Assessing Cost-Effectiveness of Cervical Cancer Screening Tests Report
VerifiedAdded on 2022/09/09
|18
|3827
|12
Report
AI Summary
This report presents a health technology assessment comparing two cervical cancer screening tests: an existing method and a proposed new method. The study utilizes decision tree analysis, incorporating epidemiological data, test performance metrics (sensitivity and specificity), and cost analysis to evaluate the cost-effectiveness of each screening approach. The report details the methods used, including interviews and questionnaires to gather data, and presents the results, highlighting differences in sensitivity, overall costs, and incremental cost-effectiveness ratios (ICERs). The analysis also addresses areas of uncertainty, such as the impact of per capita GDP on test efficacy. The findings indicate that while the proposed test has higher sensitivity and benefits, it is more expensive. The report provides recommendations based on these findings, suggesting the proposed technology for early detection and potential elimination of cervical cancer, while considering the cost implications and the willingness of society to pay for health outcomes.

ASSESSING HEALTH TECHNOLOGY1
HEALTH TECHNOLOGY ASSESSMENT
Name
Institution
Date
HEALTH TECHNOLOGY ASSESSMENT
Name
Institution
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ASSESSING HEALTH TECHNOLOGY2
ASSESSING HEALTH TECHNOLOGY
Executive Summary
Objectives
Effectiveness is one of the major aspects that must be considered when introducing a new
idea or system to the market. Effectiveness can be in terms of cost and efficiency. This was still
the case in this research study. Analyzing the effectiveness of the proposed test for screening
cancer was the main purpose of the study. Assessing the benefits of the proposed test was also a
major factor that was considered. Finally, the comparison between the existing and proposed
tests was also done.
Method
Decision Trees
In a bid to ensure that the comparison was successful, a decision tree was essential. This
was the primary method that was used in the research study. The framework was used to
compare the cost effectiveness as well as the clinical effects on HPV between existing and
proposed screening test. Both screening test involved women at the age of thirty years. The given
results were essential since they facilitated both the specificity and sensitivity of both the
proposed and existing screening tests. In addition, the given results made it possible to evaluate
the two tests. To increase the variability and accuracy of the collected data, interviews and
questionnaires were also incorporated into the study.
Data
Decision trees, epidemiological data, test performance, cost of each outcome, benefits,
utility cost analysis, cost effective analysis, analysis of the sensitivity, and incremental cost-
ASSESSING HEALTH TECHNOLOGY
Executive Summary
Objectives
Effectiveness is one of the major aspects that must be considered when introducing a new
idea or system to the market. Effectiveness can be in terms of cost and efficiency. This was still
the case in this research study. Analyzing the effectiveness of the proposed test for screening
cancer was the main purpose of the study. Assessing the benefits of the proposed test was also a
major factor that was considered. Finally, the comparison between the existing and proposed
tests was also done.
Method
Decision Trees
In a bid to ensure that the comparison was successful, a decision tree was essential. This
was the primary method that was used in the research study. The framework was used to
compare the cost effectiveness as well as the clinical effects on HPV between existing and
proposed screening test. Both screening test involved women at the age of thirty years. The given
results were essential since they facilitated both the specificity and sensitivity of both the
proposed and existing screening tests. In addition, the given results made it possible to evaluate
the two tests. To increase the variability and accuracy of the collected data, interviews and
questionnaires were also incorporated into the study.
Data
Decision trees, epidemiological data, test performance, cost of each outcome, benefits,
utility cost analysis, cost effective analysis, analysis of the sensitivity, and incremental cost-

ASSESSING HEALTH TECHNOLOGY3
effectiveness ratio. ICERs was done for all alternative screening costs that were evident
throughout the study.
Results
In both alternatives, specificity was equivalent to 94%. On the other hand, the sensitivity
score was different. The proposed screening test showed a sensitivity score of 75% while the
existing screening test recorded a score that had a sensitivity of 94%. Basing on the results, it can
therefore be confirmed that the existing cervical screening model is more sensitive than the
existing model.
Moreover, the overall cost for the proposed cervical screening test was recorded at
$466.67961 while the overall cost for the existing cancer screening cervical test was found to be
$159.769. In regard to the benefits, the results showed that the proposed and existing recorded
$36.753599 and $36.743909 respectively. The test’s incremental cost was found to be $335.311
whereas for the existing screening test was found to be $0.00969. The incremental cost-
effectiveness ratio, (the cost gained for each QALY for the proposed cervical screening was
found to be $34,603.818337.
Areas of Uncertainty
Uncertainty and variability are two major aspects in every model. Therefore, regardless
of the type of model that is build, these two aspects have to be considered. The main reason why
these two aspects are important is because they affect the interpretation and value of the model
productivity. In this case, the efficacy of the two screening costs are based on the countries per
capita, GDP. However, the benefits which are associated with the cost of both screening tests are
not identified. Therefore, basing on the results, if the price maker is $50,000, then both the
proposed and existing screening tests are cost effective.
effectiveness ratio. ICERs was done for all alternative screening costs that were evident
throughout the study.
