Chemical Composition and Properties of Gasoline and Diesel Fuels
VerifiedAdded on 2021/04/17
|15
|4027
|461
Report
AI Summary
This report provides a comprehensive comparison of gasoline and diesel fuels, focusing on their chemical compositions, properties, and combustion characteristics. It begins by introducing gasoline and diesel as key automotive fuels derived from crude oil, highlighting their differences in consistency and flammability due to varying extraction stages. The report delves into the chemical aspects, emphasizing that both fuels are primarily composed of hydrocarbons, with the number of carbon atoms determining their chemical and physical properties. Gasoline is characterized as a mixture of hydrocarbons with 5 to 12 carbon atoms, while diesel contains hydrocarbons with 8 to 21 carbon atoms, influencing their boiling points and energy densities. The report discusses the isomers of both fuels, such as octane isomers in gasoline and dodecane in diesel, explaining how their molecular structures affect combustion rates and fuel performance. The report also addresses the refining processes used to extract these fuels from petroleum, and the addition of components to gasoline to alter its chemical composition. Furthermore, the report explains how the chemical formula and the chemical reactions involved in the combustion of gasoline and diesel fuels. Finally, the report provides a comparative analysis of the chemical characteristics and properties of gasoline and diesel, emphasizing the importance of these factors in determining fuel performance and application.

Name:
Course:
Professor:
Date:
Course:
Professor:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
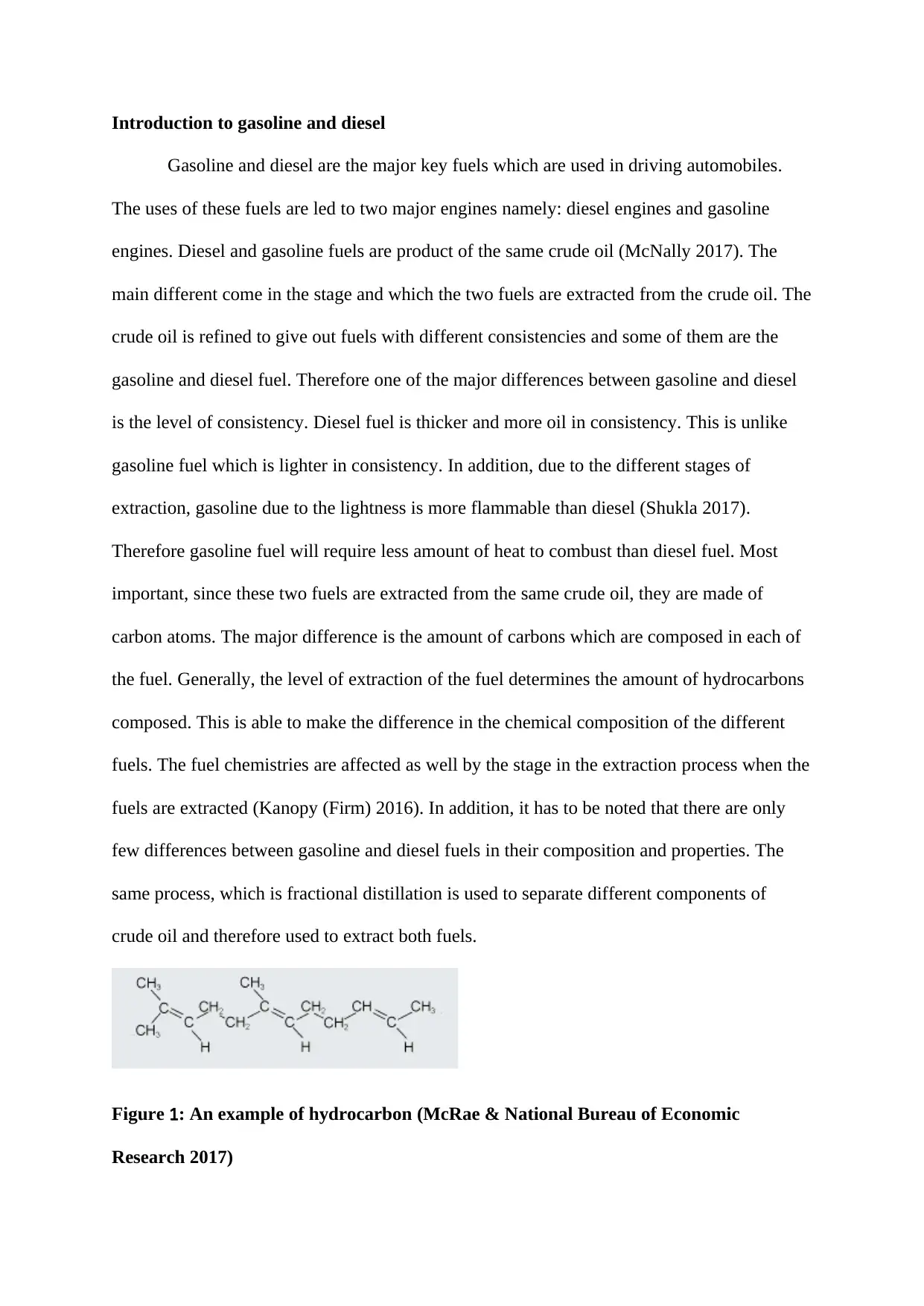
Introduction to gasoline and diesel
Gasoline and diesel are the major key fuels which are used in driving automobiles.
