NUT714 Food Safety Risk Assessment Report: Aspirin Analysis
VerifiedAdded on 2023/01/06
|11
|2119
|33
Report
AI Summary
This report delves into the critical aspects of food safety risk assessment, encompassing hazard identification, dose-response relationships, and hazard characterization. The report begins by defining risk assessment and its four key steps, followed by a discussion on the principles of toxicological risk assessment, including considerations for various substances, safe exposure levels, and the use of NOAEL and LOAEL. It then examines the dose-response assessment process, including the calculation of ADIs using safety factors. The report further explores hazard identification, differentiating between biological, chemical, and physical hazards, and proceeds to the hazard characterization process, outlining its stages from process initiation to the presentation of results. The analysis includes a discussion on the setting of safe levels, illustrated by examples related to aspirin, and concludes with a summary of the key findings, emphasizing the significance of risk assessment in ensuring public health. References are cited throughout the report to support the analysis and findings.

Running Head: RA
0
Food Safety
Student
5/6/2019
0
Food Safety
Student
5/6/2019
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RA
1
Table of Contents
Introduction.................................................................................................................................................2
Dose response assessment.......................................................................................................................3
Hazard identification and characterization...............................................................................................4
Hazard characterization:......................................................................................................................5
Conclusion...................................................................................................................................................7
References...................................................................................................................................................9
1
Table of Contents
Introduction.................................................................................................................................................2
Dose response assessment.......................................................................................................................3
Hazard identification and characterization...............................................................................................4
Hazard characterization:......................................................................................................................5
Conclusion...................................................................................................................................................7
References...................................................................................................................................................9

RA
2
Introduction
Risk assessment is the ever-evolving practice in which the scientific information on the harmful
properties of some compounds and the degree of exposure outcomes in a declaration as to the
likelihood that exposed populaces will be affected. There are four different steps in the risk
assessment process; hazard identifications, dose response valuation, exposure evaluation and risk
characterisation (Damalas, and Eleftherohorinos, 2011).
The principles associated with the toxicological risk assessment are
1. Special considerations for the different substances taken in small amounts; some of the
substances present in food are the residues transfer in foodstuffs form the packaging
material, contaminants present in environment like lead, mercury, chlorinated, cadmium,
and residual amounts of pesticides (Greim, and Snyder, 2018).
2. The establishment of the safe exposure levels established for the chemicals includes in
the risk assessment, in which the NOAELs not detected opposing effect levels) are
determined from translated into the acceptable levels of exposure such as satisfactory
daily intake or ADI (Parkhouse, et al., 1968).
3. The safety evaluations agents mixed in food for flavour presents a specific challenge,
these substances consumed in low amount and testing them for toxicity using the
toxicological strategies shows a formidable challenge and challenges. There is an
adequate margin of safety between the dietary exposure to the flavouring agent and the
NOAEL (Roderick, et al., 1993).
2
Introduction
Risk assessment is the ever-evolving practice in which the scientific information on the harmful
properties of some compounds and the degree of exposure outcomes in a declaration as to the
likelihood that exposed populaces will be affected. There are four different steps in the risk
assessment process; hazard identifications, dose response valuation, exposure evaluation and risk
characterisation (Damalas, and Eleftherohorinos, 2011).
The principles associated with the toxicological risk assessment are
1. Special considerations for the different substances taken in small amounts; some of the
substances present in food are the residues transfer in foodstuffs form the packaging
material, contaminants present in environment like lead, mercury, chlorinated, cadmium,
and residual amounts of pesticides (Greim, and Snyder, 2018).
2. The establishment of the safe exposure levels established for the chemicals includes in
the risk assessment, in which the NOAELs not detected opposing effect levels) are
determined from translated into the acceptable levels of exposure such as satisfactory
daily intake or ADI (Parkhouse, et al., 1968).
3. The safety evaluations agents mixed in food for flavour presents a specific challenge,
these substances consumed in low amount and testing them for toxicity using the
toxicological strategies shows a formidable challenge and challenges. There is an
adequate margin of safety between the dietary exposure to the flavouring agent and the
NOAEL (Roderick, et al., 1993).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

RA
3
4. Processing aids are compared of diverse substances comprising carrier or removal
solvents and the enzymes used in the food processing (Damalas, and Eleftherohorinos,
2011).
