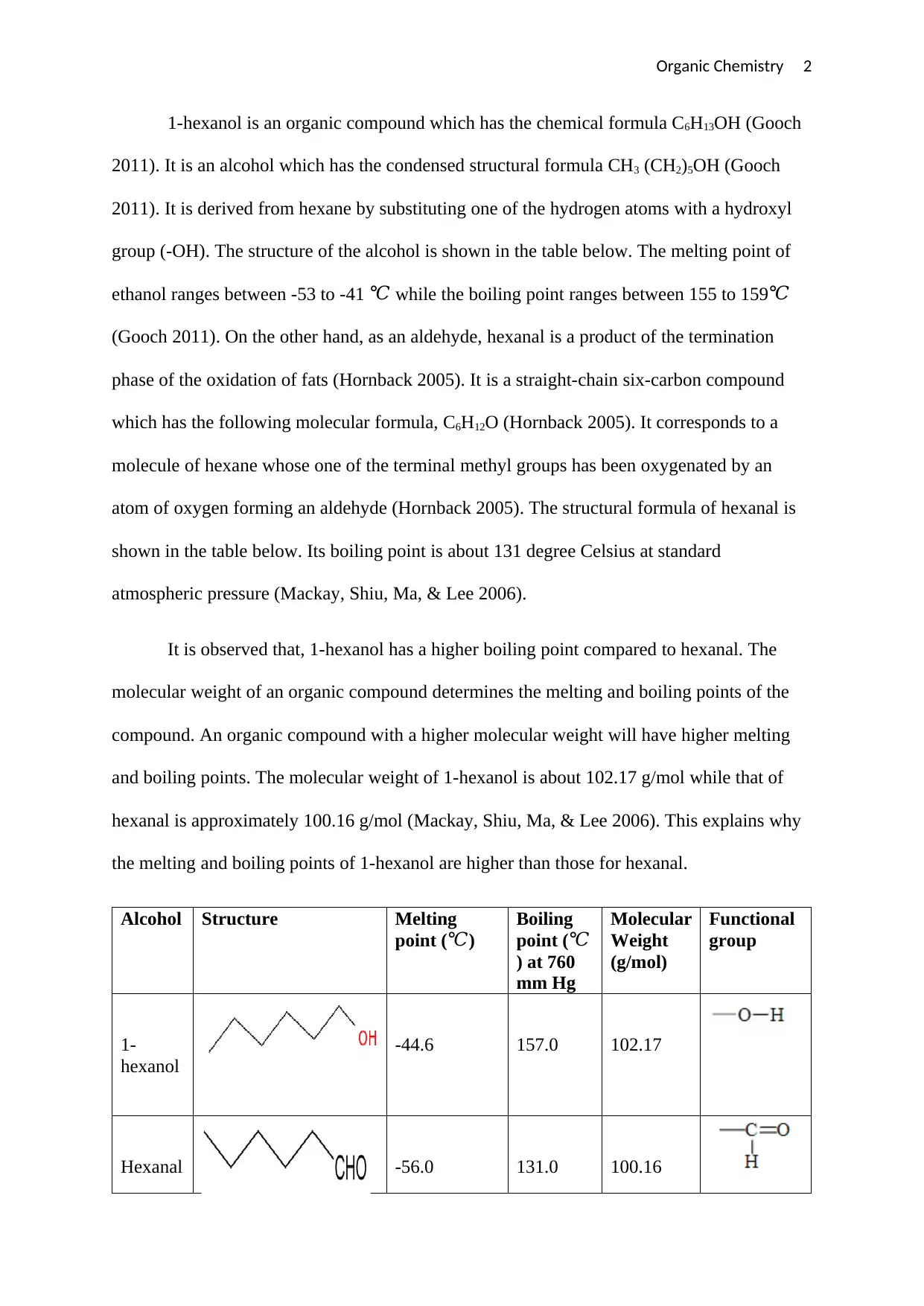
Analysis of 1-Hexanol and Hexanal Properties in Organic Chemistry
VerifiedAdded on 2022/09/18
|4
|743
|24
Homework Assignment
AI Summary
This assignment provides a detailed comparison of 1-hexanol and hexanal, two organic compounds. It begins by defining each compound, including their chemical formulas and structural representations. The study then examines their physical properties, specifically melting and boiling points, highlighting the differences between the two. The analysis delves into the factors influencing these properties, such as molecular weight and the type of functional group present (hydroxyl vs. carbonyl). The document explains how the presence of hydrogen bonds in 1-hexanol (due to the hydroxyl group) contributes to its higher boiling point compared to hexanal. The conclusion summarizes the key differences in functional groups and molecular weights, emphasizing their impact on the melting and boiling points of the compounds. References to supporting literature are also included.
1 out of 4










![[object Object]](/_next/static/media/star-bottom.7253800d.svg)