The uses of these fuels are led to two major engines namely: diesel engines and gasoline
engines. Diesel and gasoline fuels are product of the same crude oil (McNally 2017). The
main different come in the stage and which the two fuels are extracted from the crude oil. The
crude oil is refined to give out fuels with different consistencies and some of them are the
gasoline and diesel fuel. Therefore one of the major differences between gasoline and diesel
is the level of consistency. Diesel fuel is thicker and more oil in consistency. This is unlike
gasoline fuel which is lighter in consistency. In addition, due to the different stages of
extraction, gasoline due to the lightness is more flammable than diesel (Shukla 2017).
Therefore gasoline fuel will require less amount of heat to combust than diesel fuel. Most
important, since these two fuels are extracted from the same crude oil, they are made of
carbon atoms. The major difference is the amount of carbons which are composed in each of
the fuel. Generally, the level of extraction of the fuel determines the amount of hydrocarbons
composed. This is able to make the difference in the chemical composition of the different
fuels. The fuel chemistries are affected as well by the stage in the extraction process when the
fuels are extracted (Kanopy (Firm) 2016). In addition, it has to be noted that there are only
few differences between gasoline and diesel fuels in their composition and properties. The
same process, which is fractional distillation is used to separate different components of
crude oil and therefore used to extract both fuels.
Figure 1: An example of hydrocarbon (McRae & National Bureau of Economic
Research 2017)
Gasoline and diesel are the major key fuels which are used in driving automobiles.
The uses of these fuels are led to two major engines namely: diesel engines and gasoline
engines. Diesel and gasoline fuels are product of the same crude oil (McNally 2017). The
main different come in the stage and which the two fuels are extracted from the crude oil. The
crude oil is refined to give out fuels with different consistencies and some of them are the
gasoline and diesel fuel. Therefore one of the major differences between gasoline and diesel
is the level of consistency. Diesel fuel is thicker and more oil in consistency. This is unlike
gasoline fuel which is lighter in consistency. In addition, due to the different stages of
extraction, gasoline due to the lightness is more flammable than diesel (Shukla 2017).
Therefore gasoline fuel will require less amount of heat to combust than diesel fuel. Most
important, since these two fuels are extracted from the same crude oil, they are made of
carbon atoms. The major difference is the amount of carbons which are composed in each of
the fuel. Generally, the level of extraction of the fuel determines the amount of hydrocarbons
composed. This is able to make the difference in the chemical composition of the different
fuels. The fuel chemistries are affected as well by the stage in the extraction process when the
fuels are extracted (Kanopy (Firm) 2016). In addition, it has to be noted that there are only
few differences between gasoline and diesel fuels in their composition and properties. The
same process, which is fractional distillation is used to separate different components of
crude oil and therefore used to extract both fuels.
Figure 1: An example of hydrocarbon (McRae & National Bureau of Economic
Research 2017)

Chemistry on fuels
Gasoline and diesel are defined by their chemistry level. Their chemical composition is
able to define their reaction at different situations. Hydrocarbons are the major components
which are part of the fuel chemistries. The fuels are made of chains of both carbon and
hydrogen (Agarwal 2016). The different number of the carbon composed on the fuel
determines the nature of the fuel. These numbers are able to define the chemical and physical
chemistry of the specific fuel. Under this section, this paper will analyze the different
chemistries of gasoline and diesel fuels. This will involve their structure and type of isomers
which are composed on each fuel.
Gasoline chemistry
As noted early, gasoline is composed of carbon and hydrogen atoms. These chemical
elements are able to define the chemical reaction activity which this fuel is involved in.
Moreover, gasoline is a mixture of large number of hydrocarbons. Gasoline has between 5
and 12 carbon atoms. In addition, gasoline is a natural by-product of the petroleum industry
(Speight 2014). Therefore gasoline is made from a non renewable source. Fractional
distillation is the major process which is used to extract gasoline from crude oil. Gasoline is
also known as petrol in some countries and it is mainly used to power automobiles. In
definition, gasoline is a mixture of over 500 hydrocarbons. In addition, in their chemical
composition, gasoline has small amount of alkane cyclic and aromatic compounds. In
addition it has to be noted that gasoline lacks alkenes and alkynes in their composition. The
boiling points of hydrocarbons vary when the fractional distillation is used for separating
crude oil (Kent, Bommaraju & Barnicki 2017). The boiling point varies with the length of
hydrocarbons in the specific fuel. This means that the hydrocarbon in gasoline is able to
define its boiling point.
Gasoline and diesel are defined by their chemistry level. Their chemical composition is
able to define their reaction at different situations. Hydrocarbons are the major components
which are part of the fuel chemistries. The fuels are made of chains of both carbon and
hydrogen (Agarwal 2016). The different number of the carbon composed on the fuel
determines the nature of the fuel. These numbers are able to define the chemical and physical
chemistry of the specific fuel. Under this section, this paper will analyze the different
chemistries of gasoline and diesel fuels. This will involve their structure and type of isomers
which are composed on each fuel.
Gasoline chemistry
As noted early, gasoline is composed of carbon and hydrogen atoms. These chemical
elements are able to define the chemical reaction activity which this fuel is involved in.
