therefore, the research is highly significant for
future prospects.
Abstract
This research proposal discusses about the fluorescent live cell imaging done that is
used to measure or to track how fast the protein diffuse through mitochondria in a live cell or
mammalian cells. This technique would define who the DNA and RNA are tagged so that
protein identification can be done easily. The background discusses about the calcium
proteins present in ER and mitochondria and movement of these proteins. The main issue in
this research is to identify the ways the transportation is done through transportation device
that is categorised as 70S. The images that would be obtained would be marked green or blue
depending on the fluorescent tags that are being used. The tagging of DNA and RNA would
help to differentiate between nucleic acids and other elements in the cell. The tagged
elements would have strong significant on the results The images obtained would help in the
interpretation of the continuous movement of cells. The microscope view or observance
would show the clear movement of the cell in and out of mitochondria. This method is
chosen as it is highly scientific and make the research work easy for identification and
diagnosis. This method also is essential for future prospects as it can make a distinct visual
significance for the experimental material. Therefore, the research is highly significant for
future prospects.

2IMAGING TECHNIQUE
Background
Calcium-binding proteins are intricate in the signalling pathway of calcium that binds
to calcium ions, thereby playing an imperative role in many cellular processes. Calcium
binding proteins have certain spheres, binding to calcium hence it is defined to be
heterogeneous. The main function of the calcium binding proteins is to standardise the
expanse of free and unbound Ca2+ ion present in the cytosol of the cell (Dean and Palmer
2014). The parameter of Ca2+ is entitled as calcium homeostasis. The VDAC (voltage-
dependent anion channel) present in the outer mitochondrial membrane arbitrates Ca2+
metabolic flow, in addition leads to cell death signalling among the endoplasmic reticulum
(ER) in addition to mitochondrial systems. Researches have established that VDAC1 is
materially connected to the Ca2+-release channel inositol 1,4,5-trisphosphate receptor (IP3R)
of the endoplasmic reticulum with the help of molecular chaperone that is regulated by
glucose protein 75 (grp75). Functional collaboration amongst the networks was exposed by
the recombinant appearance of the IP3R, which is a ligand-binding domain present on the ER
as well as in mitochondrial surface, that openly improved Ca2+ accretion in mitochondria.
Grp75 knockdown of eliminated the stimulatory consequence, emphasising on the chaperone
facilitated conformational coupling among the IP3R in addition to the mitochondrial Ca2+
approval equipment. Since organelle Ca2+ homeostasis stimuluses essentially cellular
meanings in addition to death signalling, the main location of grp75 might characterise an
imperative point of control of fate of cell in addition to pathogenesis (Appelhans and Busch
2017). Mitochondria besides ER of eukaryotic cells are able to form two tangled
endomembrane systems, besides their vibrant interface that can control transfer of protein,
metabolic flow, cell death and intracellular Ca2+ signalling (Pedelacq and Cabantous 2019).
Mitochondrial Ca2+ can uptake, through a nevertheless to be recognised Ca2+ channel of the
inside mitochondrial membrane which is defined as the uniporter of mitochondrial Ca2+,
Background
Calcium-binding proteins are intricate in the signalling pathway of calcium that binds
to calcium ions, thereby playing an imperative role in many cellular processes. Calcium
binding proteins have certain spheres, binding to calcium hence it is defined to be
heterogeneous. The main function of the calcium binding proteins is to standardise the
expanse of free and unbound Ca2+ ion present in the cytosol of the cell (Dean and Palmer
2014). The parameter of Ca2+ is entitled as calcium homeostasis. The VDAC (voltage-
dependent anion channel) present in the outer mitochondrial membrane arbitrates Ca2+
metabolic flow, in addition leads to cell death signalling among the endoplasmic reticulum
(ER) in addition to mitochondrial systems. Researches have established that VDAC1 is
materially connected to the Ca2+-release channel inositol 1,4,5-trisphosphate receptor (IP3R)
of the endoplasmic reticulum with the help of molecular chaperone that is regulated by
glucose protein 75 (grp75). Functional collaboration amongst the networks was exposed by
the recombinant appearance of the IP3R, which is a ligand-binding domain present on the ER
as well as in mitochondrial surface, that openly improved Ca2+ accretion in mitochondria.
