KCU IP E&C Report: Future Fuels - Diesel vs. Biodiesel Comparison
VerifiedAdded on 2022/12/21
|10
|2062
|61
Report
AI Summary
This report provides a comparative analysis of diesel and biodiesel fuels, focusing on their environmental impact, production costs, and health implications. The study delves into the mechanics of diesel engines, the chemical composition of diesel and biodiesel (specifically peanut oil), and the processes involved in fuel production. The report evaluates the energy produced by each fuel type, highlighting that biodiesel from peanut oil generates more energy. It assesses the emissions associated with each fuel, including greenhouse gases like carbon dioxide, and discusses the potential of biodiesel to reduce these emissions. The report also examines the production and selling costs of both fuels and analyzes the health risks associated with each, including cancer risks. Ultimately, the report concludes that biodiesel is a more suitable option for powering engines due to its lower environmental impact and reduced health risks. The report uses information from various sources, including Biofuels Association of Australia, and Cancer.org.au.

FUTURE FUELS
By Name
Course
Instructor
Institution
Location
Date
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
FUTURE FUELS
Diesel versus Biodiesel Peanut Oil
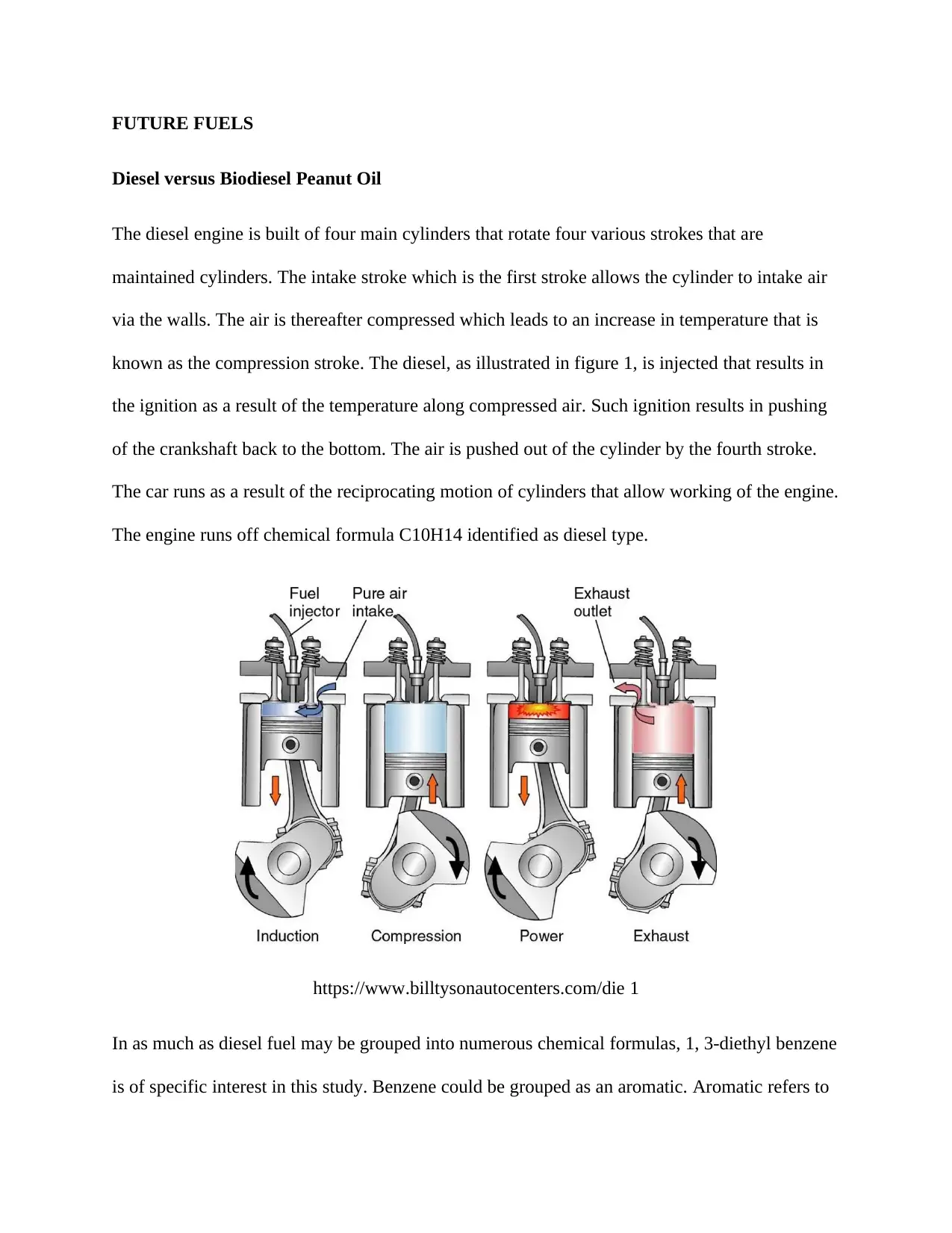
The diesel engine is built of four main cylinders that rotate four various strokes that are
maintained cylinders. The intake stroke which is the first stroke allows the cylinder to intake air
via the walls. The air is thereafter compressed which leads to an increase in temperature that is
known as the compression stroke. The diesel, as illustrated in figure 1, is injected that results in
the ignition as a result of the temperature along compressed air. Such ignition results in pushing
of the crankshaft back to the bottom. The air is pushed out of the cylinder by the fourth stroke.