Moreover, gasoline is a mixture of large number of hydrocarbons. Gasoline has between 5
and 12 carbon atoms. In addition, gasoline is a natural by-product of the petroleum industry
(Speight 2014). Therefore gasoline is made from a non renewable source. Fractional
distillation is the major process which is used to extract gasoline from crude oil. Gasoline is
also known as petrol in some countries and it is mainly used to power automobiles. In
definition, gasoline is a mixture of over 500 hydrocarbons. In addition, in their chemical
composition, gasoline has small amount of alkane cyclic and aromatic compounds. In
addition it has to be noted that gasoline lacks alkenes and alkynes in their composition. The
boiling points of hydrocarbons vary when the fractional distillation is used for separating
crude oil (Kent, Bommaraju & Barnicki 2017). The boiling point varies with the length of
hydrocarbons in the specific fuel. This means that the hydrocarbon in gasoline is able to
define its boiling point.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
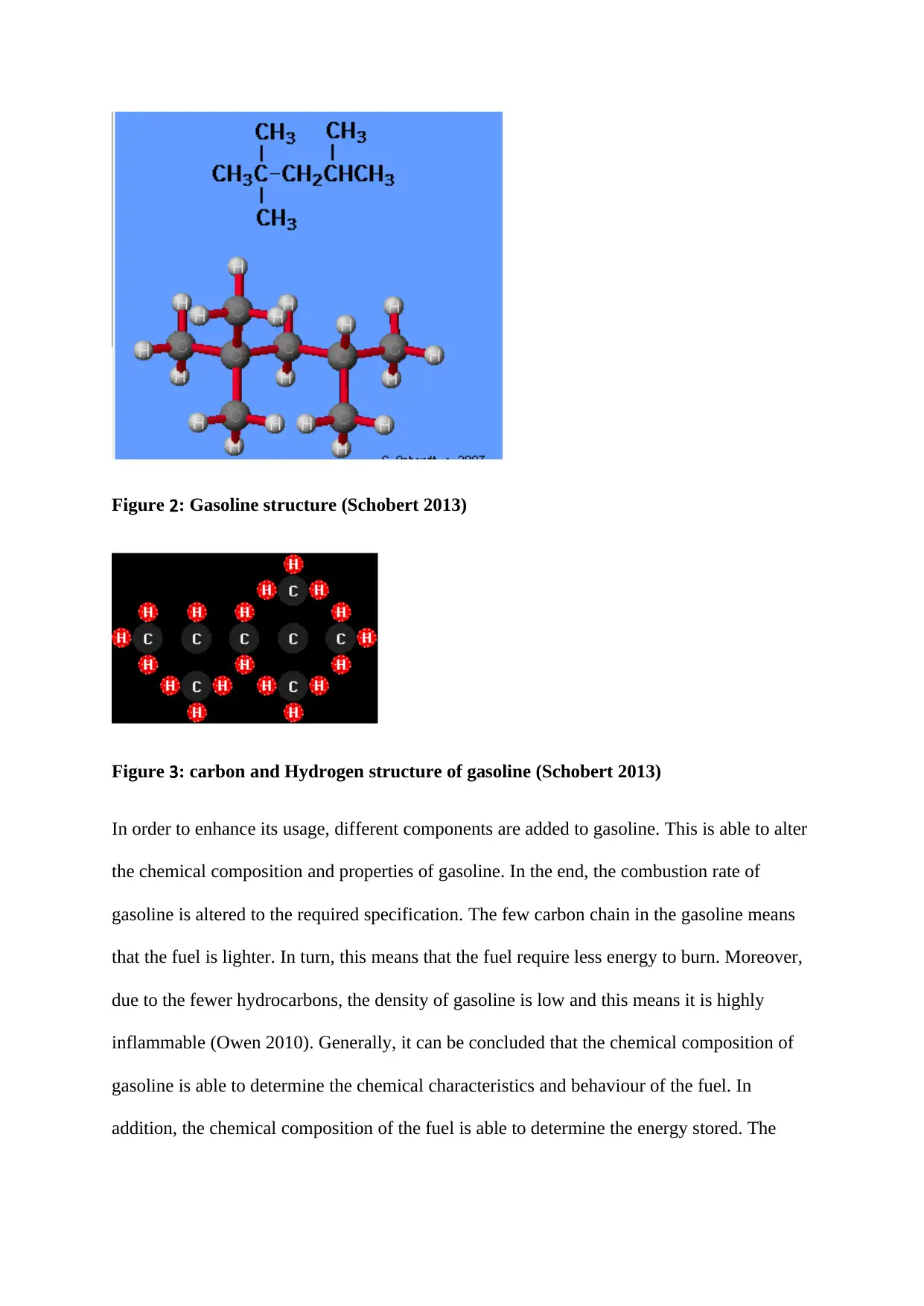
Figure 2: Gasoline structure (Schobert 2013)
Figure 3: carbon and Hydrogen structure of gasoline (Schobert 2013)
In order to enhance its usage, different components are added to gasoline. This is able to alter
the chemical composition and properties of gasoline. In the end, the combustion rate of
gasoline is altered to the required specification. The few carbon chain in the gasoline means
that the fuel is lighter. In turn, this means that the fuel require less energy to burn. Moreover,
due to the fewer hydrocarbons, the density of gasoline is low and this means it is highly
inflammable (Owen 2010). Generally, it can be concluded that the chemical composition of
gasoline is able to determine the chemical characteristics and behaviour of the fuel. In
addition, the chemical composition of the fuel is able to determine the energy stored. The
Figure 3: carbon and Hydrogen structure of gasoline (Schobert 2013)
In order to enhance its usage, different components are added to gasoline. This is able to alter
the chemical composition and properties of gasoline. In the end, the combustion rate of
gasoline is altered to the required specification. The few carbon chain in the gasoline means
that the fuel is lighter. In turn, this means that the fuel require less energy to burn. Moreover,
due to the fewer hydrocarbons, the density of gasoline is low and this means it is highly
inflammable (Owen 2010). Generally, it can be concluded that the chemical composition of
gasoline is able to determine the chemical characteristics and behaviour of the fuel. In
addition, the chemical composition of the fuel is able to determine the energy stored. The
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

energy density of the fuel is able to dictate the amount of energy stored and can be
transported in the same volume.