5. Lowest observed adverse effect levels (LOAEL) are the lowermost levels concentration
or amount of the substances, identified by the experiments or analysis. (Boyd, 1968).
6. Both NOAEL and LOAEL are based on perceived intake intensities that are established
as portion of the study plan. Neither takes into an account the form of the intake–response
curve that can be viewed at other intensities of intake (Overbosch, and Blanchard, 2014).
Dose response assessment
In order to determine ADIs, the NOAEL is classified by the safety factors to deliver a margin of
safety for the acceptable human exposure.
ADI = NOAEL (experimental dose)/Safety Factor (S)
A supplementary safety factor is involved of the LOAEL is applied. The modifying factor of 0.1
to 10 allows the risk evaluators to apply scientific judgement in order to upgrade or downgrade
the aspect cantered on the dependability and superiority of the data (Liu et al., 2013).
3
4. Processing aids are compared of diverse substances comprising carrier or removal
solvents and the enzymes used in the food processing (Damalas, and Eleftherohorinos,
2011).
5. Lowest observed adverse effect levels (LOAEL) are the lowermost levels concentration
or amount of the substances, identified by the experiments or analysis. (Boyd, 1968).
6. Both NOAEL and LOAEL are based on perceived intake intensities that are established
as portion of the study plan. Neither takes into an account the form of the intake–response
curve that can be viewed at other intensities of intake (Overbosch, and Blanchard, 2014).
Dose response assessment
In order to determine ADIs, the NOAEL is classified by the safety factors to deliver a margin of
safety for the acceptable human exposure.
ADI = NOAEL (experimental dose)/Safety Factor (S)
A supplementary safety factor is involved of the LOAEL is applied. The modifying factor of 0.1
to 10 allows the risk evaluators to apply scientific judgement in order to upgrade or downgrade
the aspect cantered on the dependability and superiority of the data (Liu et al., 2013).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
