Ethical and Legal Framework for Healthcare Research in Saudi Arabia
VerifiedAdded on 2022/11/18
|13
|771
|113
Report
AI Summary
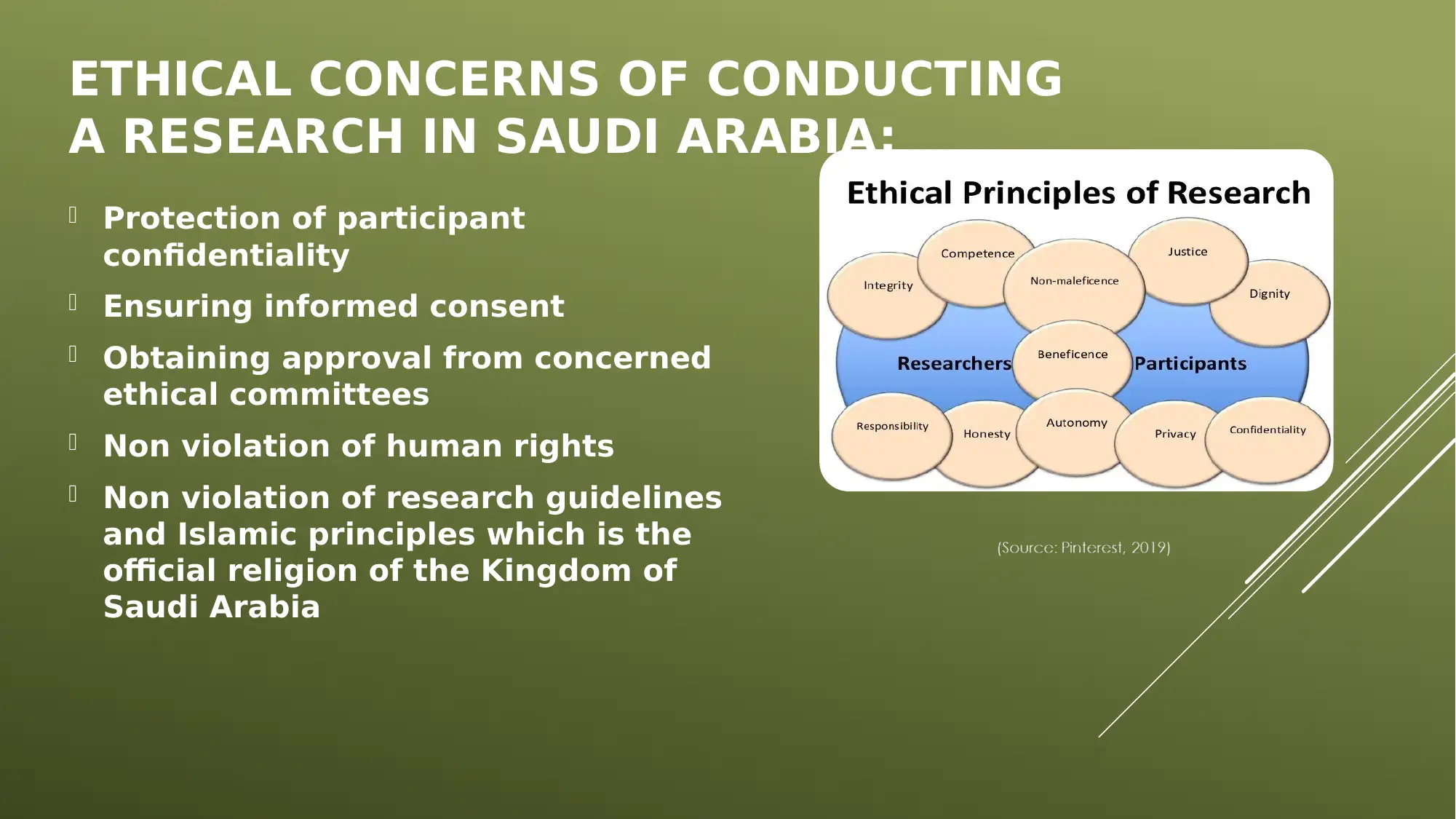
This report provides a comprehensive overview of ethics in healthcare research, focusing on ethical and legal considerations, especially within the context of Saudi Arabia. It defines research ethics, emphasizing the importance of norms, regulations, and values guiding scientific experiments. The report covers critical ethical concerns such as participant confidentiality, informed consent, and adherence to human rights and Islamic principles. It also outlines legal concerns, including compliance with the Law of Ethics of Research on Living Creatures and guidelines from the National Commission of Bioethics (NCBE) and the Saudi Food and Drug Authority. The report details best practices for informed consent, emphasizing clear communication and creating a safe environment. It also discusses the ethical and legal implications of non-compliance with research ethics, such as the cancellation of funding or legal suits. The conclusion highlights the importance of research ethics in maintaining trust, accountability, and protecting participants' interests. The report includes references to relevant literature on research ethics and legal frameworks.
1 out of 13















![[object Object]](/_next/static/media/star-bottom.7253800d.svg)