In addition, gasoline has different isomers. Isomers are defined as molecules which
have same chemical composition but do show difference in molecular structure. This means
that the isomers do have same number and type atoms and the only difference is on the
arrangement. In addition, it has to be noted that the different arrangement of the atoms is able
to result to difference in physical and chemical properties. In gasoline, the isomers are able to
exhibit different characteristics of boiling point and melting points (Maurya 2018). These key
factors affect the combustion rates of the specific isomers. Due to this factor, the selection of
gasoline isomers for fuel is very important. Additionally, the different isomers are able to
produce different energy when combusted. Different isomers of gasoline are able to burn
different. For instance, octane, which is a gasoline fuel, has 18 known isomers. The different
octane gasoline isomers are “n-octane, 2-methylheptane, 3-methylheptane, 4-methylheptane,
2.2-dimethylhexane, 2.3-dimethylhexane, 2.4-dimethylhexane, 2.5-dimethylexane, 3.3-
dimethylhexane, 3.4-dimethylhexane, 3-ethylhexane, 2.2.3-trimethylpentane, 2.2.4-
trimethylpentane, 2.3.3-trimethylpentane, 2.3.4-trimethylpentane, 2-methyl-3-ethylpentane,
3-methyl-3-ethylpentane and tetramethylbutane.” The 2.2.4-trimethylpentane which is also
known isooctane is used for the reference of octane rating scale. In addition, the 2.2.4-
trimethylpentane is part of gasoline and helps to reduce the engine knocking noise which
vehicles do exhibit sometimes (Maurya 2018). These octane isomers are derived from octane
formula of C8H18. The choice of the octane isomers is important to achieve the specific goals
of fuel such as stable burning. Some of octane isomers are able to achieve this combustion
factor better than others. To produce energy, gasoline is burned with oxygen to produce
energy and other products according to equation below.
transported in the same volume.
In addition, gasoline has different isomers. Isomers are defined as molecules which
have same chemical composition but do show difference in molecular structure. This means
that the isomers do have same number and type atoms and the only difference is on the
arrangement. In addition, it has to be noted that the different arrangement of the atoms is able
to result to difference in physical and chemical properties. In gasoline, the isomers are able to
exhibit different characteristics of boiling point and melting points (Maurya 2018). These key
factors affect the combustion rates of the specific isomers. Due to this factor, the selection of
gasoline isomers for fuel is very important. Additionally, the different isomers are able to
produce different energy when combusted. Different isomers of gasoline are able to burn
different. For instance, octane, which is a gasoline fuel, has 18 known isomers. The different
octane gasoline isomers are “n-octane, 2-methylheptane, 3-methylheptane, 4-methylheptane,
2.2-dimethylhexane, 2.3-dimethylhexane, 2.4-dimethylhexane, 2.5-dimethylexane, 3.3-
dimethylhexane, 3.4-dimethylhexane, 3-ethylhexane, 2.2.3-trimethylpentane, 2.2.4-
trimethylpentane, 2.3.3-trimethylpentane, 2.3.4-trimethylpentane, 2-methyl-3-ethylpentane,
3-methyl-3-ethylpentane and tetramethylbutane.” The 2.2.4-trimethylpentane which is also
known isooctane is used for the reference of octane rating scale. In addition, the 2.2.4-
trimethylpentane is part of gasoline and helps to reduce the engine knocking noise which
vehicles do exhibit sometimes (Maurya 2018). These octane isomers are derived from octane
formula of C8H18. The choice of the octane isomers is important to achieve the specific goals
of fuel such as stable burning. Some of octane isomers are able to achieve this combustion
factor better than others. To produce energy, gasoline is burned with oxygen to produce
energy and other products according to equation below.
