Explain Heat Transfer Processes in Solids, Fluids, Radiation - BTEC
VerifiedAdded on 2023/01/10
|2
|598
|28
Report
AI Summary
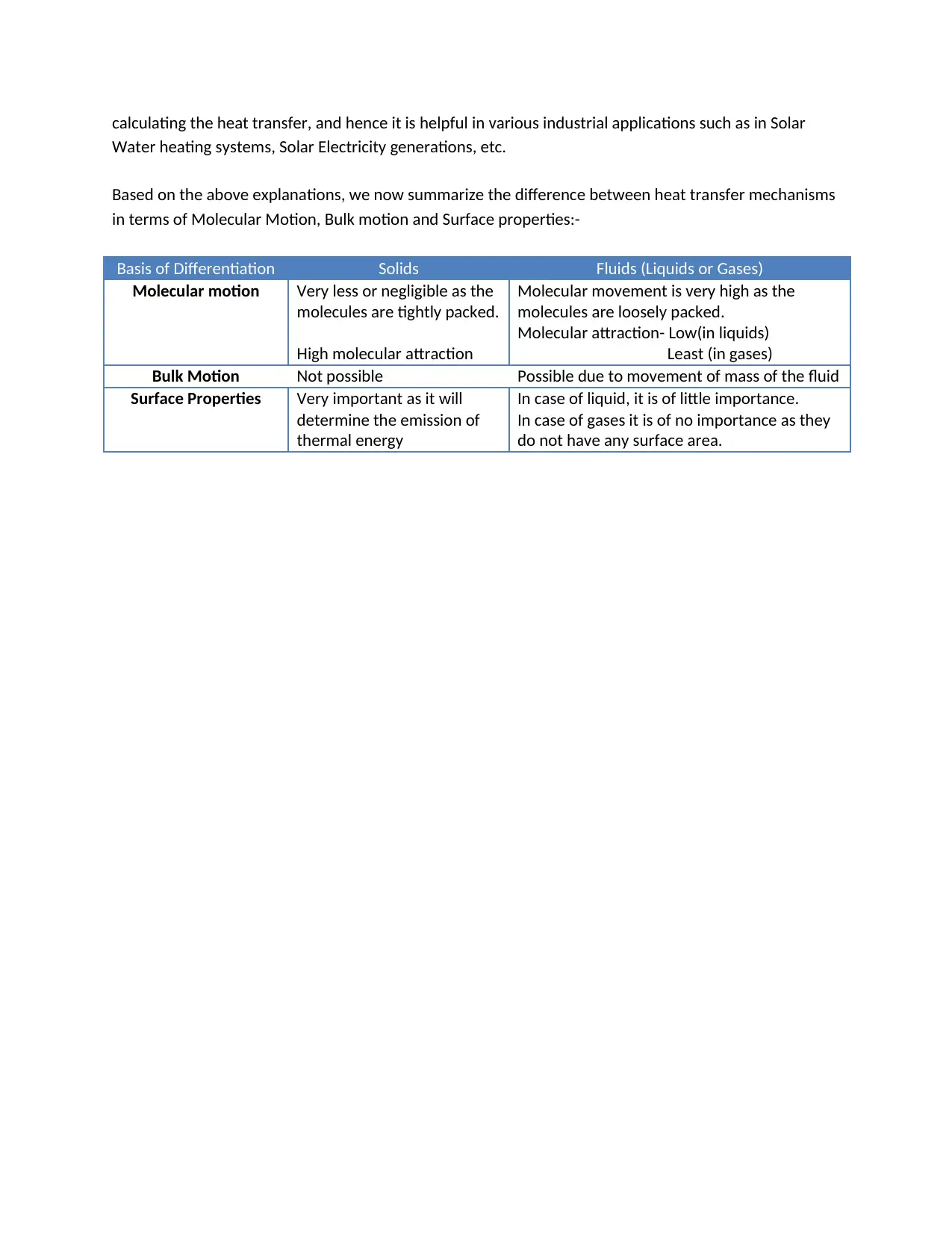
This report provides a comprehensive explanation of heat transfer mechanisms, including conduction, convection, and radiation. It details how heat is transferred within solids, fluids (liquids and gases), and through radiation, emphasizing the role of molecular motion, bulk motion, and surface properties. The report discusses the concept of thermal equilibrium and emissivity, and how these factors influence heat transfer. It also highlights the industrial applications of these heat transfer processes, such as in solar water heating and electricity generation, making it a valuable resource for understanding energy changes and their practical implications, specifically for a BTEC Level 3 Unit 14 assignment.
1 out of 2




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)