RA
4
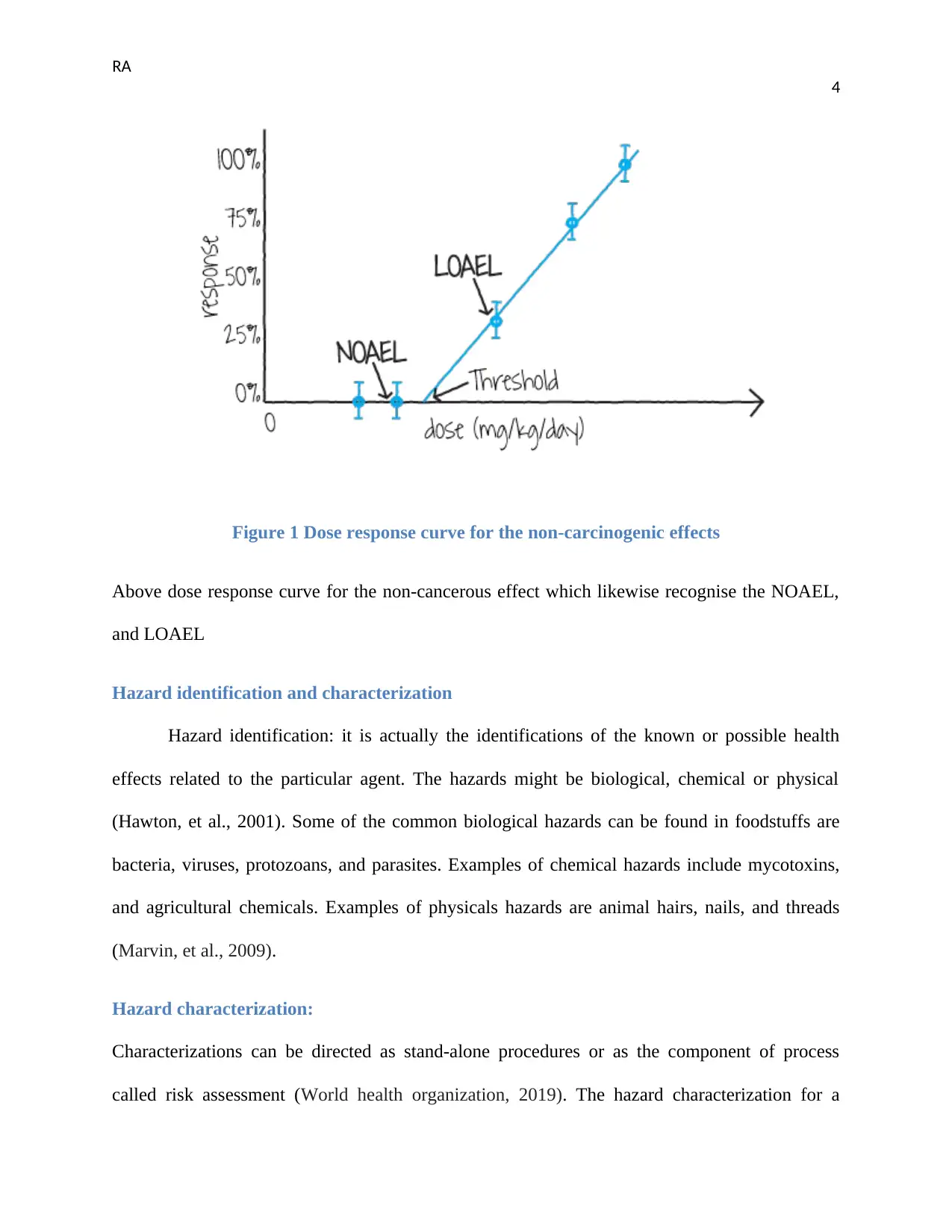
Figure 1 Dose response curve for the non-carcinogenic effects
Above dose response curve for the non-cancerous effect which likewise recognise the NOAEL,
and LOAEL
Hazard identification and characterization
Hazard identification: it is actually the identifications of the known or possible health
effects related to the particular agent. The hazards might be biological, chemical or physical
(Hawton, et al., 2001). Some of the common biological hazards can be found in foodstuffs are
bacteria, viruses, protozoans, and parasites. Examples of chemical hazards include mycotoxins,
and agricultural chemicals. Examples of physicals hazards are animal hairs, nails, and threads
(Marvin, et al., 2009).
Hazard characterization:
Characterizations can be directed as stand-alone procedures or as the component of process
called risk assessment (World health organization, 2019). The hazard characterization for a
4
Figure 1 Dose response curve for the non-carcinogenic effects
Above dose response curve for the non-cancerous effect which likewise recognise the NOAEL,
and LOAEL
Hazard identification and characterization
Hazard identification: it is actually the identifications of the known or possible health
effects related to the particular agent. The hazards might be biological, chemical or physical
(Hawton, et al., 2001). Some of the common biological hazards can be found in foodstuffs are
bacteria, viruses, protozoans, and parasites. Examples of chemical hazards include mycotoxins,
and agricultural chemicals. Examples of physicals hazards are animal hairs, nails, and threads
(Marvin, et al., 2009).
Hazard characterization:
Characterizations can be directed as stand-alone procedures or as the component of process
called risk assessment (World health organization, 2019). The hazard characterization for a
RA
5
specific pathogen might serve as a shared module or building block for the risk assessment
process directed for a range of determinations and in a collection of commodities (Havelaar,
Brul, De Jong, De Jonge, Zwietering, and Ter Kuile, 2010).