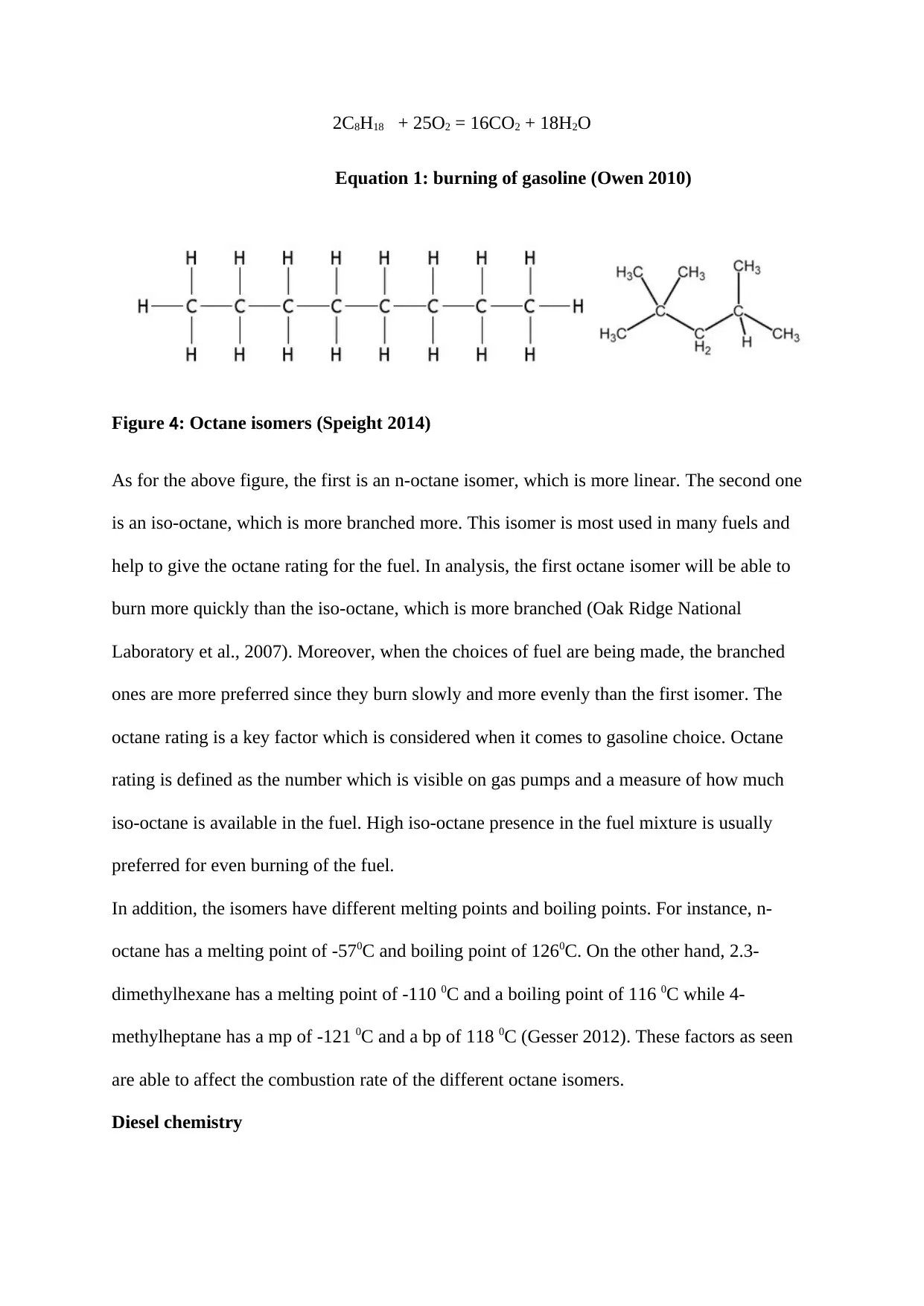
2C8H18 + 25O2 = 16CO2 + 18H2O
Equation 1: burning of gasoline (Owen 2010)
Figure 4: Octane isomers (Speight 2014)
As for the above figure, the first is an n-octane isomer, which is more linear. The second one
is an iso-octane, which is more branched more. This isomer is most used in many fuels and
help to give the octane rating for the fuel. In analysis, the first octane isomer will be able to
burn more quickly than the iso-octane, which is more branched (Oak Ridge National
Laboratory et al., 2007). Moreover, when the choices of fuel are being made, the branched
ones are more preferred since they burn slowly and more evenly than the first isomer. The
octane rating is a key factor which is considered when it comes to gasoline choice. Octane
rating is defined as the number which is visible on gas pumps and a measure of how much
iso-octane is available in the fuel. High iso-octane presence in the fuel mixture is usually
preferred for even burning of the fuel.
In addition, the isomers have different melting points and boiling points. For instance, n-
octane has a melting point of -570C and boiling point of 1260C. On the other hand, 2.3-
dimethylhexane has a melting point of -110 0C and a boiling point of 116 0C while 4-
methylheptane has a mp of -121 0C and a bp of 118 0C (Gesser 2012). These factors as seen
are able to affect the combustion rate of the different octane isomers.
Diesel chemistry
Equation 1: burning of gasoline (Owen 2010)
Figure 4: Octane isomers (Speight 2014)
As for the above figure, the first is an n-octane isomer, which is more linear. The second one
is an iso-octane, which is more branched more. This isomer is most used in many fuels and
help to give the octane rating for the fuel. In analysis, the first octane isomer will be able to
burn more quickly than the iso-octane, which is more branched (Oak Ridge National
Laboratory et al., 2007). Moreover, when the choices of fuel are being made, the branched
ones are more preferred since they burn slowly and more evenly than the first isomer. The
octane rating is a key factor which is considered when it comes to gasoline choice. Octane
rating is defined as the number which is visible on gas pumps and a measure of how much
iso-octane is available in the fuel. High iso-octane presence in the fuel mixture is usually
preferred for even burning of the fuel.
In addition, the isomers have different melting points and boiling points. For instance, n-
octane has a melting point of -570C and boiling point of 1260C. On the other hand, 2.3-
dimethylhexane has a melting point of -110 0C and a boiling point of 116 0C while 4-
methylheptane has a mp of -121 0C and a bp of 118 0C (Gesser 2012). These factors as seen
are able to affect the combustion rate of the different octane isomers.