Results
In both alternatives, specificity was equivalent to 94%. On the other hand, the sensitivity
score was different. The proposed screening test showed a sensitivity score of 75% while the
existing screening test recorded a score that had a sensitivity of 94%. Basing on the results, it can
therefore be confirmed that the existing cervical screening model is more sensitive than the
existing model.
Moreover, the overall cost for the proposed cervical screening test was recorded at
$466.67961 while the overall cost for the existing cancer screening cervical test was found to be
$159.769. In regard to the benefits, the results showed that the proposed and existing recorded
$36.753599 and $36.743909 respectively. The test’s incremental cost was found to be $335.311
whereas for the existing screening test was found to be $0.00969. The incremental cost-
effectiveness ratio, (the cost gained for each QALY for the proposed cervical screening was
found to be $34,603.818337.
Areas of Uncertainty
Uncertainty and variability are two major aspects in every model. Therefore, regardless
of the type of model that is build, these two aspects have to be considered. The main reason why
these two aspects are important is because they affect the interpretation and value of the model
productivity. In this case, the efficacy of the two screening costs are based on the countries per
capita, GDP. However, the benefits which are associated with the cost of both screening tests are
not identified. Therefore, basing on the results, if the price maker is $50,000, then both the
proposed and existing screening tests are cost effective.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
ASSESSING HEALTH TECHNOLOGY4
Discussion
From the results given, it is evident that proposed cervical cancer screening test is not
cost effective and has impacts on the set budget. The outcomes shows that the proposed cervical
screening model is $495.08 unlike the existing cervical screening test which is $117.0991.
However, the effective ration can be used to give a more expensive alternative. So, according to
the results, the society is only ready to pay $50,000 for the health consequences. Based on the
results in this case, it can be argued that the proposed test is more effective that the existing
cervical cancer screening test with a QALY value of 36.7985711. After comparing the two
values, the decision maker value was found to be greater.
Recommendations
Besides having the highest QALY value, the results shows that the proposed cervical
cancer screening model is expensive that the existing test. Moreover, the proposed cervical
screening test has also shown the high sensitivity as well as benefits than the existing cancer
screening test. Thus, the proposed cervical cancer screening test can be used in early
identification of pre-cancerous tissues. In regard to this, it will be an easy task to remove the
tissues. Finally, owing to the above discussed benefits and factors, the proposed technology is
recommended to screen cervical cancer which will also be a roadmap towards eliminating this
type of cancer among women.
Structured Report
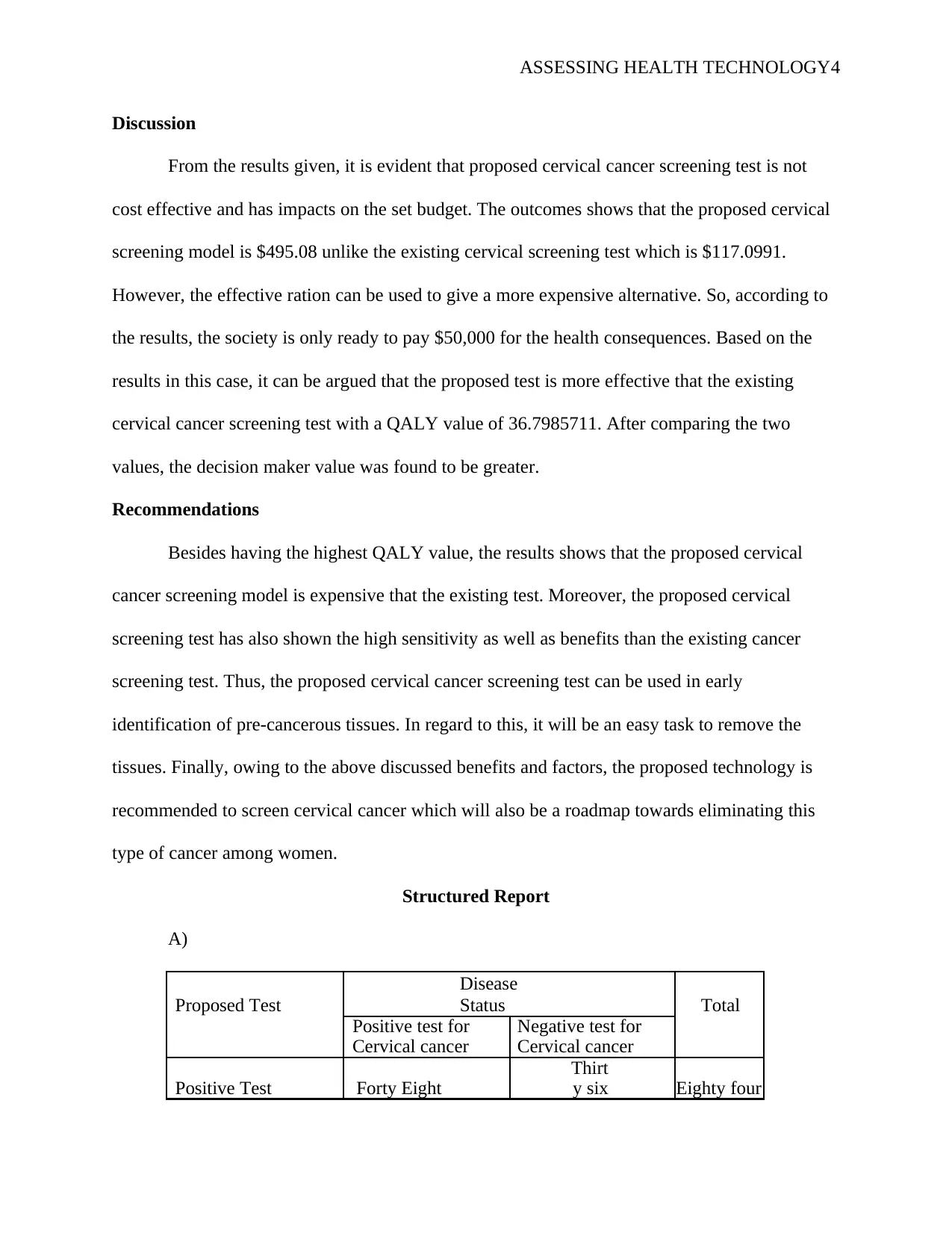
A)
Proposed Test
Disease
Status Total
Positive test for
Cervical cancer
Negative test for
Cervical cancer
Positive Test Forty Eight
Thirt
y six Eighty four
Discussion
From the results given, it is evident that proposed cervical cancer screening test is not
cost effective and has impacts on the set budget. The outcomes shows that the proposed cervical
screening model is $495.08 unlike the existing cervical screening test which is $117.0991.
However, the effective ration can be used to give a more expensive alternative. So, according to
the results, the society is only ready to pay $50,000 for the health consequences. Based on the
