Food Chemistry: Metabolism of Naphthalene, Methyl, and Ethyl Compounds
VerifiedAdded on 2020/04/07
|18
|2055
|405
Homework Assignment
AI Summary
This assignment delves into the food chemistry of naphthalene and its related compounds, exploring their properties and metabolic pathways. The student analyzes naphthalene's structure, comparing it with alkylated derivatives like 1-methyl and 2-methyl naphthalene, and 1-ethyl and 2-ethyl naphthalene, examining the presence of aromatic rings and side chains. The assignment details the metabolic pathways of these compounds, including the breakdown of naphthalene into various metabolites like 1,2-Dihydroxyl-naphthalene, and the subsequent formation of compounds such as salicylaldehyde, salicylate, catechol, and ultimately, succinate and Acetyl CoA. The metabolic pathways of 1-methyl and 2-methyl are also explored, highlighting the role of enzymes like xanthine oxidase and cytochrome P450 oxidase. The student also discusses the impact of adding alkyl groups on the aromatic ring and the differences in metabolism between alkyl side chains and condensed aromatic rings, differentiating between alkylated PAHs, non-alkylated PAHs, and naked PAHs. The analysis highlights the importance of these compounds in environmental science and food chemistry, including their sources, characteristics, and environmental implications.

Food Chemistry 1
FOOD CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
FOOD CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Food Chemistry 2
Question 1
● Naphthalene is a compound.
○ With substituent
○ Without substituent
■ Explain why?
Naphthalene is a compound with substituents. This is because, in its chemical reactions, it shows
aromatic behavior related to benzene and it simple substituents. And the reaction is basically the
substitution of the hydrogen atom by hydrogen atom (Cerniglia, 2013).
Question 1
● Naphthalene is a compound.
○ With substituent
○ Without substituent
■ Explain why?
Naphthalene is a compound with substituents. This is because, in its chemical reactions, it shows
aromatic behavior related to benzene and it simple substituents. And the reaction is basically the
substitution of the hydrogen atom by hydrogen atom (Cerniglia, 2013).

Food Chemistry 3
● Naphthalene is:
○ Naked PAHs
○ Alkylated PAH
○ Has Aromatic ring
■ Explain why?
Naphthalene has an aromatic ring. This is because aromatic rings organic compound which
usually occurs as the substructure of more complex molecules and these aromatic compounds are
indole, cyclotetradecaheptaene, and benzene, but Naphthalene is the simplest condensed ring
compound of hydrocarbon made up of two benzene ring.
● Naphthalene has:
○ A side chain
○ No side chain
○ Substituted
○ Unsubstituted
■ Explain why?
Naphthalene has substituted. This is because it contains two composed compound of benzene
rings having common two adjacent carbon atoms. It also contains hydrocarbon raw material
which gives a host substitution products which are employed in making of synthetic and dyestuff
resins (Chen, 2012).
● Naphthalene is
○ Mineral Oils
○ MOAH
● Naphthalene is:
○ Naked PAHs
○ Alkylated PAH
○ Has Aromatic ring
■ Explain why?
Naphthalene has an aromatic ring. This is because aromatic rings organic compound which
usually occurs as the substructure of more complex molecules and these aromatic compounds are
indole, cyclotetradecaheptaene, and benzene, but Naphthalene is the simplest condensed ring
compound of hydrocarbon made up of two benzene ring.
● Naphthalene has:
○ A side chain
○ No side chain
○ Substituted
○ Unsubstituted
■ Explain why?
Naphthalene has substituted. This is because it contains two composed compound of benzene
rings having common two adjacent carbon atoms. It also contains hydrocarbon raw material
which gives a host substitution products which are employed in making of synthetic and dyestuff
resins (Chen, 2012).
● Naphthalene is
○ Mineral Oils
○ MOAH
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Food Chemistry 4
○ PAHs
■ Explain why?
Naphthalene is MOAH(mineral oil aromatic hydrocarbons). This is true from the above
discussions that Naphthalene is oil aromatic hydrocarbon compound. MOAH is a part under 1-
and 2- methylnaphthalene .