Diesel chemistry
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

In addition, diesel as well is used as an automobile fuel, which is a by-product of
petroleum. Moreover, it is achieved through fractional distillation. Compared to gasoline is
denser and has a higher boiling point than water. In terms of the chemical composition, is
composed of hydrocarbons which have long chain of carbon where they have carbon atoms
of between 8 and 21 (Roussak & Gesser 2013). Therefore compared to gasoline, diesel has
more carbon atoms which make is heavier than gasoline. The major compounds of diesel
include paraffins, isoparafins, napthenes, olefins aromatic hydrocarbons. Moreover, diesel is
categorized according to different grades which are based on different uses which have to be
taken. In addition due to its chemical status, diesel burn without sparks due to the
compression at the air inlet when burning it.
In addition, in terms of its chemical composition, diesel is composed of about 75
percent saturated hydrocarbons and 25 aromatic hydrocarbons. The chemical formula of
diesel range from C10H20 to C15H28 (Roussak & Gesser 2013). The average type of diesel fuel
by the use of chemical formula is C12H24. The temperature of diesel is able to vary according
to the available environment. In addition, the range of boiling point between 150 and 380oC.
Figure 5: Diesel hydrocarbon structure (Maurya 2018)
In addition, the chemical formation of diesel is composed of many components which
are not individually made. Although diesel can be viewed as natural product the end product
diesel fuel is man-made product (Dane, Voorhees & National Renewable Energy Laboratory
(U.S.) 2010). During fractional distillation, diesel fuel is created at the end of the tower. Due
to the higher density, diesel fuel is oily and sometimes is referred to as diesel oil. Due to the
high number of hydrocarbons, diesel evaporates much slower compared to gasoline fuel
petroleum. Moreover, it is achieved through fractional distillation. Compared to gasoline is
denser and has a higher boiling point than water. In terms of the chemical composition, is
composed of hydrocarbons which have long chain of carbon where they have carbon atoms
of between 8 and 21 (Roussak & Gesser 2013). Therefore compared to gasoline, diesel has
more carbon atoms which make is heavier than gasoline. The major compounds of diesel
include paraffins, isoparafins, napthenes, olefins aromatic hydrocarbons. Moreover, diesel is
categorized according to different grades which are based on different uses which have to be
taken. In addition due to its chemical status, diesel burn without sparks due to the
compression at the air inlet when burning it.
In addition, in terms of its chemical composition, diesel is composed of about 75
percent saturated hydrocarbons and 25 aromatic hydrocarbons. The chemical formula of
diesel range from C10H20 to C15H28 (Roussak & Gesser 2013). The average type of diesel fuel
by the use of chemical formula is C12H24. The temperature of diesel is able to vary according
to the available environment. In addition, the range of boiling point between 150 and 380oC.
Figure 5: Diesel hydrocarbon structure (Maurya 2018)
In addition, the chemical formation of diesel is composed of many components which
are not individually made. Although diesel can be viewed as natural product the end product
diesel fuel is man-made product (Dane, Voorhees & National Renewable Energy Laboratory
(U.S.) 2010). During fractional distillation, diesel fuel is created at the end of the tower. Due
to the higher density, diesel fuel is oily and sometimes is referred to as diesel oil. Due to the
high number of hydrocarbons, diesel evaporates much slower compared to gasoline fuel
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

(Speight 2014). The higher melting point is as well as a result of the high number o
hydrocarbons, which ranges between 200 and 300 oC. In its combustion, diesel is able to burn
in presence of air to produce energy, carbon dioxide and water. The following equation
shows the chemical reaction of dodecane which is type of diesel in presence of air
C12H26 + 18.5O2 = 12CO2 + 13H2O + energy
Equation 2 Burning of diesel (Holt 2008)
Diesel has different types of grades and which have different chemical composition.
The typical molecule of biodiesel, which is one of the grades of diesel, has long chain of
carbon atom, which has hydrogen atoms attached to the carbon atoms. The following figure
shows the typical representation of a biodiesel. This type of diesel has an end which is known
as an ester functional group. This is the part which is on the far end of the following figure. In
addition, due to its density, diesel is able to yield more energy per gallon. According to
research, a gallon of diesel is able to produce 10% more energy than gasoline (Armitage
2009). The different ranges of diesel are able to meet different fuel requirements. The ranges
have different chemical and physical characteristics which ensure that different uses are met.
Figure 6: Structure of diesel (Schobert 2013)
Moreover, like other fuels and elements, diesel has its own isomers. As noted, the
different isomers have different arrangement of atoms which lead to them having different
chemical and physical characteristics. One of the diesel isomer is dodecane. This is a alkane
hydrocarbon which is represented by a chemical forma of CH3(CH2)10CH3, or simply C12H26,
hydrocarbons, which ranges between 200 and 300 oC. In its combustion, diesel is able to burn
in presence of air to produce energy, carbon dioxide and water. The following equation
shows the chemical reaction of dodecane which is type of diesel in presence of air
C12H26 + 18.5O2 = 12CO2 + 13H2O + energy
Equation 2 Burning of diesel (Holt 2008)
Diesel has different types of grades and which have different chemical composition.