results in this case, it can be argued that the proposed test is more effective that the existing
cervical cancer screening test with a QALY value of 36.7985711. After comparing the two
values, the decision maker value was found to be greater.
Recommendations
Besides having the highest QALY value, the results shows that the proposed cervical
cancer screening model is expensive that the existing test. Moreover, the proposed cervical
screening test has also shown the high sensitivity as well as benefits than the existing cancer
screening test. Thus, the proposed cervical cancer screening test can be used in early
identification of pre-cancerous tissues. In regard to this, it will be an easy task to remove the
tissues. Finally, owing to the above discussed benefits and factors, the proposed technology is
recommended to screen cervical cancer which will also be a roadmap towards eliminating this
type of cancer among women.
Structured Report
A)
Proposed Test
Disease
Status Total
Positive test for
Cervical cancer
Negative test for
Cervical cancer
Positive Test Forty Eight
Thirt
y six Eighty four
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ASSESSING HEALTH TECHNOLOGY5
Negative Test Two
Five
hund
red
and
Sixty
four
Five
hundred
and sixty
six
Total fifty Six hundred
Five
hundred
and sixty
B)
According to Theron et al (2016, p. 1001), true positive rate, which is in most cases
known as the recall or sensitivity, plays a critical role in measuring the percentage of the real
positives which are correctly identified in a specific test. In this particular case, true positive will
be define as situations in which a screened patients has cancer and the test shows positive results
(Bernardi, eta al., 2018, p. 28, Wang, et al., 2018, p.739). The research involved 48 true positives
respondents who were financial and healthcare professionals (N= 48). Qualitative data collection
methods were used in this research. Some of the crucial qualitative data collection techniques
that were incorporated into the study have discussed in the former section of this report.
Consequently, false positive is referred to as a condition in whereby the screened patients
do not cervical cancer (Johnson-Davis et al., 2015, p. 100, Nwachukwu & Bozic, 2015, p. 1119).
However, the test in this case is usually negative. The number of patients in this case was 36.
True negative is defined as a situation in which the screened patients did not have the
disease and the test was negative (Makady et al, 2017, 530, Claxton et al., 2015, p. 14, Brazier
& Tsuchiya, 2015, p. 560). The number of patients in this case was 564.
Finally positive was a situation in which the screened patients did not have the disease but the
test was positive (Woods et al., 2016, p. 930, DeJean et al., 2016, p. 1310). The number of
patients in this case was 2. This information was obtained in two major ways, interviews and
questionnaires.
Negative Test Two
Five
hund
red
and
Sixty
four
Five
hundred
and sixty
six
Total fifty Six hundred
Five
hundred
and sixty
B)
According to Theron et al (2016, p. 1001), true positive rate, which is in most cases
known as the recall or sensitivity, plays a critical role in measuring the percentage of the real
positives which are correctly identified in a specific test. In this particular case, true positive will
be define as situations in which a screened patients has cancer and the test shows positive results
(Bernardi, eta al., 2018, p. 28, Wang, et al., 2018, p.739). The research involved 48 true positives
respondents who were financial and healthcare professionals (N= 48). Qualitative data collection
methods were used in this research. Some of the crucial qualitative data collection techniques
that were incorporated into the study have discussed in the former section of this report.
