Functional Groups: Role in Macromolecules - Biology Essay
VerifiedAdded on 2022/08/11
|5
|747
|22
Essay
AI Summary
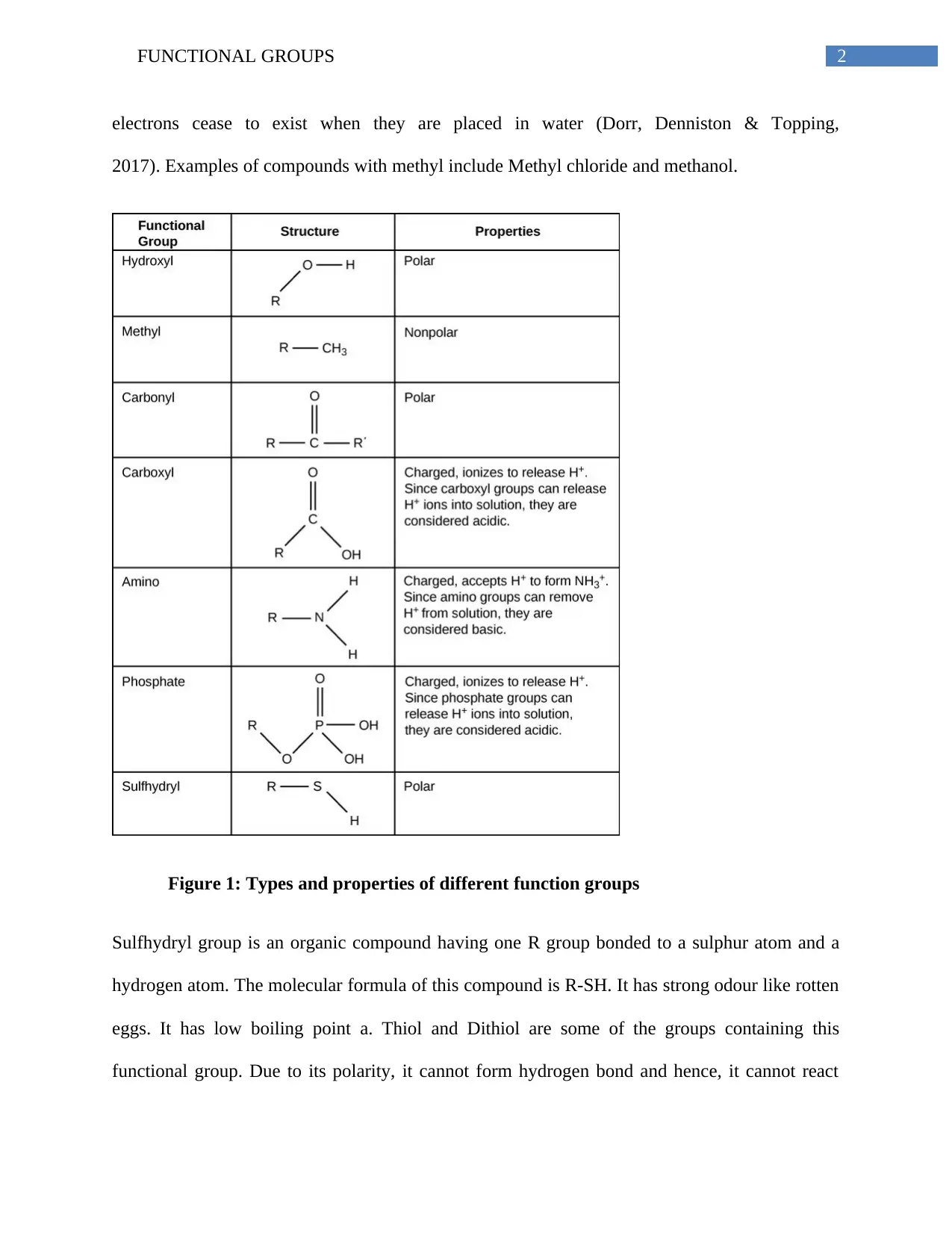
This essay explores the significance of functional groups in organic molecules. It delves into the structure and properties of six key functional groups: hydroxyl, carbonyl, methyl, sulfhydryl, amino, and phosphate. The essay examines how each group contributes to the characteristics of macromolecules such as proteins, lipids, carbohydrates, and nucleic acids. It highlights the polarity, reactivity, and bonding capabilities of each group, providing examples like alcohols, aldehydes, ketones, and amino acids. The essay emphasizes the role of these functional groups in influencing chemical reactions, solubility, and the overall behavior of organic compounds, ultimately concluding that these groups are fundamental to the formation and function of biological molecules. The essay uses references to support the information presented.
1 out of 5











![[object Object]](/_next/static/media/star-bottom.7253800d.svg)