The typical molecule of biodiesel, which is one of the grades of diesel, has long chain of
carbon atom, which has hydrogen atoms attached to the carbon atoms. The following figure
shows the typical representation of a biodiesel. This type of diesel has an end which is known
as an ester functional group. This is the part which is on the far end of the following figure. In
addition, due to its density, diesel is able to yield more energy per gallon. According to
research, a gallon of diesel is able to produce 10% more energy than gasoline (Armitage
2009). The different ranges of diesel are able to meet different fuel requirements. The ranges
have different chemical and physical characteristics which ensure that different uses are met.
Figure 6: Structure of diesel (Schobert 2013)
Moreover, like other fuels and elements, diesel has its own isomers. As noted, the
different isomers have different arrangement of atoms which lead to them having different
chemical and physical characteristics. One of the diesel isomer is dodecane. This is a alkane
hydrocarbon which is represented by a chemical forma of CH3(CH2)10CH3, or simply C12H26,

which is a oily liquid and part of paraffin series. This isomer has a record of 255 isomers
(Owen 2010). The different isomers have different characteristics. Additionally, the different
isomers have different uses according to their characteristics (Armitage 2009). This helps to
meet the different need and provide key merits which are required in the diesel use. The
development of these isomers is able to provide different fuels with different characteristics.
This ensures that the different needs of the fuels are met depending on the need of use and
combustion required. Generally, the different isomers ensure that the different fuel
characteristics provide wide range of use of the diesel fuel.
Refining process
Petroleum processes are used in refining crude oil and able to produce gasoline, diesel
among other fuels and products. Fractional distillation has been used for long for refining
crude oil in order to produce gasoline, diesel and other products. Under the fractional
distillation, the crude oil is heated up and then the favour vaporizes (Holt 2008). After that
the vapour is condensed to achieve the different fuels. The chemistry of gasoline and diesel
are able to affect their operations. Both physical and chemical characteristics define the
different uses of the fuels (Meyers 2009). These play a key role when refining the fuels and
determine at which level the fuel will be extracted. Physical and chemical transformation of
the crude oil is able to take place during the refining process. Distinct processes are able to
happen at different specific facilities or process units.
Physical process in refining
First, distillation process is utilized before the chemical refining of the crude oil is carried
out. The distillation is able to separate the liquid crude of the solid particles. In the distillation
process, the crude is divided into different sections (Meyers 2009). This helps to easily enable
the separation of the crude according to their density. The fuels with high number of carbon
are widely used in this step of processing. Crude distillation is able to separate the crude oil
(Owen 2010). The different isomers have different characteristics. Additionally, the different
isomers have different uses according to their characteristics (Armitage 2009). This helps to
meet the different need and provide key merits which are required in the diesel use. The
development of these isomers is able to provide different fuels with different characteristics.
This ensures that the different needs of the fuels are met depending on the need of use and
combustion required. Generally, the different isomers ensure that the different fuel
characteristics provide wide range of use of the diesel fuel.
Refining process
Petroleum processes are used in refining crude oil and able to produce gasoline, diesel
among other fuels and products. Fractional distillation has been used for long for refining
crude oil in order to produce gasoline, diesel and other products. Under the fractional
distillation, the crude oil is heated up and then the favour vaporizes (Holt 2008). After that
the vapour is condensed to achieve the different fuels. The chemistry of gasoline and diesel
are able to affect their operations. Both physical and chemical characteristics define the
different uses of the fuels (Meyers 2009). These play a key role when refining the fuels and
determine at which level the fuel will be extracted. Physical and chemical transformation of
the crude oil is able to take place during the refining process. Distinct processes are able to
happen at different specific facilities or process units.
Physical process in refining
First, distillation process is utilized before the chemical refining of the crude oil is carried
out. The distillation is able to separate the liquid crude of the solid particles. In the distillation
process, the crude is divided into different sections (Meyers 2009). This helps to easily enable
the separation of the crude according to their density. The fuels with high number of carbon
are widely used in this step of processing. Crude distillation is able to separate the crude oil
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

into different ranges of the fuels boiling points so that they can be further processed. During
refining process, the level of carbon and density is able to affect the level at which each of the
fuel will be collected. When using the fractional distillation, the chemical composition is key
at the level when the fuel will be collected. First, gasoline will evaporate first before diesel
(Keyworth & National Petroleum Refiners Association 2010). This is because gasoline is
lighter and has low boiling point than diesel. Fractional distillation is used for the refining of
crude oil since it components have different boiling points. Some of the important processes
used for the distillation include the atmospheric distillation and vacuum distillation. The
distillation process is able to separate the fuels according to different ranges of their
hydrocarbons. Therefore under this process, the chemical hydrocarbon structure of the fuel is
important. Generally, it can be noted that the chemical structure of the fuels influence the
physical process of distillation. After distillation, the fuels are composed at different ranges
of their boiling points due to the differences in carbon atoms at each fuel.
Figure 7: Refining process (Demirbas 2008)
Additionally, chemical composition of the fuels plays an important role in refining of
the fuels. Chemical processes are able to enhance the refining process by further refining the
fuels to their different specific fuels. One of the major chemical processes used for refining
refining process, the level of carbon and density is able to affect the level at which each of the
fuel will be collected. When using the fractional distillation, the chemical composition is key
at the level when the fuel will be collected. First, gasoline will evaporate first before diesel
(Keyworth & National Petroleum Refiners Association 2010). This is because gasoline is
lighter and has low boiling point than diesel. Fractional distillation is used for the refining of
crude oil since it components have different boiling points. Some of the important processes
used for the distillation include the atmospheric distillation and vacuum distillation. The
distillation process is able to separate the fuels according to different ranges of their
hydrocarbons. Therefore under this process, the chemical hydrocarbon structure of the fuel is
important. Generally, it can be noted that the chemical structure of the fuels influence the
physical process of distillation. After distillation, the fuels are composed at different ranges
of their boiling points due to the differences in carbon atoms at each fuel.