Grp75 knockdown of eliminated the stimulatory consequence, emphasising on the chaperone
facilitated conformational coupling among the IP3R in addition to the mitochondrial Ca2+
approval equipment. Since organelle Ca2+ homeostasis stimuluses essentially cellular
meanings in addition to death signalling, the main location of grp75 might characterise an
imperative point of control of fate of cell in addition to pathogenesis (Appelhans and Busch
2017). Mitochondria besides ER of eukaryotic cells are able to form two tangled
endomembrane systems, besides their vibrant interface that can control transfer of protein,
metabolic flow, cell death and intracellular Ca2+ signalling (Pedelacq and Cabantous 2019).
Mitochondrial Ca2+ can uptake, through a nevertheless to be recognised Ca2+ channel of the
inside mitochondrial membrane which is defined as the uniporter of mitochondrial Ca2+,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3IMAGING TECHNIQUE
controlling procedures as miscellaneous as aerobic metabolism. It can release of caspase
cofactors, in addition to reaction control of adjoining ER and plasma membrane Ca2+
channels. A consequence of the well-organized mitochondrial Ca2+ uptake for the duration of
IP3-induced Ca2+ discharge is adjacent apposition of ER in addition to outer mitochondrial
membranes. The molecular factors of this crosstalk, nevertheless, are immobile principally
unidentified. present, PACS2, that associated with ER vesicular categorisation protein, was
projected to connexion the ER to mitochondria. Fluorescent live cell imaging would help in
analysis the movement of cellular components (Larson 2019).
The giveaway of PACS2 caused stress facilitated separation of the organelles, that
was replicated by the reticence of Ca2+ signal transmission. Along with this, the copious
voltage-dependent anion channel 1 (VDAC1) can also play a role in the interaction. It was
identified that it is required to be present at ER–mitochondrial links in addition to facilitate
Ca2+ channelling to the intermembrane space from the high level of Ca2+ micro domain
produced by the inaugural of the inositol 1,4,5-trisphosphate receptor (IP3R) (Lang et al.
2015). Along with this, VDAC1 arbitrates metabolic flow all through the VDAC channel
establishing an ATP micro domain that is near to the ER in addition to sarcoplasmic
reticulum Ca2+ ATPases in addition to in cooperation VDAC1 in addition to VDAC2 that are
taking part in metabolic in addition to apoptotic protein groups (Weinhäupl et al. 2018).
Define problem
The molecular equipment required for mitochondrial Ca2+ transport is considered to
be highly obscure. Hypothesis that is to be tested is that gathering of all the materials in the
matrix as well as the release of consequent happen through the movement of transportation of
devices, which are functionally categorised in the 70S on the other hand were not ever been
molecularly recognised even though there were widespread exertions in this approach.
controlling procedures as miscellaneous as aerobic metabolism. It can release of caspase
cofactors, in addition to reaction control of adjoining ER and plasma membrane Ca2+
channels. A consequence of the well-organized mitochondrial Ca2+ uptake for the duration of
IP3-induced Ca2+ discharge is adjacent apposition of ER in addition to outer mitochondrial
membranes. The molecular factors of this crosstalk, nevertheless, are immobile principally
unidentified. present, PACS2, that associated with ER vesicular categorisation protein, was
projected to connexion the ER to mitochondria. Fluorescent live cell imaging would help in
analysis the movement of cellular components (Larson 2019).
The giveaway of PACS2 caused stress facilitated separation of the organelles, that
was replicated by the reticence of Ca2+ signal transmission. Along with this, the copious
voltage-dependent anion channel 1 (VDAC1) can also play a role in the interaction. It was
identified that it is required to be present at ER–mitochondrial links in addition to facilitate
Ca2+ channelling to the intermembrane space from the high level of Ca2+ micro domain
produced by the inaugural of the inositol 1,4,5-trisphosphate receptor (IP3R) (Lang et al.
2015). Along with this, VDAC1 arbitrates metabolic flow all through the VDAC channel
establishing an ATP micro domain that is near to the ER in addition to sarcoplasmic
reticulum Ca2+ ATPases in addition to in cooperation VDAC1 in addition to VDAC2 that are
taking part in metabolic in addition to apoptotic protein groups (Weinhäupl et al. 2018).