Consequently, false positive is referred to as a condition in whereby the screened patients
do not cervical cancer (Johnson-Davis et al., 2015, p. 100, Nwachukwu & Bozic, 2015, p. 1119).
However, the test in this case is usually negative. The number of patients in this case was 36.
True negative is defined as a situation in which the screened patients did not have the
disease and the test was negative (Makady et al, 2017, 530, Claxton et al., 2015, p. 14, Brazier
& Tsuchiya, 2015, p. 560). The number of patients in this case was 564.
Finally positive was a situation in which the screened patients did not have the disease but the
test was positive (Woods et al., 2016, p. 930, DeJean et al., 2016, p. 1310). The number of
patients in this case was 2. This information was obtained in two major ways, interviews and
questionnaires.

ASSESSING HEALTH TECHNOLOGY6
Interviews
Use of Interviews was one of the most crucial data collection techniques that were used
in the study. As demonstrated in various research studies, this type of data collection is among
the most successful techniques that researchers always rely on. Basically, the method involved
analyzing the behaviors, and trends of both the proposed and existing cervical cancer screening
tests. The data recorded in this case was analyzed after the data collection phase was over. This
was specifically during the data analysis stage.
Questionnaires
Questionnaires were also critical in this study. It involved both health and financial
professionals giving their responses about the proposed and existing cervical cancer screening
tests. The following steps were crucial in ensuring that the questionnaires were well formulated.
One of these steps was making a decision on the type of questions that were incorporated into the
study. To achieve this, the type of information that was required for the study was also analyzed.
Due to the sensitivity of the data analysis phase, the second step majorly dealt with planning of
the questionnaires (Huisman et al., 2019, pp.125). Basically, this step involved narrowing down
the formulated questions to ensure that they focused on answering the main research questions
which was crucial in achieving the goals and objectives of the research study (Korhonen et al.,
2016, p. 1954, Simkins et al., 2019, p. 13492).
C) Sensitivity and specificity (4 marks)
Sensitivity is given as
= true pos. / (true pos. / false neg.)
Therefore, the sensitivity in this case was given by
Sensitivity = 48/ (48 + 2)
Interviews
Use of Interviews was one of the most crucial data collection techniques that were used
in the study. As demonstrated in various research studies, this type of data collection is among
the most successful techniques that researchers always rely on. Basically, the method involved
analyzing the behaviors, and trends of both the proposed and existing cervical cancer screening
tests. The data recorded in this case was analyzed after the data collection phase was over. This
was specifically during the data analysis stage.
Questionnaires
Questionnaires were also critical in this study. It involved both health and financial
professionals giving their responses about the proposed and existing cervical cancer screening
tests. The following steps were crucial in ensuring that the questionnaires were well formulated.
One of these steps was making a decision on the type of questions that were incorporated into the
study. To achieve this, the type of information that was required for the study was also analyzed.
Due to the sensitivity of the data analysis phase, the second step majorly dealt with planning of
the questionnaires (Huisman et al., 2019, pp.125). Basically, this step involved narrowing down
the formulated questions to ensure that they focused on answering the main research questions
which was crucial in achieving the goals and objectives of the research study (Korhonen et al.,
2016, p. 1954, Simkins et al., 2019, p. 13492).
C) Sensitivity and specificity (4 marks)
Sensitivity is given as
= true pos. / (true pos. / false neg.)
Therefore, the sensitivity in this case was given by
Sensitivity = 48/ (48 + 2)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ASSESSING HEALTH TECHNOLOGY7
Sensitivity = 44 / 53
Sensitivity = 0.75 which is equivalent to 75 percent of sensitivity.
On the other hand, specificity is given by
Specificity = true negative / false positive + false positive
Therefore;
Specificity = 560 / 560 + 34
Specificity = 560 / 594
Specificity = 0.94 (The proposed cervical cancer screening had 94 percent specificity.
C.
Part A
The assumptions of both the sensitivity and specificity of the depended on the prevalence
of the population that was screened (Press et al., 2017, p.10, Meisner et al., 2019, p. 02087, Keup
et al., 2018, p. 210). Although there were different laboratory and women cohorts for the
proposed cervical cancer screening test, the prevalence for both interventions were the same as
depicted by the recorded results. Therefore, its sensitivity and specificity was not affected
regardless of the laboratory of cohort that was used.
Part B.
Sensitivity = 44 / 53
Sensitivity = 0.75 which is equivalent to 75 percent of sensitivity.
On the other hand, specificity is given by
Specificity = true negative / false positive + false positive
Therefore;
Specificity = 560 / 560 + 34
Specificity = 560 / 594
Specificity = 0.94 (The proposed cervical cancer screening had 94 percent specificity.
C.