Figure 7: Refining process (Demirbas 2008)
Additionally, chemical composition of the fuels plays an important role in refining of
the fuels. Chemical processes are able to enhance the refining process by further refining the
fuels to their different specific fuels. One of the major chemical processes used for refining
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

crude oil and especially gasoline and diesel is the conversion or cracking process (Demirbas
2008). Tin this process, the heavy crude oil and fuel specific parts are broken down into
lighter refineries. These finer fractions are then further processed or blended to produce the
required chemical composition of fuels. The major processes which are included in this stage
include the hydrocracking and fluid catalytic cracking (FCC). Next in the chemical processes,
the upgrading process takes place. Under this stage, the molecular structure of the fuels is
rearranged to improve the properties of the fuels. This process helps to enhance the value of
the gasoline and diesel components (Argonne National Lab et al., 2011). Isomerisation and
catalytic reforming are major processes which are included in this stage.
Chemical reactions
In addition, another key process which is included in the chemical process is the
treatment of the fuels. This process is used to remove hetero-atoms impurities. This is seen as
a purification process of the fuel and it is used to remove aromatics compounds from the
refining streams. Examples of processes which may be applied at this stage include FCC
feeding hydroheating, reformer feed hydroheating, Gasoline and distillate hydroheating and
benzene saturation. Additionally other processes which are included in refining of gasoline
and diesel include the separation and blending processes (Ramirez-Corredores & Borole
2011). The separation process is able to use both physical and chemical separation. The
different parts of the fuels are further refined for quality controls. Fractionation and aromatic
extraction are some of the key processes used in this process. Blending process helps to
combine blendstocks and produce finished products of the fuels. This stage ensures that the
fuels are able to meet the different specific requirements and meet required environmental
standards.
Conclusion
2008). Tin this process, the heavy crude oil and fuel specific parts are broken down into
lighter refineries. These finer fractions are then further processed or blended to produce the
required chemical composition of fuels. The major processes which are included in this stage
include the hydrocracking and fluid catalytic cracking (FCC). Next in the chemical processes,
the upgrading process takes place. Under this stage, the molecular structure of the fuels is
rearranged to improve the properties of the fuels. This process helps to enhance the value of
the gasoline and diesel components (Argonne National Lab et al., 2011). Isomerisation and
catalytic reforming are major processes which are included in this stage.
Chemical reactions
In addition, another key process which is included in the chemical process is the
treatment of the fuels. This process is used to remove hetero-atoms impurities. This is seen as
a purification process of the fuel and it is used to remove aromatics compounds from the
refining streams. Examples of processes which may be applied at this stage include FCC
feeding hydroheating, reformer feed hydroheating, Gasoline and distillate hydroheating and
benzene saturation. Additionally other processes which are included in refining of gasoline
and diesel include the separation and blending processes (Ramirez-Corredores & Borole
2011). The separation process is able to use both physical and chemical separation. The
different parts of the fuels are further refined for quality controls. Fractionation and aromatic
extraction are some of the key processes used in this process. Blending process helps to
combine blendstocks and produce finished products of the fuels. This stage ensures that the
fuels are able to meet the different specific requirements and meet required environmental
standards.
Conclusion

In conclusion, both gasoline and crude oil have most of the characteristics similar
since they are all produced from same crude oil. The numbers of carbons are usually the
major differences in the two fuels. Moreover, another difference can be traced on the refining
stage when the fuels are extracted. Moreover, the chemical and physical characteristics are
able to differ in the two fuels. As seen the two have different ranges of number of carbons.
This makes their physical characteristics and chemical characteristics different. In addition,
the different types of fuels have their own isomers. The isomers bear different characteristics
due to difference in the arrangement of their atoms. While purchasing the fuels, it is
important to consider the type of isomer which is preferred. The isomers of these fuels are
able to differ in term of their purpose and use. Physical and chemical characteristics are able
to influence the refining processes which are involved. Distillation is one of the major
physical processes used in refining of the fuels. This process helps to separate the fuels
according to their boiling point ranges. In chemical refining, different processes are involves.
Some of them include hydrocraking, cracking, isomerisation, hydroheating among other
processes.
since they are all produced from same crude oil. The numbers of carbons are usually the
major differences in the two fuels. Moreover, another difference can be traced on the refining
stage when the fuels are extracted. Moreover, the chemical and physical characteristics are
able to differ in the two fuels. As seen the two have different ranges of number of carbons.
This makes their physical characteristics and chemical characteristics different. In addition,
the different types of fuels have their own isomers. The isomers bear different characteristics
due to difference in the arrangement of their atoms. While purchasing the fuels, it is
important to consider the type of isomer which is preferred. The isomers of these fuels are
able to differ in term of their purpose and use. Physical and chemical characteristics are able
to influence the refining processes which are involved. Distillation is one of the major
physical processes used in refining of the fuels. This process helps to separate the fuels
according to their boiling point ranges. In chemical refining, different processes are involves.
Some of them include hydrocraking, cracking, isomerisation, hydroheating among other
processes.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 15
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