Define problem
The molecular equipment required for mitochondrial Ca2+ transport is considered to
be highly obscure. Hypothesis that is to be tested is that gathering of all the materials in the
matrix as well as the release of consequent happen through the movement of transportation of
devices, which are functionally categorised in the 70S on the other hand were not ever been
molecularly recognised even though there were widespread exertions in this approach.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4IMAGING TECHNIQUE
Hence, this paper extensively chose to discuss on this issue by emerging and
considering the outer mitochondria membrane that are critically considered as a determinant
of the accumulation of mitochondrial Ca2+. Therefore, the quality of both these proteins have
an imperative role on ER as well as on mitochondria.
Solve the problem
The utmost extensively functional method for molecular imaging of live cells can be
done by using fluorescent proteins (FPs) so that the cellular structures gets lighted up like
biomolecules in addition to organelles as well for some proteins.
Sample preparation:
It includes three dimensional imaging with high spatial resolution, temporary high
resolution, low photo toxicity for lengthy imaging which is >106, biological tissues and cell,
one molecule sensitivity, concurrent replication of numerous molecular boards, no perturbing
labelling approaches and proteins imaging that is under control of innate genomic promoters
(Wang et al. 2018).
Imaging technique
The cell sample was taken and was tagged with fluorescence to check the activity of
the biomarker. The cells were incubated for 10 minute and was observed under fluorescent
microscope (Sadaghiani, Verhelst and Bogyo, 2017). The highlight protein shows the protein
specific for ER and mitochondria and their movement can be tracked.
Hence, this paper extensively chose to discuss on this issue by emerging and
considering the outer mitochondria membrane that are critically considered as a determinant
of the accumulation of mitochondrial Ca2+. Therefore, the quality of both these proteins have
an imperative role on ER as well as on mitochondria.
Solve the problem
The utmost extensively functional method for molecular imaging of live cells can be
done by using fluorescent proteins (FPs) so that the cellular structures gets lighted up like
biomolecules in addition to organelles as well for some proteins.
Sample preparation:
It includes three dimensional imaging with high spatial resolution, temporary high
resolution, low photo toxicity for lengthy imaging which is >106, biological tissues and cell,
one molecule sensitivity, concurrent replication of numerous molecular boards, no perturbing
labelling approaches and proteins imaging that is under control of innate genomic promoters
(Wang et al. 2018).
Imaging technique
The cell sample was taken and was tagged with fluorescence to check the activity of
the biomarker. The cells were incubated for 10 minute and was observed under fluorescent
microscope (Sadaghiani, Verhelst and Bogyo, 2017). The highlight protein shows the protein
specific for ER and mitochondria and their movement can be tracked.

5IMAGING TECHNIQUE
Fig 1: Labelling of nucleic acid molecules. a and b) fixing of samples c) nucleate
formation and multiple nucleate tagging, d) non fluorescent until tagged e) live cell
imaging (Lang et al. 2015)
The tagging of protein can be done on fixed samples that comprises DNA and RNA
oligonucleotides tagged with a single fluorophore. It can also be tagged with a multiple
smaller oligonucleotide; of which each are labelled with a single fluorophore (Wong 2015,).
Hence, the nucleotides give increased specificity by two adaptor oligonucleotides such as
nucleate the development of a pronged in addition to multiply labelled oligonucleotides
equivalent to DNA origami (Sartor et al. 2020). The fluorophore-quencher the conjugate and
remains no fluorescent till there is any hybridization with sequence that is of interest (Fig 1).
The fusion proteins can then identify each hairpin motif in addition to bind with great
similarity, producing a transcription with 48 GFP molecules in addition to qualifying
repetitive single-molecule imaging of protein in live cell (Narayanan et al. 2014).
Fig 1: Labelling of nucleic acid molecules. a and b) fixing of samples c) nucleate
formation and multiple nucleate tagging, d) non fluorescent until tagged e) live cell
imaging (Lang et al. 2015)
The tagging of protein can be done on fixed samples that comprises DNA and RNA
oligonucleotides tagged with a single fluorophore. It can also be tagged with a multiple
smaller oligonucleotide; of which each are labelled with a single fluorophore (Wong 2015,).