The car runs as a result of the reciprocating motion of cylinders that allow working of the engine.
The engine runs off chemical formula C10H14 identified as diesel type.
https://www.billtysonautocenters.com/die 1
In as much as diesel fuel may be grouped into numerous chemical formulas, 1, 3-diethyl benzene
is of specific interest in this study. Benzene could be grouped as an aromatic. Aromatic refers to
Diesel versus Biodiesel Peanut Oil
The diesel engine is built of four main cylinders that rotate four various strokes that are
maintained cylinders. The intake stroke which is the first stroke allows the cylinder to intake air
via the walls. The air is thereafter compressed which leads to an increase in temperature that is
known as the compression stroke. The diesel, as illustrated in figure 1, is injected that results in
the ignition as a result of the temperature along compressed air. Such ignition results in pushing
of the crankshaft back to the bottom. The air is pushed out of the cylinder by the fourth stroke.
The car runs as a result of the reciprocating motion of cylinders that allow working of the engine.
The engine runs off chemical formula C10H14 identified as diesel type.
https://www.billtysonautocenters.com/die 1
In as much as diesel fuel may be grouped into numerous chemical formulas, 1, 3-diethyl benzene
is of specific interest in this study. Benzene could be grouped as an aromatic. Aromatic refers to
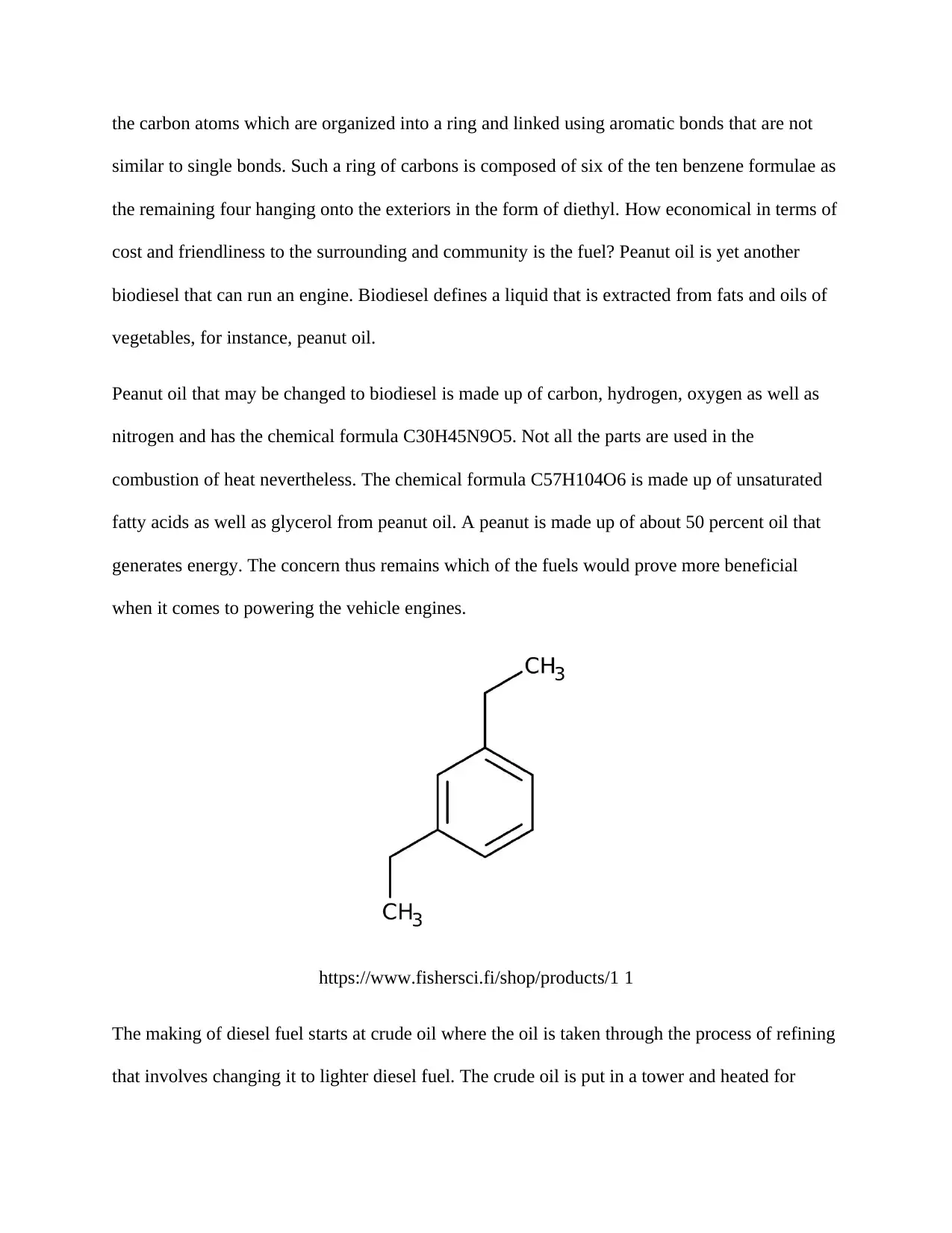
the carbon atoms which are organized into a ring and linked using aromatic bonds that are not
similar to single bonds. Such a ring of carbons is composed of six of the ten benzene formulae as
the remaining four hanging onto the exteriors in the form of diethyl. How economical in terms of
cost and friendliness to the surrounding and community is the fuel? Peanut oil is yet another