Part A
The assumptions of both the sensitivity and specificity of the depended on the prevalence
of the population that was screened (Press et al., 2017, p.10, Meisner et al., 2019, p. 02087, Keup
et al., 2018, p. 210). Although there were different laboratory and women cohorts for the
proposed cervical cancer screening test, the prevalence for both interventions were the same as
depicted by the recorded results. Therefore, its sensitivity and specificity was not affected
regardless of the laboratory of cohort that was used.
Part B.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
ASSESSING HEALTH TECHNOLOGY8
ASSESSING HEALTH TECHNOLOGY9
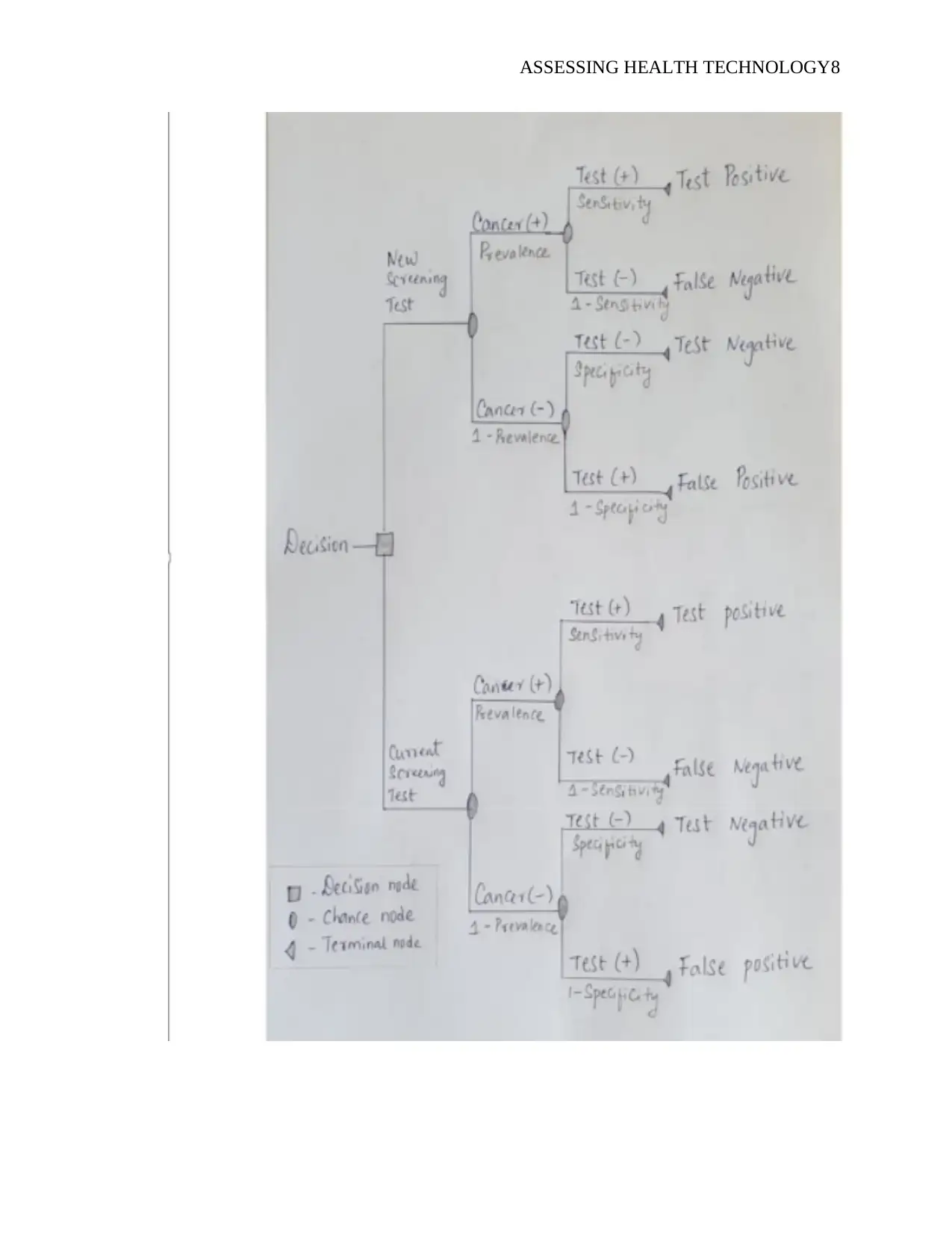
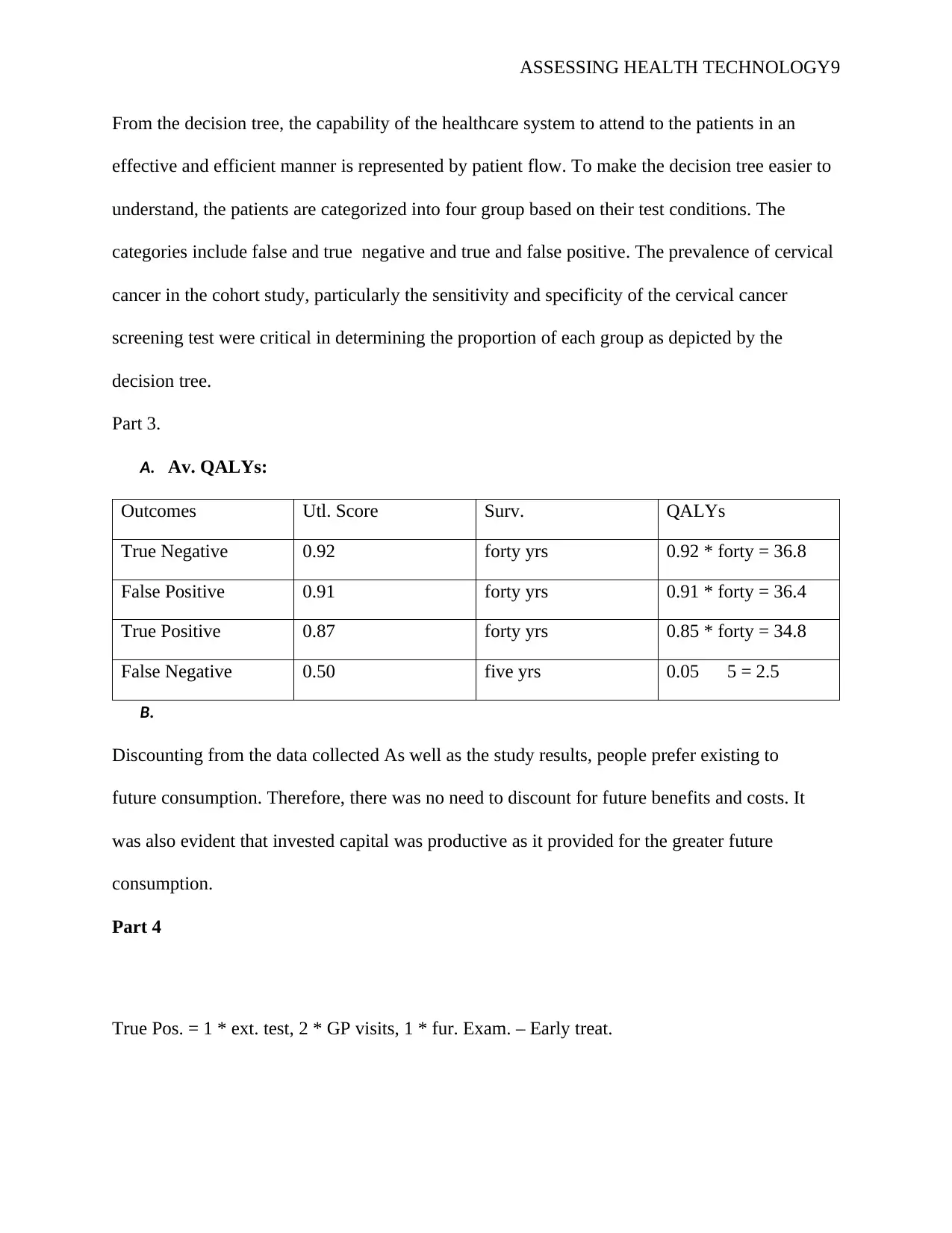
From the decision tree, the capability of the healthcare system to attend to the patients in an
effective and efficient manner is represented by patient flow. To make the decision tree easier to
understand, the patients are categorized into four group based on their test conditions. The
categories include false and true negative and true and false positive. The prevalence of cervical
cancer in the cohort study, particularly the sensitivity and specificity of the cervical cancer
screening test were critical in determining the proportion of each group as depicted by the
decision tree.
Part 3.
A. Av. QALYs:
Outcomes Utl. Score Surv. QALYs