Hence, the nucleotides give increased specificity by two adaptor oligonucleotides such as
nucleate the development of a pronged in addition to multiply labelled oligonucleotides
equivalent to DNA origami (Sartor et al. 2020). The fluorophore-quencher the conjugate and
remains no fluorescent till there is any hybridization with sequence that is of interest (Fig 1).
The fusion proteins can then identify each hairpin motif in addition to bind with great
similarity, producing a transcription with 48 GFP molecules in addition to qualifying
repetitive single-molecule imaging of protein in live cell (Narayanan et al. 2014).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
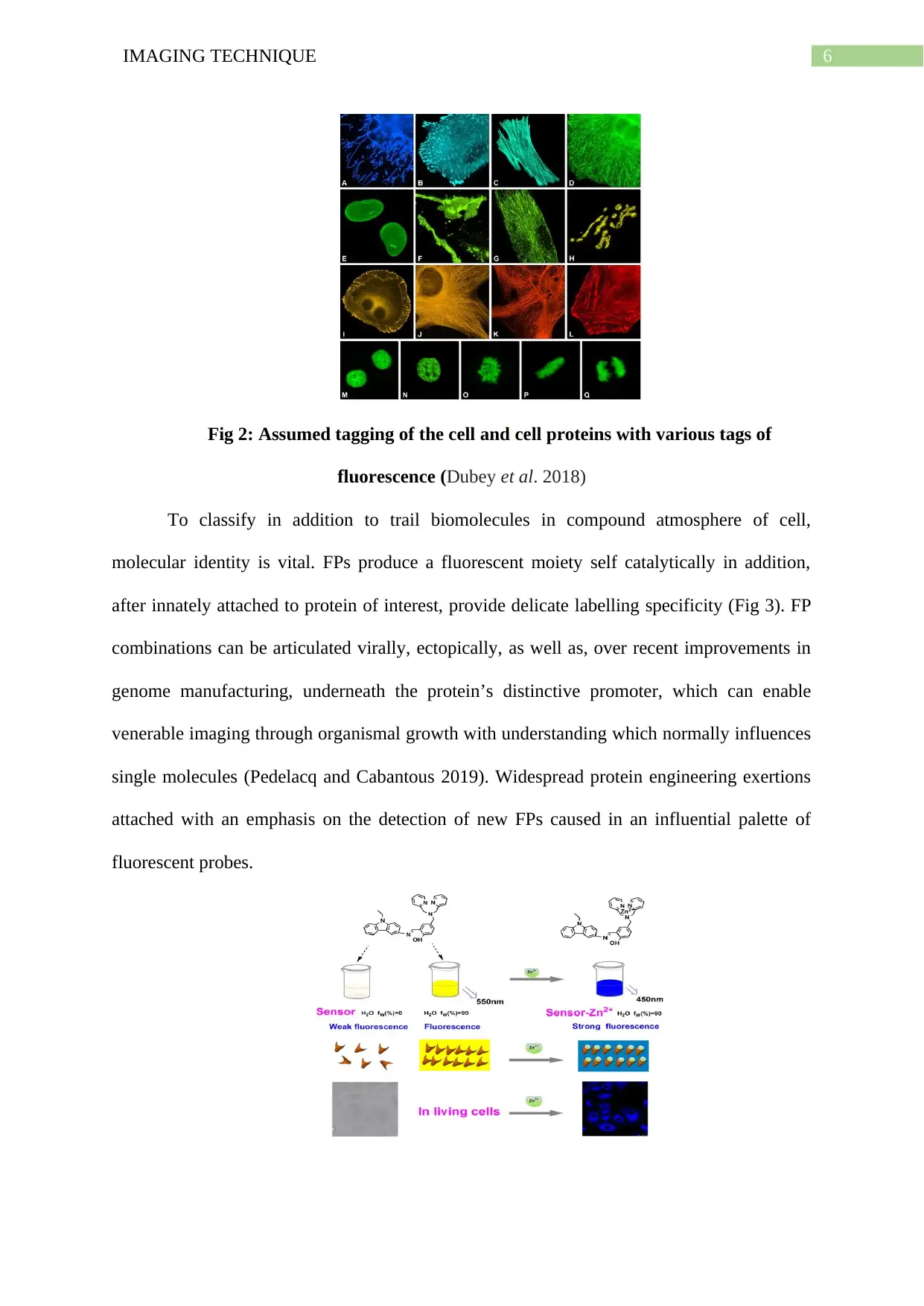
6IMAGING TECHNIQUE
Fig 2: Assumed tagging of the cell and cell proteins with various tags of
fluorescence (Dubey et al. 2018)
To classify in addition to trail biomolecules in compound atmosphere of cell,
molecular identity is vital. FPs produce a fluorescent moiety self catalytically in addition,
after innately attached to protein of interest, provide delicate labelling specificity (Fig 3). FP
combinations can be articulated virally, ectopically, as well as, over recent improvements in
genome manufacturing, underneath the protein’s distinctive promoter, which can enable
venerable imaging through organismal growth with understanding which normally influences
single molecules (Pedelacq and Cabantous 2019). Widespread protein engineering exertions
attached with an emphasis on the detection of new FPs caused in an influential palette of
fluorescent probes.