biodiesel that can run an engine. Biodiesel defines a liquid that is extracted from fats and oils of
vegetables, for instance, peanut oil.
Peanut oil that may be changed to biodiesel is made up of carbon, hydrogen, oxygen as well as
nitrogen and has the chemical formula C30H45N9O5. Not all the parts are used in the
combustion of heat nevertheless. The chemical formula C57H104O6 is made up of unsaturated
fatty acids as well as glycerol from peanut oil. A peanut is made up of about 50 percent oil that
generates energy. The concern thus remains which of the fuels would prove more beneficial
when it comes to powering the vehicle engines.
https://www.fishersci.fi/shop/products/1 1
The making of diesel fuel starts at crude oil where the oil is taken through the process of refining
that involves changing it to lighter diesel fuel. The crude oil is put in a tower and heated for
similar to single bonds. Such a ring of carbons is composed of six of the ten benzene formulae as
the remaining four hanging onto the exteriors in the form of diethyl. How economical in terms of
cost and friendliness to the surrounding and community is the fuel? Peanut oil is yet another
biodiesel that can run an engine. Biodiesel defines a liquid that is extracted from fats and oils of
vegetables, for instance, peanut oil.
Peanut oil that may be changed to biodiesel is made up of carbon, hydrogen, oxygen as well as
nitrogen and has the chemical formula C30H45N9O5. Not all the parts are used in the
combustion of heat nevertheless. The chemical formula C57H104O6 is made up of unsaturated
fatty acids as well as glycerol from peanut oil. A peanut is made up of about 50 percent oil that
generates energy. The concern thus remains which of the fuels would prove more beneficial
when it comes to powering the vehicle engines.