● MOAH consists of
○ Substituted aromatic hydrocarbon
○ Polycyclic aromatic hydrocarbon
○ Naked PAHs
■ Explain why?
MOAH consisted of polycyclic aromatic hydrocarbon. This due to the fact that the mineral oil
product from the crude oil via distillation methods and has a fraction of MOAH including n-
alkane , cycloalkane, and this MOAH basically has alkylated polyaromatic hydrocarbon
(Vermerris, 2015).
● MOAH consists of
○ alkylated PAHs
○ Non-alkylated PAHs
○ Naked PAHs
■ Explain why?
○ PAHs
■ Explain why?
Naphthalene is MOAH(mineral oil aromatic hydrocarbons). This is true from the above
discussions that Naphthalene is oil aromatic hydrocarbon compound. MOAH is a part under 1-
and 2- methylnaphthalene .
● MOAH consists of
○ Substituted aromatic hydrocarbon
○ Polycyclic aromatic hydrocarbon
○ Naked PAHs
■ Explain why?
MOAH consisted of polycyclic aromatic hydrocarbon. This due to the fact that the mineral oil
product from the crude oil via distillation methods and has a fraction of MOAH including n-
alkane , cycloalkane, and this MOAH basically has alkylated polyaromatic hydrocarbon
(Vermerris, 2015).
● MOAH consists of
○ alkylated PAHs
○ Non-alkylated PAHs
○ Naked PAHs
■ Explain why?
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Food Chemistry 5
MOAH consisted of alkylated PAHs. Because the mineral oil basically from crude oil which is
MOAH usually has alkylated polyaromatic hydrocarbon which alkylated PAHs in full.
● Commercially available 1-methyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
Is with a substituent. This is because 1-methyl will be part (substituent) of the benzene which is
a constituent of Naphthalene. But it is a hydrocarbon without an aromatic ring since the aromatic
rings are found in 2-methyl.
● Commercially available 2-methyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
MOAH consisted of alkylated PAHs. Because the mineral oil basically from crude oil which is
MOAH usually has alkylated polyaromatic hydrocarbon which alkylated PAHs in full.
● Commercially available 1-methyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
Is with a substituent. This is because 1-methyl will be part (substituent) of the benzene which is
a constituent of Naphthalene. But it is a hydrocarbon without an aromatic ring since the aromatic
rings are found in 2-methyl.
● Commercially available 2-methyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent

Food Chemistry 6
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
Commercially available 2-methyl has aroma ring. 2-methyl of the naphthalene shows aromatic
nature related to benzene and its simple end product.
● Commercially available 1-ethyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
Commercially available 1-ethyl is substituted. This is because this compound still has the
benzene parts which makes naphthalene. It is the only ethyl which is introduced at the first
carbon as seen in the figure below;
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
Commercially available 2-methyl has aroma ring. 2-methyl of the naphthalene shows aromatic
nature related to benzene and its simple end product.
● Commercially available 1-ethyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
Commercially available 1-ethyl is substituted. This is because this compound still has the
benzene parts which makes naphthalene. It is the only ethyl which is introduced at the first
carbon as seen in the figure below;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Food Chemistry 7
Fig 1: shows 1-ethylenaphthalene (Vermerris, W)
● Commercially available 2-ethyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
The commercially available 2-ethylenaphthalene is with a side chain. This is because the branch
of the ethyl group attached at the second carbon atom will form a chain as seen in the figure
below;
Fig 2: showing a side chain hydrocarbon (Vermerris, W)
Fig 1: shows 1-ethylenaphthalene (Vermerris, W)
● Commercially available 2-ethyl
○ Is With a side chain
○ Has No side chain
○ Is With substituent
○ Is Without substituent
○ Has Aromatic ring
○ Is Substituted
○ Is Unsubstituted
■ Explain why?
The commercially available 2-ethylenaphthalene is with a side chain. This is because the branch
of the ethyl group attached at the second carbon atom will form a chain as seen in the figure
below;
Fig 2: showing a side chain hydrocarbon (Vermerris, W)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Food Chemistry 8
● Methylated metabolites can be metabolized much faster?