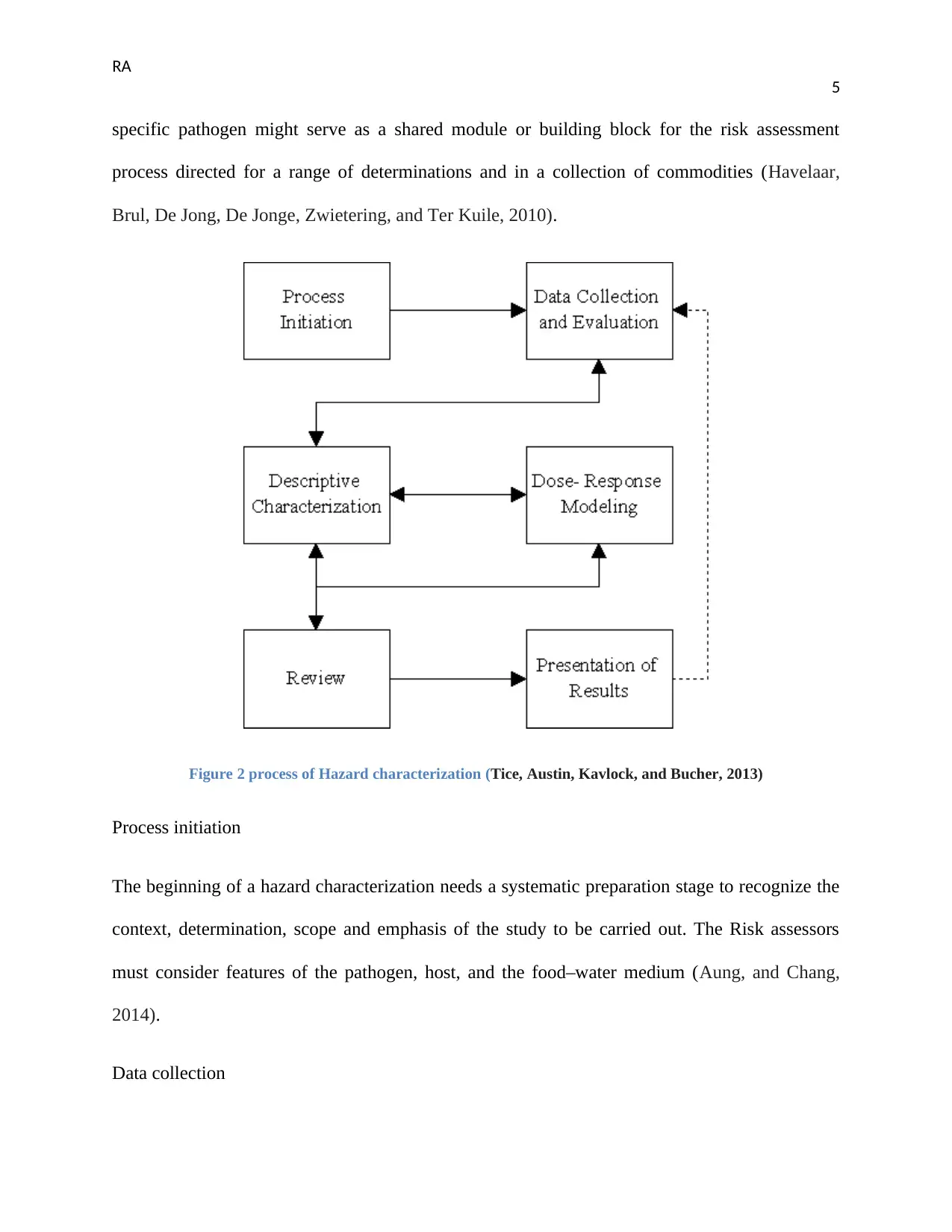
Figure 2 process of Hazard characterization (Tice, Austin, Kavlock, and Bucher, 2013)
Process initiation
The beginning of a hazard characterization needs a systematic preparation stage to recognize the
context, determination, scope and emphasis of the study to be carried out. The Risk assessors
must consider features of the pathogen, host, and the food–water medium (Aung, and Chang,
2014).
Data collection
5
specific pathogen might serve as a shared module or building block for the risk assessment
process directed for a range of determinations and in a collection of commodities (Havelaar,
Brul, De Jong, De Jonge, Zwietering, and Ter Kuile, 2010).
Figure 2 process of Hazard characterization (Tice, Austin, Kavlock, and Bucher, 2013)
Process initiation
The beginning of a hazard characterization needs a systematic preparation stage to recognize the
context, determination, scope and emphasis of the study to be carried out. The Risk assessors
must consider features of the pathogen, host, and the food–water medium (Aung, and Chang,
2014).
Data collection
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

RA
6
Hazard characterizations are naturally developed by amassing information from a range of
information sources, applying a plethora of examination protocols. The gratitude of the strengths
and limits of the numerous data foundations is critical to choosing appropriate data for practice,
and to founding the improbability related with dose-response replicas that are industrialized from
dissimilar data sets and test practice (Havelaar, et al., 2010).
Evaluation
Risk assessors should assess both the quality of the obtainable sources of info for the
determination of the examination, and the resources of characterizing the insecurity of all the
facts used. Dignified superiority control of raw facts and its following treatment is wanted,
nonetheless also highly reliant on obtainability and the application to which the facts are applied
(Tice, Austin, Kavlock, and Bucher, 2013).