https://www.fishersci.fi/shop/products/1 1
The making of diesel fuel starts at crude oil where the oil is taken through the process of refining
that involves changing it to lighter diesel fuel. The crude oil is put in a tower and heated for
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
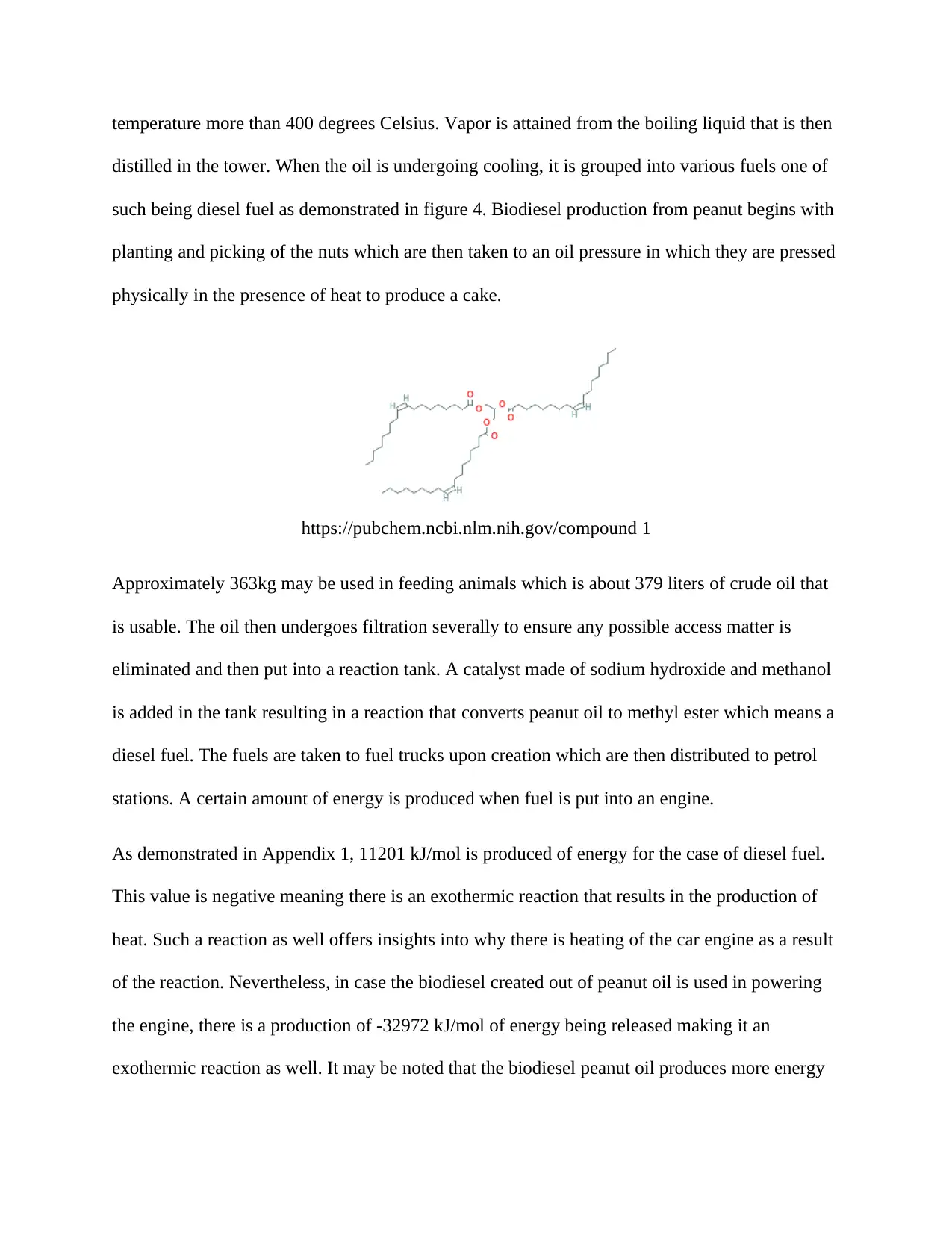
temperature more than 400 degrees Celsius. Vapor is attained from the boiling liquid that is then
distilled in the tower. When the oil is undergoing cooling, it is grouped into various fuels one of
such being diesel fuel as demonstrated in figure 4. Biodiesel production from peanut begins with
planting and picking of the nuts which are then taken to an oil pressure in which they are pressed
physically in the presence of heat to produce a cake.
https://pubchem.ncbi.nlm.nih.gov/compound 1
Approximately 363kg may be used in feeding animals which is about 379 liters of crude oil that
is usable. The oil then undergoes filtration severally to ensure any possible access matter is
eliminated and then put into a reaction tank. A catalyst made of sodium hydroxide and methanol
is added in the tank resulting in a reaction that converts peanut oil to methyl ester which means a
diesel fuel. The fuels are taken to fuel trucks upon creation which are then distributed to petrol
stations. A certain amount of energy is produced when fuel is put into an engine.