True Negative 0.92 forty yrs 0.92 * forty = 36.8
False Positive 0.91 forty yrs 0.91 * forty = 36.4
True Positive 0.87 forty yrs 0.85 * forty = 34.8
False Negative 0.50 five yrs 0.05 5 = 2.5
B.
Discounting from the data collected As well as the study results, people prefer existing to
future consumption. Therefore, there was no need to discount for future benefits and costs. It
was also evident that invested capital was productive as it provided for the greater future
consumption.
Part 4
True Pos. = 1 * ext. test, 2 * GP visits, 1 * fur. Exam. – Early treat.
From the decision tree, the capability of the healthcare system to attend to the patients in an
effective and efficient manner is represented by patient flow. To make the decision tree easier to
understand, the patients are categorized into four group based on their test conditions. The
categories include false and true negative and true and false positive. The prevalence of cervical
cancer in the cohort study, particularly the sensitivity and specificity of the cervical cancer
screening test were critical in determining the proportion of each group as depicted by the
decision tree.
Part 3.
A. Av. QALYs:
Outcomes Utl. Score Surv. QALYs
True Negative 0.92 forty yrs 0.92 * forty = 36.8
False Positive 0.91 forty yrs 0.91 * forty = 36.4
True Positive 0.87 forty yrs 0.85 * forty = 34.8
False Negative 0.50 five yrs 0.05 5 = 2.5
B.