Fig 2: Assumed tagging of the cell and cell proteins with various tags of
fluorescence (Dubey et al. 2018)
To classify in addition to trail biomolecules in compound atmosphere of cell,
molecular identity is vital. FPs produce a fluorescent moiety self catalytically in addition,
after innately attached to protein of interest, provide delicate labelling specificity (Fig 3). FP
combinations can be articulated virally, ectopically, as well as, over recent improvements in
genome manufacturing, underneath the protein’s distinctive promoter, which can enable
venerable imaging through organismal growth with understanding which normally influences
single molecules (Pedelacq and Cabantous 2019). Widespread protein engineering exertions
attached with an emphasis on the detection of new FPs caused in an influential palette of
fluorescent probes.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7IMAGING TECHNIQUE
Fig 3: potential steps for probing live cells. Cell are tagged with fluroscent probe
and observed under microscope (Battaglia and Biocompatibles UK Ltd, 2016)
The most fascinating belongings about this science is engineering exertions have
highly been fruitful at directing some possessions, like brightness, nevertheless have exposed
difficulty in photo physical possessions such as, photo switching, as well as dark-state
conversion, which are often perplexing (Larson 2019). The feature might be subjugated for
particular microscopy presentations, for outmoded imaging, where they generally bound
photon output.
Significance and future aspects
The brighter probes are highly important in the field of research as it can adopt low
copy of expression. This would help in identifying the matrix material that are released by
transportation device and gets categorised as 70S. The FPs probe are bright green that
explains the movement of the two proteins have continuous diffusion overtime (Larson
2019). The outcome can be summarised that the chief physiological part of uptake of Ca2+
and transfer of proteins occurs throughout the channels. It was evaluated by the switch of
metabolic activity of the mitochondria, related to production rate of ATP. Certainly,
imperative metabolic enzymes contained in the background of isocitrate-dehydrogenases as
well as α-ketoglutarate-along with the pyruvate-, gets stimulated by Ca2+, with diverse tools
(Pedelacq and Cabantous 2019). The first step was through a Ca2+-dependent
dephosphorylating stage, while the others through uninterrupted obligatory to a supervisory
site. Consequent research has discovered that it is an essential imperative instance where only
one of the appliance supervisory mitochondrial metabolism. Certainly, metabolite
transporters of the inner membrane of ER and mitochondria, like aralar1 in addition to citrin,
retain a Ca2+ binding site in the part of the protruding protein in the intermembrane area, that
is accountable for stimulus reliant augmentation of substrate accretion in the matrix.
Fig 3: potential steps for probing live cells. Cell are tagged with fluroscent probe
and observed under microscope (Battaglia and Biocompatibles UK Ltd, 2016)
The most fascinating belongings about this science is engineering exertions have
highly been fruitful at directing some possessions, like brightness, nevertheless have exposed
difficulty in photo physical possessions such as, photo switching, as well as dark-state
conversion, which are often perplexing (Larson 2019). The feature might be subjugated for
particular microscopy presentations, for outmoded imaging, where they generally bound
photon output.