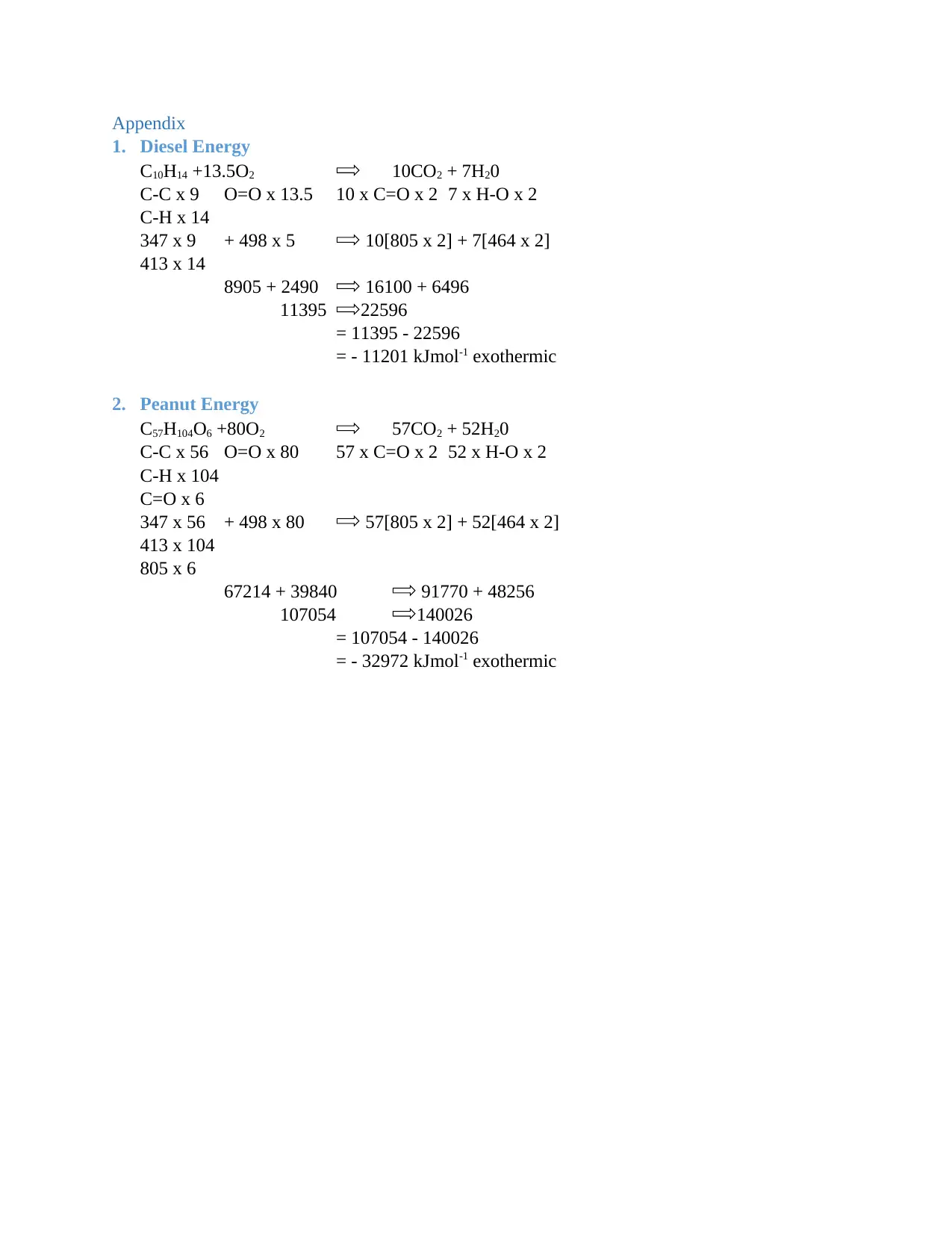
As demonstrated in Appendix 1, 11201 kJ/mol is produced of energy for the case of diesel fuel.
This value is negative meaning there is an exothermic reaction that results in the production of
heat. Such a reaction as well offers insights into why there is heating of the car engine as a result
of the reaction. Nevertheless, in case the biodiesel created out of peanut oil is used in powering
the engine, there is a production of -32972 kJ/mol of energy being released making it an
exothermic reaction as well. It may be noted that the biodiesel peanut oil produces more energy
distilled in the tower. When the oil is undergoing cooling, it is grouped into various fuels one of
such being diesel fuel as demonstrated in figure 4. Biodiesel production from peanut begins with
planting and picking of the nuts which are then taken to an oil pressure in which they are pressed
physically in the presence of heat to produce a cake.
https://pubchem.ncbi.nlm.nih.gov/compound 1
Approximately 363kg may be used in feeding animals which is about 379 liters of crude oil that
is usable. The oil then undergoes filtration severally to ensure any possible access matter is
eliminated and then put into a reaction tank. A catalyst made of sodium hydroxide and methanol
is added in the tank resulting in a reaction that converts peanut oil to methyl ester which means a
diesel fuel. The fuels are taken to fuel trucks upon creation which are then distributed to petrol
stations. A certain amount of energy is produced when fuel is put into an engine.
As demonstrated in Appendix 1, 11201 kJ/mol is produced of energy for the case of diesel fuel.