Descriptive characterization
Descriptive Evocative hazard characterization assists to structure and current the available data
on the range of human disease associated with a specific pathogen, and how this is subjective by
the features of a host, a pathogen and the matrix, as designated. This is founded on the qualitative
or a semi-quantitative examination of the obtainable evidence, and may take the diverse
pathogenic mechanisms into an account (Aung, and Chang, 2014)
Dose response Modelling
Alongside with the descriptive investigation of medical or epidemiological info or data, and
mathematical type modelling has been supported to provide help in emerging a dose response
association, in specific when extrapolation to lesser doses is essential. In the ground of food
6
Hazard characterizations are naturally developed by amassing information from a range of
information sources, applying a plethora of examination protocols. The gratitude of the strengths
and limits of the numerous data foundations is critical to choosing appropriate data for practice,
and to founding the improbability related with dose-response replicas that are industrialized from
dissimilar data sets and test practice (Havelaar, et al., 2010).
Evaluation
Risk assessors should assess both the quality of the obtainable sources of info for the
determination of the examination, and the resources of characterizing the insecurity of all the
facts used. Dignified superiority control of raw facts and its following treatment is wanted,
nonetheless also highly reliant on obtainability and the application to which the facts are applied
(Tice, Austin, Kavlock, and Bucher, 2013).
Descriptive characterization
Descriptive Evocative hazard characterization assists to structure and current the available data
on the range of human disease associated with a specific pathogen, and how this is subjective by
the features of a host, a pathogen and the matrix, as designated. This is founded on the qualitative
or a semi-quantitative examination of the obtainable evidence, and may take the diverse
pathogenic mechanisms into an account (Aung, and Chang, 2014)
Dose response Modelling
Alongside with the descriptive investigation of medical or epidemiological info or data, and
mathematical type modelling has been supported to provide help in emerging a dose response
association, in specific when extrapolation to lesser doses is essential. In the ground of food
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RA
7
microbiology, it is presently accepted that mathematical replicas might enable the dose-response
valuation exercise, and deliver useful info though accounting for changeability and uncertainty
(Marvin, et al., 2009).
Review
Peer and community review of outcomes is an important part of the procedure. Interdisciplinary
communication is important to the procedure of risk assessment, and must be lengthy to the
review procedure. Specialists in the biological procedures involved must review the simple
concepts and fundamental assumptions applied in a hazard characterization (Brown Matthew,
1967).
Presentation of results
Transparency needs that outcomes are both nearby and comprehensible. Therefore, consideration
must be given to the practice of the presentation of the hazard characterization outcomes for both
technical and the non-technical viewers (Tice et al., 2013). ‘
General consideration
The level of hazard will upsurge as the probability of harm and its seriousness upsurges. The
probability of harm happening might be exaggerated by how frequently the task is finished, in
what circumstances, how many individuals are unprotected to the threat and for what period.
Access to the threat must be restricted till the hazard can be dropped to an acceptable level. The
risk levels can be set by implementing the colour threats or rating system. The green colour will
display that there is no risk, and red colour means that there is high risk (Poma, et al., 2017).
Steps after RA
7
microbiology, it is presently accepted that mathematical replicas might enable the dose-response
valuation exercise, and deliver useful info though accounting for changeability and uncertainty
(Marvin, et al., 2009).
Review
Peer and community review of outcomes is an important part of the procedure. Interdisciplinary
communication is important to the procedure of risk assessment, and must be lengthy to the
review procedure. Specialists in the biological procedures involved must review the simple
concepts and fundamental assumptions applied in a hazard characterization (Brown Matthew,
1967).
Presentation of results
Transparency needs that outcomes are both nearby and comprehensible. Therefore, consideration
must be given to the practice of the presentation of the hazard characterization outcomes for both
technical and the non-technical viewers (Tice et al., 2013). ‘
General consideration
The level of hazard will upsurge as the probability of harm and its seriousness upsurges. The
probability of harm happening might be exaggerated by how frequently the task is finished, in
what circumstances, how many individuals are unprotected to the threat and for what period.