Discounting from the data collected As well as the study results, people prefer existing to
future consumption. Therefore, there was no need to discount for future benefits and costs. It
was also evident that invested capital was productive as it provided for the greater future
consumption.
Part 4
True Pos. = 1 * ext. test, 2 * GP visits, 1 * fur. Exam. – Early treat.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ASSESSING HEALTH TECHNOLOGY10
Based on the above formula, the treatment in this case would therefore cost --1 * $50 + (2 *
$35) + $1,000 = $1,120
False pos. = 1 * exist. test, 2 * GP visits, 1 * fur. Exam. – No.treat.
Based on the above formula, the treatment in this case would therefore cost --1 * $50 + (2 *
$35) + $500 = $620
True. Neg = 1 * ext. test, 1* GP visit.
Based on the above formula, the treatment in this case would therefore cost –1* $50 +$35 =
$85
False Neg. = 1 * ext. test, 1 * GP visit, 1 * del. Treat.
Based on the above formula, the treatment in this case would therefore cost—1 * $50 + 1 *
$35 +1 * 50,000 =$50,085.
Estimated costs for an individual in proposed test.
True pos. = 1 * prop. test, 2 * GP visits, 1 * fur. Exam. –early treat.
Based on the above formula, the treatment in this case would therefore cost—1 * $400 + (2 *
$35) + 1 * 500 = $970
False pos. = 1 * prop. test, 2 * GP visits, 1 * fur. Exam. – No treat.
Based on the above formula, the treatment in this case would therefore cost --1 * $400 + (2 *
$35) + $500 = $970
True Negative = 1 * prop. test, 1* GP visit.
Based on the above formula, the treatment in this case would therefore cost –1* $400 +$35 =
$435
False Neg. = 1 * prop. Test, 1 * GP visit, 1 * del. Treat.
Based on the above formula, the treatment in this case would therefore cost --1 * $50 + (2 *
$35) + $1,000 = $1,120
False pos. = 1 * exist. test, 2 * GP visits, 1 * fur. Exam. – No.treat.
Based on the above formula, the treatment in this case would therefore cost --1 * $50 + (2 *
$35) + $500 = $620
True. Neg = 1 * ext. test, 1* GP visit.
Based on the above formula, the treatment in this case would therefore cost –1* $50 +$35 =
$85
False Neg. = 1 * ext. test, 1 * GP visit, 1 * del. Treat.
Based on the above formula, the treatment in this case would therefore cost—1 * $50 + 1 *
$35 +1 * 50,000 =$50,085.
Estimated costs for an individual in proposed test.
True pos. = 1 * prop. test, 2 * GP visits, 1 * fur. Exam. –early treat.
Based on the above formula, the treatment in this case would therefore cost—1 * $400 + (2 *
$35) + 1 * 500 = $970
False pos. = 1 * prop. test, 2 * GP visits, 1 * fur. Exam. – No treat.
Based on the above formula, the treatment in this case would therefore cost --1 * $400 + (2 *
$35) + $500 = $970
True Negative = 1 * prop. test, 1* GP visit.
Based on the above formula, the treatment in this case would therefore cost –1* $400 +$35 =
$435
False Neg. = 1 * prop. Test, 1 * GP visit, 1 * del. Treat.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
ASSESSING HEALTH TECHNOLOGY11
Based on the above formula, the treatment in this case would therefore cost—1 * $400 + 1 *
$35 +1 * 50,000 =$50,435.
Part 5: (10 marks)
A) Table 3:
Description of the Parameter Existing Test Proposed Test
cervical cancer Prevalence 0.0040 0.0040
Test Sensitivity 0.750 0.830
Test Specificity 0.94 0.94407
True Positive Cost $1,120.45 $1,470.00
False Positive Cost $620.00 $970.00
True Negative Cost $85.00 $435.00
False negative Cost $50,085.00 $50,435.00
QALYs Existing Test Proposed Test
True Positive $34.8.00 $34.8.00
False Positive 36.79857115 36.75857115
True Negative 36.778 36.5456
False negative 0.500 0.500
B)
Based on the above formula, the treatment in this case would therefore cost—1 * $400 + 1 *
$35 +1 * 50,000 =$50,435.