This value is negative meaning there is an exothermic reaction that results in the production of
heat. Such a reaction as well offers insights into why there is heating of the car engine as a result
of the reaction. Nevertheless, in case the biodiesel created out of peanut oil is used in powering
the engine, there is a production of -32972 kJ/mol of energy being released making it an
exothermic reaction as well. It may be noted that the biodiesel peanut oil produces more energy
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

in comparison with diesel from observation. More energy being produced translates to more
power of the car.
There are two kinds of emissions that may impact it within the surrounding. One such is
greenhouse gas emission which is inclusive of carbon dioxide that is released into the
atmosphere. The carbon dioxide may be trapped into the earth’s atmosphere upon being released
resulting in more heat that generates climate change as well as the greenhouse effect. Climate
change is defined as the alteration in the weather pattern that is the change in the atmospheric
rainfall, humidity, wind as well the as temperature. The greenhouse effects define warming on
the surface of the earth as well as suspended air. The major greenhouse gas tends to be carbon
dioxide that most of the automobiles generate from using fuels.
Going by the Australian Government Initiative, the mean combined carbon dioxide emissions in
2007 for a new light vehicle that is sold in Australia was about 182 gram/km. Nevertheless, as
per Bioenergy Australia, a litre of biodiesel lowers net carbon dioxide emissions by more than
95% hence a litre of biodiesel will be able to save to the tune of 2.5kg of carbon dioxide.
Suppose 20% of the used diesel is substituted by biodiesel is Australia, this may save about 118
million tons of the emitted carbon dioxide every year. The demand for diesel, however, in 2014
in Australia was quoted to be 229 million liters of fuel. Besides, 17kg of carbon dioxide every
liter is released into the atmosphere each time it is burnt in an engine. Hence, biodiesel may
lower the number of emissions of greenhouse gas of carbon dioxide that will be of benefit to the
surrounding.
power of the car.
There are two kinds of emissions that may impact it within the surrounding. One such is
greenhouse gas emission which is inclusive of carbon dioxide that is released into the
atmosphere. The carbon dioxide may be trapped into the earth’s atmosphere upon being released
resulting in more heat that generates climate change as well as the greenhouse effect. Climate
change is defined as the alteration in the weather pattern that is the change in the atmospheric
rainfall, humidity, wind as well the as temperature. The greenhouse effects define warming on
the surface of the earth as well as suspended air. The major greenhouse gas tends to be carbon
dioxide that most of the automobiles generate from using fuels.
Going by the Australian Government Initiative, the mean combined carbon dioxide emissions in
2007 for a new light vehicle that is sold in Australia was about 182 gram/km. Nevertheless, as
per Bioenergy Australia, a litre of biodiesel lowers net carbon dioxide emissions by more than
95% hence a litre of biodiesel will be able to save to the tune of 2.5kg of carbon dioxide.
Suppose 20% of the used diesel is substituted by biodiesel is Australia, this may save about 118
million tons of the emitted carbon dioxide every year. The demand for diesel, however, in 2014
in Australia was quoted to be 229 million liters of fuel. Besides, 17kg of carbon dioxide every
liter is released into the atmosphere each time it is burnt in an engine. Hence, biodiesel may
lower the number of emissions of greenhouse gas of carbon dioxide that will be of benefit to the
surrounding.

https://www.e-education.psu.edu/eme801/n 1
In addition to the environment as well as social perceptions on the topic, the production costs
price, as well as selling prices, may as well be analyzed. The production price and selling price
may change each year as a result of updates as well as advancements in technology. The update
in the knowledge and machinery that is acquired throughout the years as well as aid in the
variation of cost price and production. The production price was about $0.8 per liter for diesel
fuel in 2006. The cost price for the production of biodiesel peanut oil was about $0.7 per liter in
2006 meaning that the difference in the cost of production between the two fuels is $0.1. This
might not necessarily sound like a big gap between the two fuels but upon production in large
fuel quantities, the gap gains significance.
Still, it may be noted that the selling prices of the fuel tend to be different completely. The
purchase cost of diesel in 2001 was $1. 5/liter while biodiesel cost $.2. The cost prices of the two
fuels may be noted to be more than the selling price since, during the discussed time,
manufacturing of the fuel was being updated to befit the year and the technology advancements.