○ True
○ False
■ Explain why?
True. This is because the methylate in metabolites will increase the rate of reaction therefore by
increasing the rate of chemical reaction it will make the metabolites to be metabolized faster.
Question 2
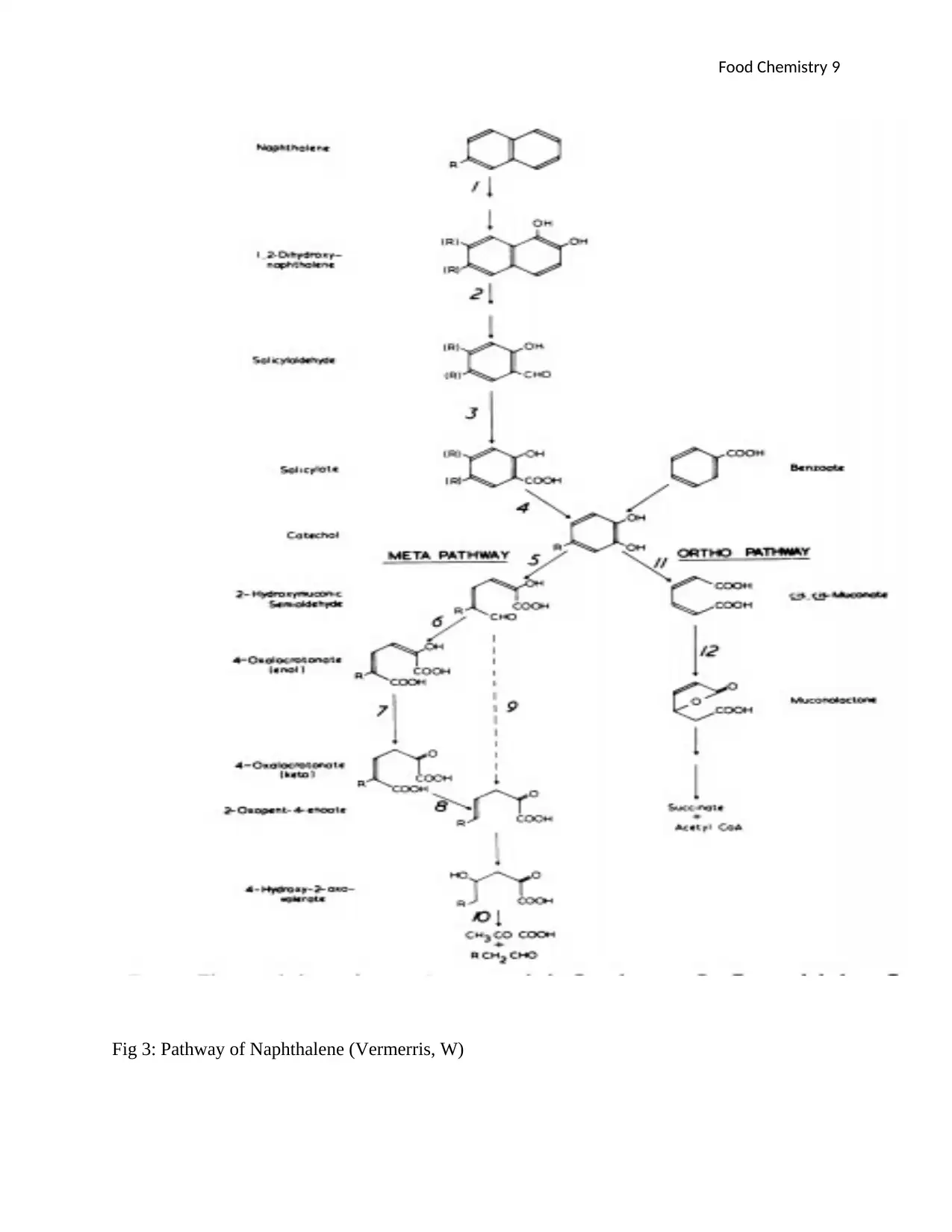
The Naphthalene pathway is illustrated by the fig 2 below;
The metabolic pathway of Naphthalene entails several stages where Naphthalene is broken down
into 1,2-Dihydroxyl-naphthalene (Ritchie, 2012). This compound is then broken down by the
help of some enzymes like naphthalene oxygenase to form salicylaldehyde which is broken
further to salicylate by the same enzyme. From this point, salicylaldehyde dehydrogenase will
disintegrate salicylate to form catechol which is then broken down by the help of salicylate
hydroxylase enzyme into two major pathways (META and ORTHO pathways). And these then
disintegrate as shown in figure 2 below until succinate+ Acetyle CoA is formed at the ORTHO
pathways and 4Hydroxy-2-oxo-volerate at the META pathways.