Significance and future aspects
The brighter probes are highly important in the field of research as it can adopt low
copy of expression. This would help in identifying the matrix material that are released by
transportation device and gets categorised as 70S. The FPs probe are bright green that
explains the movement of the two proteins have continuous diffusion overtime (Larson
2019). The outcome can be summarised that the chief physiological part of uptake of Ca2+
and transfer of proteins occurs throughout the channels. It was evaluated by the switch of
metabolic activity of the mitochondria, related to production rate of ATP. Certainly,
imperative metabolic enzymes contained in the background of isocitrate-dehydrogenases as
well as α-ketoglutarate-along with the pyruvate-, gets stimulated by Ca2+, with diverse tools
(Pedelacq and Cabantous 2019). The first step was through a Ca2+-dependent
dephosphorylating stage, while the others through uninterrupted obligatory to a supervisory
site. Consequent research has discovered that it is an essential imperative instance where only
one of the appliance supervisory mitochondrial metabolism. Certainly, metabolite
transporters of the inner membrane of ER and mitochondria, like aralar1 in addition to citrin,
retain a Ca2+ binding site in the part of the protruding protein in the intermembrane area, that
is accountable for stimulus reliant augmentation of substrate accretion in the matrix.

8IMAGING TECHNIQUE
In future this policy would help in the easy indentation of the Ca2+ protein channel
and calculation of their activities undergoing all through the process of metabolism causing
energy production. The efforts highlight the better appreciation of the photo physical
possessions of FPs in addition to ways such things effect imaging requests.
In future this policy would help in the easy indentation of the Ca2+ protein channel
and calculation of their activities undergoing all through the process of metabolism causing
energy production. The efforts highlight the better appreciation of the photo physical
possessions of FPs in addition to ways such things effect imaging requests.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9IMAGING TECHNIQUE
References
Appelhans, T. and Busch, K.B., 2017. Dynamic imaging of mitochondrial membrane proteins
in specific sub-organelle membrane locations. Biophysical reviews, 9(4), pp.345-352.
Battaglia, G., Biocompatibles UK Ltd, 2016. Method of monitoring live cells. U.S. Patent
Application 15/155,308.
Dean, K.M. and Palmer, A.E., 2014. Advances in fluorescence labeling strategies for
dynamic cellular imaging. Nature chemical biology, 10(7), p.512.
Dubey, V., Ahmad, A., Singh, R., Wolfson, D.L., Basnet, P., Acharya, G., Mehta, D.S. and
Ahluwalia, B.S., 2018. Multi-modal chip-based fluorescence and quantitative phase
microscopy for studying inflammation in macrophages. Optics express, 26(16), pp.19864-
19876.
Lang, F., Qin, Z., Li, F., Zhang, H., Fang, Z. and Hao, E., 2015. Apoptotic cell death induced
by resveratrol is partially mediated by the autophagy pathway in human ovarian cancer cells.
PloS one, 10(6).
Larson, E.R., 2019. Timing Is Everything: Tandem Fluorescent Timers Expand Our
Understanding of Protein Longevity. Plant physiology, 180(2), pp.699-700.
Narayanan, K., Yen, S.K., Dou, Q., Padmanabhan, P., Sudhaharan, T., Ahmed, S., Ying, J.Y.
and Selvan, S.T., 2013. Mimicking cellular transport mechanism in stem cells through
endosomal escape of new peptide-coated quantum dots. Scientific reports, 3, p.2184.
Pedelacq, J.D. and Cabantous, S., 2019. Development and Applications of Superfolder and
Split Fluorescent Protein Detection Systems in Biology. International journal of molecular
sciences, 20(14), p.3479.
Sadaghiani, A.M., Verhelst, S.H. and Bogyo, M., 2017. Tagging and detection strategies for
activity-based proteomics. Current opinion in chemical biology, 11(1), pp.20-28.
References
Appelhans, T. and Busch, K.B., 2017. Dynamic imaging of mitochondrial membrane proteins
in specific sub-organelle membrane locations. Biophysical reviews, 9(4), pp.345-352.
Battaglia, G., Biocompatibles UK Ltd, 2016. Method of monitoring live cells. U.S. Patent
Application 15/155,308.
Dean, K.M. and Palmer, A.E., 2014. Advances in fluorescence labeling strategies for
dynamic cellular imaging. Nature chemical biology, 10(7), p.512.