Hence, by looking at the production of fuels, it may be noted the prices tend to be close but when
In addition to the environment as well as social perceptions on the topic, the production costs
price, as well as selling prices, may as well be analyzed. The production price and selling price
may change each year as a result of updates as well as advancements in technology. The update
in the knowledge and machinery that is acquired throughout the years as well as aid in the
variation of cost price and production. The production price was about $0.8 per liter for diesel
fuel in 2006. The cost price for the production of biodiesel peanut oil was about $0.7 per liter in
2006 meaning that the difference in the cost of production between the two fuels is $0.1. This
might not necessarily sound like a big gap between the two fuels but upon production in large
fuel quantities, the gap gains significance.
Still, it may be noted that the selling prices of the fuel tend to be different completely. The
purchase cost of diesel in 2001 was $1. 5/liter while biodiesel cost $.2. The cost prices of the two
fuels may be noted to be more than the selling price since, during the discussed time,
manufacturing of the fuel was being updated to befit the year and the technology advancements.
Hence, by looking at the production of fuels, it may be noted the prices tend to be close but when
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

considering the quantity that is produced, there tends to be a large variation. Still, the prices of
biodiesel, as well as diesel, may note that the biodiesel fuel burns less money off the pockets in
comparison with diesel.
Recent research has demonstrated all diesel as well as biodiesel may result in cancer. The use of
biodiesel in traditional engine significantly lowers emissions of hydrocarbons, sulfates, carbon
monoxide, aromatic hydrocarbons as well as particulate matter. Upon inhaling such chemicals,
long term health complications tend to have a higher development risk. Such risks may be
inclusive of bladder cancer and lung solution. As per the Cancer Council website, diesel engine
exhaust has been rated as the second most cancer-causing agent works are unveiled behind the
exposure to ultraviolet radiation. It is approximated about 1.2 million Australian employees had
direct exposures in 2011. Biodiesel fuels may lower the chemicals within the air as well as the
compounds that lower cancer. Risks of cancer are lowered by 94 percent by using pure biodiesel
fuels in engines.
Research states that burning of pure biodiesel lowers the emission by more than 75 percent in
comparison with petroleum diesel. Using a mixture of 20 percent diesel lowers the emission of
carbon dioxide by 15 percent. Diesel fuels are made up of sulfur that may be emitted into the air,
nevertheless, biodiesel doesn't mean the sulfur dioxide emission is ignored. Hence, diesel fuels
may harm human health as a result of the chemicals it produces into the atmosphere thus
biodiesel manufactured from peanut oil would tend to be more favorable in adoption in engines.
Biodiesel is hence is likely to be more suitable for running a car as diesel produces more
greenhouse gas emissions within environment, for instance, carbon dioxide into the air that traps
the heat into effects of climate change in the atmosphere. Still, the price of production of fuels is
insignificantly different even though on a larger scale they tend to be distant apart. The selling
biodiesel, as well as diesel, may note that the biodiesel fuel burns less money off the pockets in
comparison with diesel.
Recent research has demonstrated all diesel as well as biodiesel may result in cancer. The use of
biodiesel in traditional engine significantly lowers emissions of hydrocarbons, sulfates, carbon
monoxide, aromatic hydrocarbons as well as particulate matter. Upon inhaling such chemicals,
long term health complications tend to have a higher development risk. Such risks may be
inclusive of bladder cancer and lung solution. As per the Cancer Council website, diesel engine
exhaust has been rated as the second most cancer-causing agent works are unveiled behind the
exposure to ultraviolet radiation. It is approximated about 1.2 million Australian employees had
direct exposures in 2011. Biodiesel fuels may lower the chemicals within the air as well as the
compounds that lower cancer. Risks of cancer are lowered by 94 percent by using pure biodiesel
fuels in engines.
Research states that burning of pure biodiesel lowers the emission by more than 75 percent in
comparison with petroleum diesel. Using a mixture of 20 percent diesel lowers the emission of
carbon dioxide by 15 percent. Diesel fuels are made up of sulfur that may be emitted into the air,
nevertheless, biodiesel doesn't mean the sulfur dioxide emission is ignored. Hence, diesel fuels
may harm human health as a result of the chemicals it produces into the atmosphere thus
biodiesel manufactured from peanut oil would tend to be more favorable in adoption in engines.