● Methylated metabolites can be metabolized much faster?
○ True
○ False
■ Explain why?
True. This is because the methylate in metabolites will increase the rate of reaction therefore by
increasing the rate of chemical reaction it will make the metabolites to be metabolized faster.
Question 2
The Naphthalene pathway is illustrated by the fig 2 below;
The metabolic pathway of Naphthalene entails several stages where Naphthalene is broken down
into 1,2-Dihydroxyl-naphthalene (Ritchie, 2012). This compound is then broken down by the
help of some enzymes like naphthalene oxygenase to form salicylaldehyde which is broken
further to salicylate by the same enzyme. From this point, salicylaldehyde dehydrogenase will
disintegrate salicylate to form catechol which is then broken down by the help of salicylate
hydroxylase enzyme into two major pathways (META and ORTHO pathways). And these then
disintegrate as shown in figure 2 below until succinate+ Acetyle CoA is formed at the ORTHO
pathways and 4Hydroxy-2-oxo-volerate at the META pathways.
Food Chemistry 9
Fig 3: Pathway of Naphthalene (Vermerris, W)
Fig 3: Pathway of Naphthalene (Vermerris, W)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Food Chemistry 10
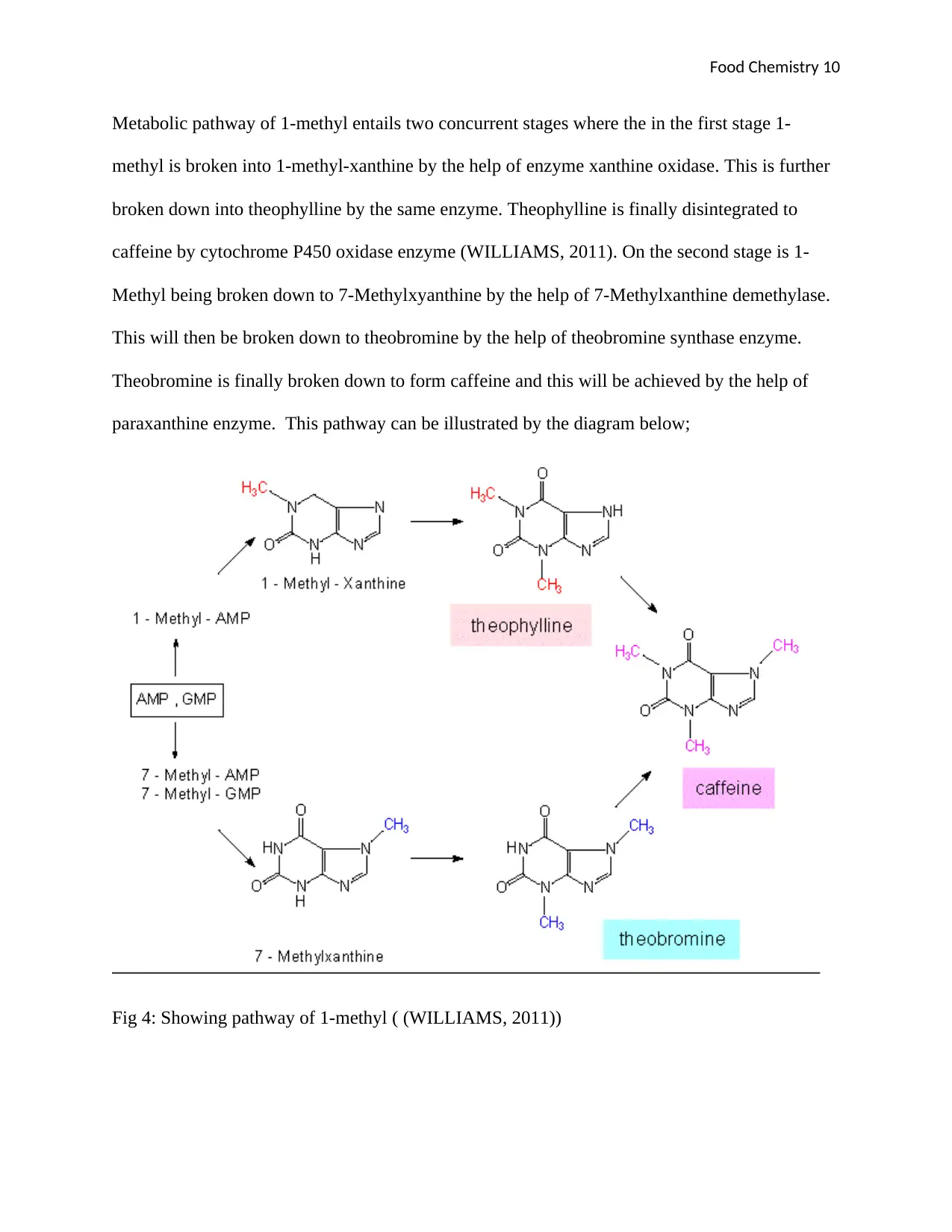