Access to the threat must be restricted till the hazard can be dropped to an acceptable level. The
risk levels can be set by implementing the colour threats or rating system. The green colour will
display that there is no risk, and red colour means that there is high risk (Poma, et al., 2017).
Steps after RA

RA
8
Setting safe levels of salicylate (aspirin)
The usual dosages of aspirin for mild pain are 350 or 650 mg per four hours or 500 mg per 6
hours. If there is any symptom of heart attack observed 160 to 325 mf of non-enteric coated
aspirin must be chewed instantly. The preventing does of another stroke IQ is 75 to 100 mg
every day. Therapeutic levels for aspirin are 150-300 mcg/mL. The legislations associated with
aspirin use are that the specific warning of threats of aspirin overuses should be printed on the
packets and on the leaflets in the packets (Brown and Matthew 1967).
Conclusion
Risk assessment is actually the method in which the clinical data on the harmful properties the
compounds and the rate of exposure used to identify the outcome of the problem or risk. In order
to determine ADI, the NOAEL is characterised by the safety aspects to provide a sideline of the
security for the suitable human experience. Hazard identification is the recognition of the
identified or conceivable health properties related to the specific agent. Hazard characterization
process includes process initiation, data collection, descriptive characterization, dose response
modelling, review, and presentation of the outcomes. The safe uses can be set according to the
intensity of pain and gender and age.
8
Setting safe levels of salicylate (aspirin)
The usual dosages of aspirin for mild pain are 350 or 650 mg per four hours or 500 mg per 6
hours. If there is any symptom of heart attack observed 160 to 325 mf of non-enteric coated
aspirin must be chewed instantly. The preventing does of another stroke IQ is 75 to 100 mg
every day. Therapeutic levels for aspirin are 150-300 mcg/mL. The legislations associated with
aspirin use are that the specific warning of threats of aspirin overuses should be printed on the
packets and on the leaflets in the packets (Brown and Matthew 1967).
Conclusion
Risk assessment is actually the method in which the clinical data on the harmful properties the
compounds and the rate of exposure used to identify the outcome of the problem or risk. In order
to determine ADI, the NOAEL is characterised by the safety aspects to provide a sideline of the
security for the suitable human experience. Hazard identification is the recognition of the
identified or conceivable health properties related to the specific agent. Hazard characterization
process includes process initiation, data collection, descriptive characterization, dose response
modelling, review, and presentation of the outcomes. The safe uses can be set according to the
intensity of pain and gender and age.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

RA
9
References
Aung, M.M. and Chang, Y.S., 2014. Traceability in a food supply chain: Safety and quality
perspectives. Food control, 39, pp.172-184.
Boyd, E. M. (1968). "Analgesic abuse: maximal tolerated daily doses of acetylsalicylic acid."
Canadian Medical Association Journal, 99(16): 7908.
Brown SS, C. J., Matthew H. (1967). "Plasma salicylate levels in acute poisoning in adults."
British Medical Journal 2(5554):738-9.
Damalas, C.A. and Eleftherohorinos, I.G., 2011. Pesticide exposure, safety issues, and risk
assessment indicators. International journal of environmental research and public health, 8(5),
pp.1402-1419.
Greim, H. and Snyder, R. eds., 2018. Toxicology and risk assessment: a comprehensive
introduction. John Wiley & Sons.
Havelaar, A.H., Brul, S., De Jong, A., De Jonge, R., Zwietering, M.H. and Ter Kuile, B.H.,
2010. Future challenges to microbial food safety. International Journal of Food
Microbiology, 139, pp.S79-S94.
Hawton, K., E. Townsend, et al. (2001). "Effects of legislation restricting pack sizes of
paracetamol and salicylate on self-poisoning in the United Kingdom: before and after study."
British Medical Journal ,322(1203) 1203-7.
Liu, X., Song, Q., Tang, Y., Li, W., Xu, J., Wu, J., Wang, F. and Brookes, P.C., 2013. Human
health risk assessment of heavy metals in soil–vegetable system: a multi-medium
analysis. Science of the Total Environment, 463, pp.530-540.
9
References
Aung, M.M. and Chang, Y.S., 2014. Traceability in a food supply chain: Safety and quality
perspectives. Food control, 39, pp.172-184.
Boyd, E. M. (1968). "Analgesic abuse: maximal tolerated daily doses of acetylsalicylic acid."