Part 5: (10 marks)
A) Table 3:
Description of the Parameter Existing Test Proposed Test
cervical cancer Prevalence 0.0040 0.0040
Test Sensitivity 0.750 0.830
Test Specificity 0.94 0.94407
True Positive Cost $1,120.45 $1,470.00
False Positive Cost $620.00 $970.00
True Negative Cost $85.00 $435.00
False negative Cost $50,085.00 $50,435.00
QALYs Existing Test Proposed Test
True Positive $34.8.00 $34.8.00
False Positive 36.79857115 36.75857115
True Negative 36.778 36.5456
False negative 0.500 0.500
B)
ASSESSING HEALTH TECHNOLOGY12
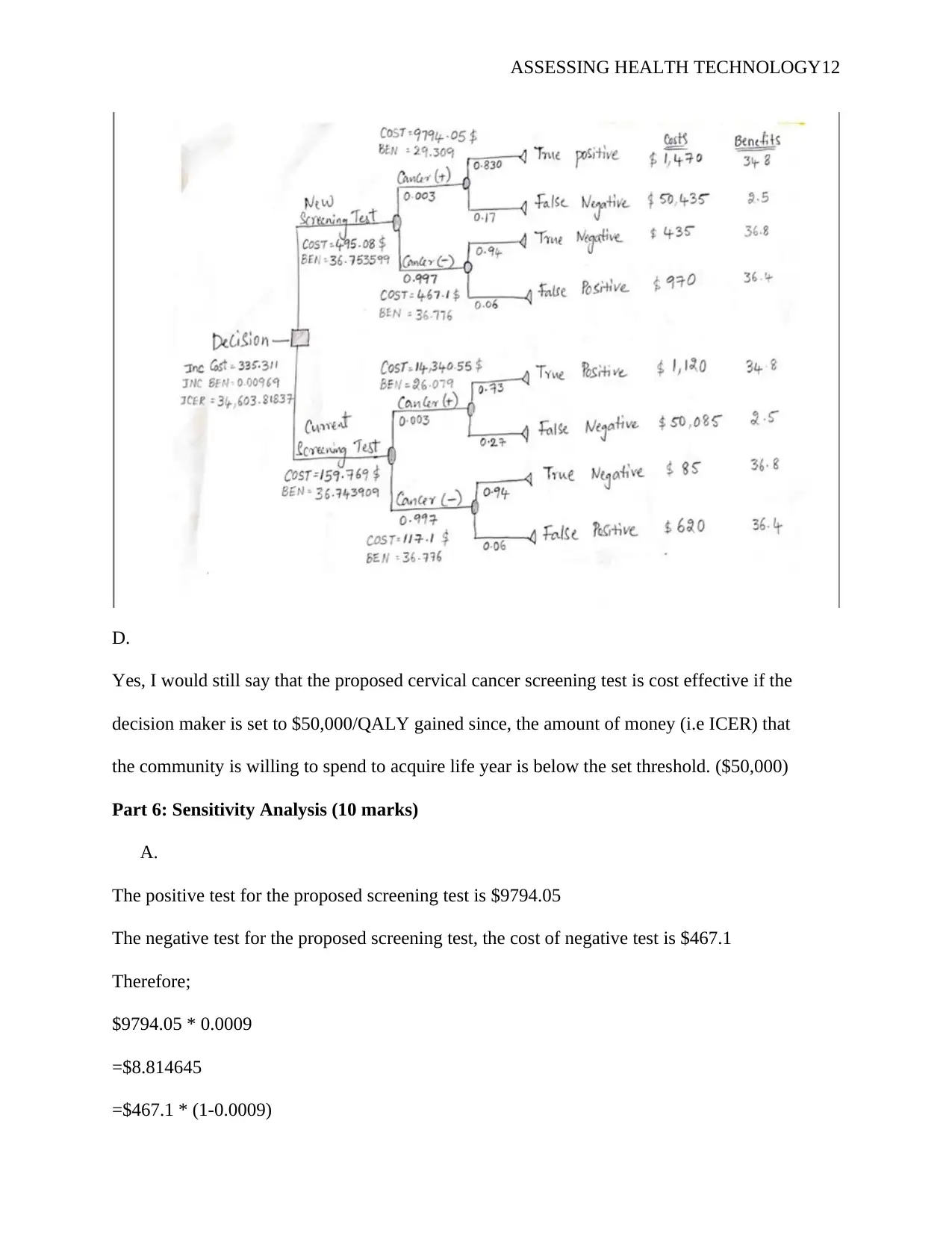
D.
Yes, I would still say that the proposed cervical cancer screening test is cost effective if the
decision maker is set to $50,000/QALY gained since, the amount of money (i.e ICER) that
the community is willing to spend to acquire life year is below the set threshold. ($50,000)
Part 6: Sensitivity Analysis (10 marks)
A.
The positive test for the proposed screening test is $9794.05
The negative test for the proposed screening test, the cost of negative test is $467.1
Therefore;
$9794.05 * 0.0009
=$8.814645
=$467.1 * (1-0.0009)
D.
Yes, I would still say that the proposed cervical cancer screening test is cost effective if the
decision maker is set to $50,000/QALY gained since, the amount of money (i.e ICER) that
the community is willing to spend to acquire life year is below the set threshold. ($50,000)
Part 6: Sensitivity Analysis (10 marks)
A.
The positive test for the proposed screening test is $9794.05
The negative test for the proposed screening test, the cost of negative test is $467.1
Therefore;
$9794.05 * 0.0009
=$8.814645
=$467.1 * (1-0.0009)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 18
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





![Case Study Report: Business Decision Analysis - [University Name]](/_next/image/?url=https%3A%2F%2Fdesklib.com%2Fmedia%2Fimages%2Fnm%2F0fe1229012094541bae6dce0f9e36c59.jpg&w=256&q=75)