Metabolic pathway of 1-methyl entails two concurrent stages where the in the first stage 1-
methyl is broken into 1-methyl-xanthine by the help of enzyme xanthine oxidase. This is further
broken down into theophylline by the same enzyme. Theophylline is finally disintegrated to
caffeine by cytochrome P450 oxidase enzyme (WILLIAMS, 2011). On the second stage is 1-
Methyl being broken down to 7-Methylxyanthine by the help of 7-Methylxanthine demethylase.
This will then be broken down to theobromine by the help of theobromine synthase enzyme.
Theobromine is finally broken down to form caffeine and this will be achieved by the help of
paraxanthine enzyme. This pathway can be illustrated by the diagram below;
Fig 4: Showing pathway of 1-methyl ( (WILLIAMS, 2011))
Metabolic pathway of 1-methyl entails two concurrent stages where the in the first stage 1-
methyl is broken into 1-methyl-xanthine by the help of enzyme xanthine oxidase. This is further
broken down into theophylline by the same enzyme. Theophylline is finally disintegrated to
caffeine by cytochrome P450 oxidase enzyme (WILLIAMS, 2011). On the second stage is 1-
Methyl being broken down to 7-Methylxyanthine by the help of 7-Methylxanthine demethylase.
This will then be broken down to theobromine by the help of theobromine synthase enzyme.