Dubey, V., Ahmad, A., Singh, R., Wolfson, D.L., Basnet, P., Acharya, G., Mehta, D.S. and
Ahluwalia, B.S., 2018. Multi-modal chip-based fluorescence and quantitative phase
microscopy for studying inflammation in macrophages. Optics express, 26(16), pp.19864-
19876.
Lang, F., Qin, Z., Li, F., Zhang, H., Fang, Z. and Hao, E., 2015. Apoptotic cell death induced
by resveratrol is partially mediated by the autophagy pathway in human ovarian cancer cells.
PloS one, 10(6).
Larson, E.R., 2019. Timing Is Everything: Tandem Fluorescent Timers Expand Our
Understanding of Protein Longevity. Plant physiology, 180(2), pp.699-700.
Narayanan, K., Yen, S.K., Dou, Q., Padmanabhan, P., Sudhaharan, T., Ahmed, S., Ying, J.Y.
and Selvan, S.T., 2013. Mimicking cellular transport mechanism in stem cells through
endosomal escape of new peptide-coated quantum dots. Scientific reports, 3, p.2184.
Pedelacq, J.D. and Cabantous, S., 2019. Development and Applications of Superfolder and
Split Fluorescent Protein Detection Systems in Biology. International journal of molecular
sciences, 20(14), p.3479.
Sadaghiani, A.M., Verhelst, S.H. and Bogyo, M., 2017. Tagging and detection strategies for
activity-based proteomics. Current opinion in chemical biology, 11(1), pp.20-28.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10IMAGING TECHNIQUE
Sartor, A.M., Dahlberg, P.D., Wang, J., Saurabh, S., Shapiro, L. and Moerner, W.E., 2020,
February. Cryogenic single-molecule active control microscopy with a photoactivatable
fluorescent protein. In Single Molecule Spectroscopy and Superresolution Imaging XIII (Vol.
11246, p. 112460G). International Society for Optics and Photonics.
Wang, C.X., Wu, B., Zhou, W., Wang, Q., Yu, H., Deng, K., Li, J.M., Zhuo, R.X. and
Huang, S.W., 2018. Turn-on fluorescent probe-encapsulated micelle as colloidally stable
nano-chemosensor for highly selective detection of Al3+ in aqueous solution and living cell
imaging. Sensors and Actuators B: Chemical, 271, pp.225-238.
Weinhäupl, K., Lindau, C., Hessel, A., Wang, Y., Schütze, C., Jores, T., Melchionda, L.,
Schönfisch, B., Kalbacher, H., Bersch, B. and Rapaport, D., 2018. Structural basis of
membrane protein chaperoning through the mitochondrial intermembrane space. Cell, 175(5),
pp.1365-1379.
Wong, S.T., 2015, February. Image informatics in systems biology applications. In
Electronic Imaging and Multimedia Technology IV (Vol. 5637, pp. 138-145). International
Society for Optics and Photonics.
Sartor, A.M., Dahlberg, P.D., Wang, J., Saurabh, S., Shapiro, L. and Moerner, W.E., 2020,
February. Cryogenic single-molecule active control microscopy with a photoactivatable
fluorescent protein. In Single Molecule Spectroscopy and Superresolution Imaging XIII (Vol.
11246, p. 112460G). International Society for Optics and Photonics.
Wang, C.X., Wu, B., Zhou, W., Wang, Q., Yu, H., Deng, K., Li, J.M., Zhuo, R.X. and
Huang, S.W., 2018. Turn-on fluorescent probe-encapsulated micelle as colloidally stable
nano-chemosensor for highly selective detection of Al3+ in aqueous solution and living cell
imaging. Sensors and Actuators B: Chemical, 271, pp.225-238.
Weinhäupl, K., Lindau, C., Hessel, A., Wang, Y., Schütze, C., Jores, T., Melchionda, L.,
Schönfisch, B., Kalbacher, H., Bersch, B. and Rapaport, D., 2018. Structural basis of
membrane protein chaperoning through the mitochondrial intermembrane space. Cell, 175(5),
pp.1365-1379.
Wong, S.T., 2015, February. Image informatics in systems biology applications. In
Electronic Imaging and Multimedia Technology IV (Vol. 5637, pp. 138-145). International
Society for Optics and Photonics.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