Canadian Medical Association Journal, 99(16): 7908.
Brown SS, C. J., Matthew H. (1967). "Plasma salicylate levels in acute poisoning in adults."
British Medical Journal 2(5554):738-9.
Damalas, C.A. and Eleftherohorinos, I.G., 2011. Pesticide exposure, safety issues, and risk
assessment indicators. International journal of environmental research and public health, 8(5),
pp.1402-1419.
Greim, H. and Snyder, R. eds., 2018. Toxicology and risk assessment: a comprehensive
introduction. John Wiley & Sons.
Havelaar, A.H., Brul, S., De Jong, A., De Jonge, R., Zwietering, M.H. and Ter Kuile, B.H.,
2010. Future challenges to microbial food safety. International Journal of Food
Microbiology, 139, pp.S79-S94.
Hawton, K., E. Townsend, et al. (2001). "Effects of legislation restricting pack sizes of
paracetamol and salicylate on self-poisoning in the United Kingdom: before and after study."
British Medical Journal ,322(1203) 1203-7.
Liu, X., Song, Q., Tang, Y., Li, W., Xu, J., Wu, J., Wang, F. and Brookes, P.C., 2013. Human
health risk assessment of heavy metals in soil–vegetable system: a multi-medium
analysis. Science of the Total Environment, 463, pp.530-540.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RA
10
Marvin, H.J.P., Kleter, G.A., Prandini, A., Dekkers, S. and Bolton, D.J., 2009. Early
identification systems for emerging foodborne hazards. Food and Chemical Toxicology, 47(5),
pp.915-926.
Overbosch, P. and Blanchard, S., 2014. Principles and systems for quality and food safety
management. In Food Safety Management (pp. 537-558). Academic Press.
Poma, G., Cuykx, M., Amato, E., Calaprice, C., Focant, J.F. and Covaci, A., 2017. Evaluation of
hazardous chemicals in edible insects and insect-based food intended for human consumption. Food and
chemical toxicology, 100, pp.70-79.
Roderick, P. J., H. C. Wilkes, et al. (1993). "The gastrointestinal toxicity of aspirin: an overview
of randomised controlled trials." British Journal of Clinical Pharmacology 35(3): 219-26.
Tice, R.R., Austin, C.P., Kavlock, R.J. and Bucher, J.R., 2013. Improving the human hazard
characterization of chemicals: a Tox21 update. Environmental health perspectives, 121(7),
pp.756-765.
World Health Organisation. 2019. Hazard characterization for pathogens in food and water
[ONLINE]. Available from: https://www.who.int/foodsafety/publications/mra_3/en/ [Accessed
6th May 2019]
10
Marvin, H.J.P., Kleter, G.A., Prandini, A., Dekkers, S. and Bolton, D.J., 2009. Early
identification systems for emerging foodborne hazards. Food and Chemical Toxicology, 47(5),
pp.915-926.
Overbosch, P. and Blanchard, S., 2014. Principles and systems for quality and food safety
management. In Food Safety Management (pp. 537-558). Academic Press.
Poma, G., Cuykx, M., Amato, E., Calaprice, C., Focant, J.F. and Covaci, A., 2017. Evaluation of
hazardous chemicals in edible insects and insect-based food intended for human consumption. Food and
chemical toxicology, 100, pp.70-79.
Roderick, P. J., H. C. Wilkes, et al. (1993). "The gastrointestinal toxicity of aspirin: an overview
of randomised controlled trials." British Journal of Clinical Pharmacology 35(3): 219-26.
Tice, R.R., Austin, C.P., Kavlock, R.J. and Bucher, J.R., 2013. Improving the human hazard
characterization of chemicals: a Tox21 update. Environmental health perspectives, 121(7),
pp.756-765.
World Health Organisation. 2019. Hazard characterization for pathogens in food and water
[ONLINE]. Available from: https://www.who.int/foodsafety/publications/mra_3/en/ [Accessed
6th May 2019]
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




![Chemical Risk Assessment Report: Benzo[a]pyrene in Biosolids](/_next/image/?url=https%3A%2F%2Fdesklib.com%2Fmedia%2Fimages%2Fck%2F80b1cd28273045d0abdea30a147a23b4.jpg&w=256&q=75)