Theobromine is finally broken down to form caffeine and this will be achieved by the help of
paraxanthine enzyme. This pathway can be illustrated by the diagram below;
Fig 4: Showing pathway of 1-methyl ( (WILLIAMS, 2011))
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Food Chemistry 11
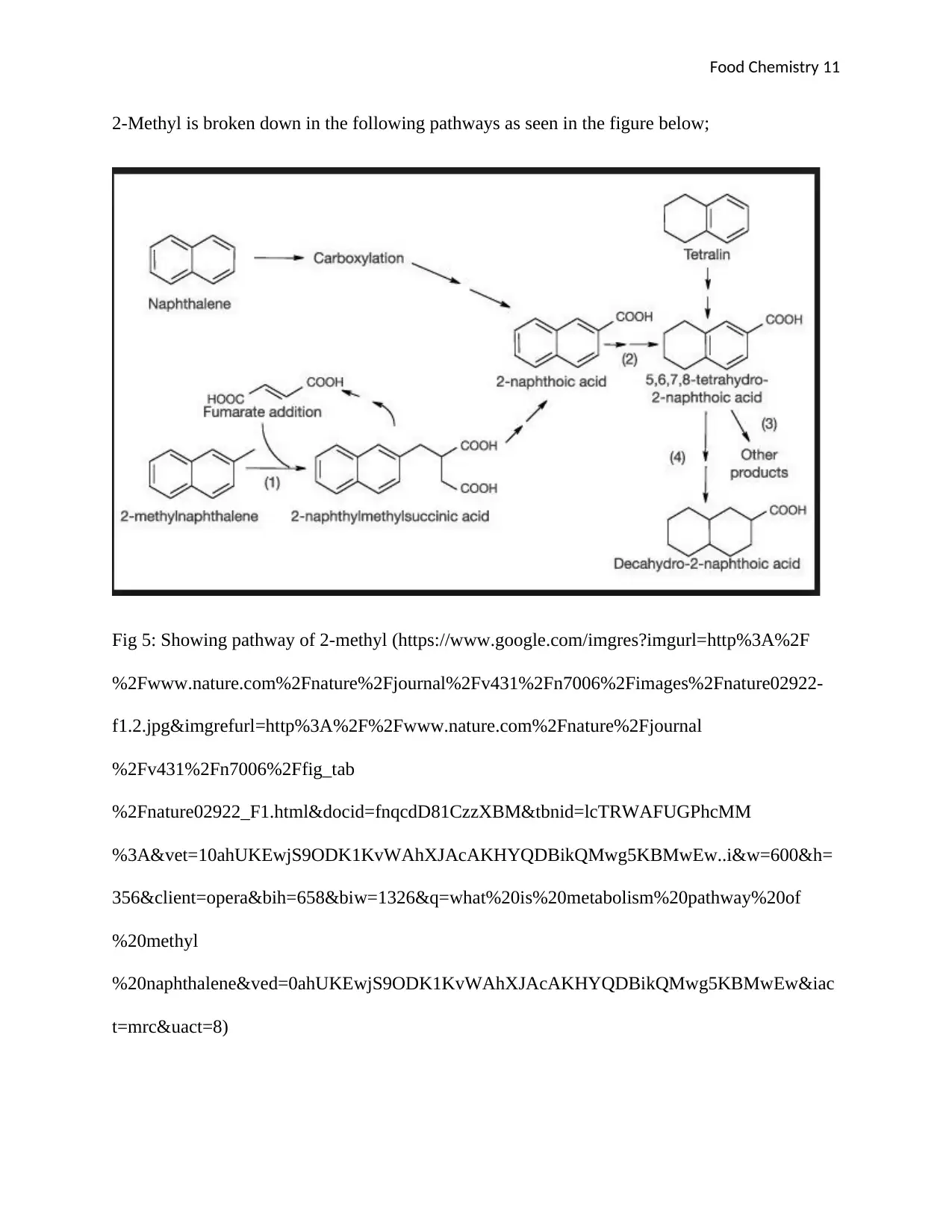
2-Methyl is broken down in the following pathways as seen in the figure below;
Fig 5: Showing pathway of 2-methyl (https://www.google.com/imgres?imgurl=http%3A%2F
%2Fwww.nature.com%2Fnature%2Fjournal%2Fv431%2Fn7006%2Fimages%2Fnature02922-
f1.2.jpg&imgrefurl=http%3A%2F%2Fwww.nature.com%2Fnature%2Fjournal
%2Fv431%2Fn7006%2Ffig_tab
%2Fnature02922_F1.html&docid=fnqcdD81CzzXBM&tbnid=lcTRWAFUGPhcMM
%3A&vet=10ahUKEwjS9ODK1KvWAhXJAcAKHYQDBikQMwg5KBMwEw..i&w=600&h=
356&client=opera&bih=658&biw=1326&q=what%20is%20metabolism%20pathway%20of
%20methyl
%20naphthalene&ved=0ahUKEwjS9ODK1KvWAhXJAcAKHYQDBikQMwg5KBMwEw&iac
t=mrc&uact=8)
2-Methyl is broken down in the following pathways as seen in the figure below;
Fig 5: Showing pathway of 2-methyl (https://www.google.com/imgres?imgurl=http%3A%2F
%2Fwww.nature.com%2Fnature%2Fjournal%2Fv431%2Fn7006%2Fimages%2Fnature02922-
f1.2.jpg&imgrefurl=http%3A%2F%2Fwww.nature.com%2Fnature%2Fjournal
%2Fv431%2Fn7006%2Ffig_tab
%2Fnature02922_F1.html&docid=fnqcdD81CzzXBM&tbnid=lcTRWAFUGPhcMM
%3A&vet=10ahUKEwjS9ODK1KvWAhXJAcAKHYQDBikQMwg5KBMwEw..i&w=600&h=
356&client=opera&bih=658&biw=1326&q=what%20is%20metabolism%20pathway%20of
%20methyl
%20naphthalene&ved=0ahUKEwjS9ODK1KvWAhXJAcAKHYQDBikQMwg5KBMwEw&iac
t=mrc&uact=8)

Food Chemistry 12
Just like the 1-Methyl, 2-Methyl is also broken down in two concurrent stages, where the first
stage is 2-Methylnaphthalene is broken down to 2-naphthylmethylsuccinic acid this is promoted
by addition of Fumarate, this is then broken down to 2-naphthoic acid by the help of synthetase
enzyme. This naphthoic acid is combined with the first stage which entails the breaking of
Naphthalene to Carboxylation and then forms 2-naphthoic acid too. 2-naphthoic acid is then
broken down to form 5,6,7,8 tetrahyhydro-2-naphthoic acid, this will be achieved by the use of
synthetese enzyme. 5,6,7,8 tetrahyhydro-2-naphthoic acid is further broken down to form other
products and Decahydro-2-naphtholic acid as the final product during metabolic reaction.