Biodiesel is hence is likely to be more suitable for running a car as diesel produces more
greenhouse gas emissions within environment, for instance, carbon dioxide into the air that traps
the heat into effects of climate change in the atmosphere. Still, the price of production of fuels is
insignificantly different even though on a larger scale they tend to be distant apart. The selling
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

price of such fuels demonstrates a large variation of $1.3 with the biodiesel being a cheaper
option. A conclusion can be made that biodiesel fuel would be better in powering an engine in
comparison with diesel fuel. This is since diesel fuel produced more chemical products into the
air that may enhance the risk of cancer. With a view of the different perceptions of the topic on
fuels, it may be outlined that biodiesel tends to be more beneficial for the atmosphere. Hence,
when it narrows down to the fuel that one would select in making a better future.
option. A conclusion can be made that biodiesel fuel would be better in powering an engine in
comparison with diesel fuel. This is since diesel fuel produced more chemical products into the
air that may enhance the risk of cancer. With a view of the different perceptions of the topic on
fuels, it may be outlined that biodiesel tends to be more beneficial for the atmosphere. Hence,
when it narrows down to the fuel that one would select in making a better future.

References
Biofuels Association of Australia. (n.d.). Effect of biodiesel on emissions - Biofuels
Association of Australia. [online] Available at:
http://biofuelsassociation.com.au/biofuels/biodiesel/effect-of-biodiesel-on-emissions/
[Accessed 3 Aug. 2019].
Britannica Kids. (n.d.). greenhouse effect. [online] Available at:
https://kids.britannica.com/kids/article/greenhouse-effect/403919 [Accessed 19 Aug.
2019].
Cancer.org.au. (n.d.). [online] Available at: https://www.cancer.org.au/preventing-
cancer/workplace-cancer/diesel.html [Accessed 16 Aug. 2019].
Greenvehicleguide.gov.au. (n.d.). Vehicle emissions | Green Vehicle Guide. [online]
Available at: https://www.greenvehicleguide.gov.au/pages/Information/VehicleEmissions
[Accessed 20 Jul. 2019].
Inforse.org. (n.d.). BIODIESEL. [online] Available at:
http://www.inforse.org/europe/dieret/altfuels/biodiesel.htm [Accessed 7 Aug. 2019].
Biofuels Association of Australia. (n.d.). Effect of biodiesel on emissions - Biofuels
Association of Australia. [online] Available at:
http://biofuelsassociation.com.au/biofuels/biodiesel/effect-of-biodiesel-on-emissions/
[Accessed 3 Aug. 2019].
Britannica Kids. (n.d.). greenhouse effect. [online] Available at:
https://kids.britannica.com/kids/article/greenhouse-effect/403919 [Accessed 19 Aug.
2019].
Cancer.org.au. (n.d.). [online] Available at: https://www.cancer.org.au/preventing-
cancer/workplace-cancer/diesel.html [Accessed 16 Aug. 2019].
Greenvehicleguide.gov.au. (n.d.). Vehicle emissions | Green Vehicle Guide. [online]
Available at: https://www.greenvehicleguide.gov.au/pages/Information/VehicleEmissions
[Accessed 20 Jul. 2019].
Inforse.org. (n.d.). BIODIESEL. [online] Available at:
http://www.inforse.org/europe/dieret/altfuels/biodiesel.htm [Accessed 7 Aug. 2019].
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Appendix
1. Diesel Energy
C10H14 +13.5O2 10CO2 + 7H20
C-C x 9 O=O x 13.5 10 x C=O x 2 7 x H-O x 2
C-H x 14
347 x 9 + 498 x 5 10[805 x 2] + 7[464 x 2]
413 x 14
8905 + 2490 16100 + 6496
11395 22596
= 11395 - 22596
= - 11201 kJmol-1 exothermic
2. Peanut Energy
C57H104O6 +80O2 57CO2 + 52H20
C-C x 56 O=O x 80 57 x C=O x 2 52 x H-O x 2
C-H x 104
C=O x 6
347 x 56 + 498 x 80 57[805 x 2] + 52[464 x 2]
413 x 104
805 x 6
67214 + 39840 91770 + 48256
107054 140026
= 107054 - 140026
= - 32972 kJmol-1 exothermic
1. Diesel Energy
C10H14 +13.5O2 10CO2 + 7H20
C-C x 9 O=O x 13.5 10 x C=O x 2 7 x H-O x 2
C-H x 14
347 x 9 + 498 x 5 10[805 x 2] + 7[464 x 2]
413 x 14
8905 + 2490 16100 + 6496
11395 22596
= 11395 - 22596
= - 11201 kJmol-1 exothermic
2. Peanut Energy
C57H104O6 +80O2 57CO2 + 52H20
C-C x 56 O=O x 80 57 x C=O x 2 52 x H-O x 2
C-H x 104
C=O x 6
347 x 56 + 498 x 80 57[805 x 2] + 52[464 x 2]
413 x 104
805 x 6
67214 + 39840 91770 + 48256
107054 140026
= 107054 - 140026
= - 32972 kJmol-1 exothermic
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