When alkyl group is added to an aromatic ring the rate of metabolism will reduce. This is
because the aqueous solubility will be reduced, with the reduction of the solubility aqueous
substance on addition of alkyl group to aromatic ring (Chen, 2012). Information indicates that in
within a defined lipophilicity range, increase alkyl group on aromatic ring will result to
decreased solubility. With reduced solubility the rate of metabolism will as well reduce.
In both naphthalene and 1-methyl, 2-methyl, 1-ethyl, 2 ethyl there is at least a ring of carbon
atoms, for example in Naphthalene there are two rings of carbon atoms ( benzene ) which when
combined form Naphthalene as seen in the figure below;
Just like the 1-Methyl, 2-Methyl is also broken down in two concurrent stages, where the first
stage is 2-Methylnaphthalene is broken down to 2-naphthylmethylsuccinic acid this is promoted
by addition of Fumarate, this is then broken down to 2-naphthoic acid by the help of synthetase
enzyme. This naphthoic acid is combined with the first stage which entails the breaking of
Naphthalene to Carboxylation and then forms 2-naphthoic acid too. 2-naphthoic acid is then
broken down to form 5,6,7,8 tetrahyhydro-2-naphthoic acid, this will be achieved by the use of
synthetese enzyme. 5,6,7,8 tetrahyhydro-2-naphthoic acid is further broken down to form other
products and Decahydro-2-naphtholic acid as the final product during metabolic reaction.
When alkyl group is added to an aromatic ring the rate of metabolism will reduce. This is
because the aqueous solubility will be reduced, with the reduction of the solubility aqueous
substance on addition of alkyl group to aromatic ring (Chen, 2012). Information indicates that in
within a defined lipophilicity range, increase alkyl group on aromatic ring will result to
decreased solubility. With reduced solubility the rate of metabolism will as well reduce.
In both naphthalene and 1-methyl, 2-methyl, 1-ethyl, 2 ethyl there is at least a ring of carbon
atoms, for example in Naphthalene there are two rings of carbon atoms ( benzene ) which when
combined form Naphthalene as seen in the figure below;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 18
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

