Comprehensive Analysis of Nitrogen Metabolism in the Human Body
VerifiedAdded on 2022/11/15
|9
|1466
|168
Report
AI Summary
This report provides a detailed overview of nitrogen metabolism in the human body, beginning with the dietary intake of nitrogen through protein consumption. It explains how amino acids are broken down, releasing nitrogen in the form of ammonia, and how the carbon backbones are converted into glucose. The report describes the digestion process, including the role of enzymes in the stomach and small intestine, and the absorption of amino acids. It explores the transamination of amino acids in the liver, the role of glutamate, and the oxidative deamination process. The urea cycle is explained step-by-step, including the production of carbamoyl phosphate, the conversion of ornithine to citrulline, and the final excretion of urea. The report also highlights the interconnectedness of the urea cycle with the Krebs cycle through the malate-aspartate shuttle and the regulatory mechanisms of the urea cycle, including the roles of carbamoyl phosphate synthetase I and N-acetyl glutamate. The report concludes with a discussion of the key enzymes and pathways involved in maintaining nitrogen balance within the body and provides relevant references.

Running head: NITROGEN METABOLISM IN HUMAN BODY
NITROGEN METABOLISM IN HUMAN BODY
Name of the Student:
Name of the University:
Author Note:
NITROGEN METABOLISM IN HUMAN BODY
Name of the Student:
Name of the University:
Author Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
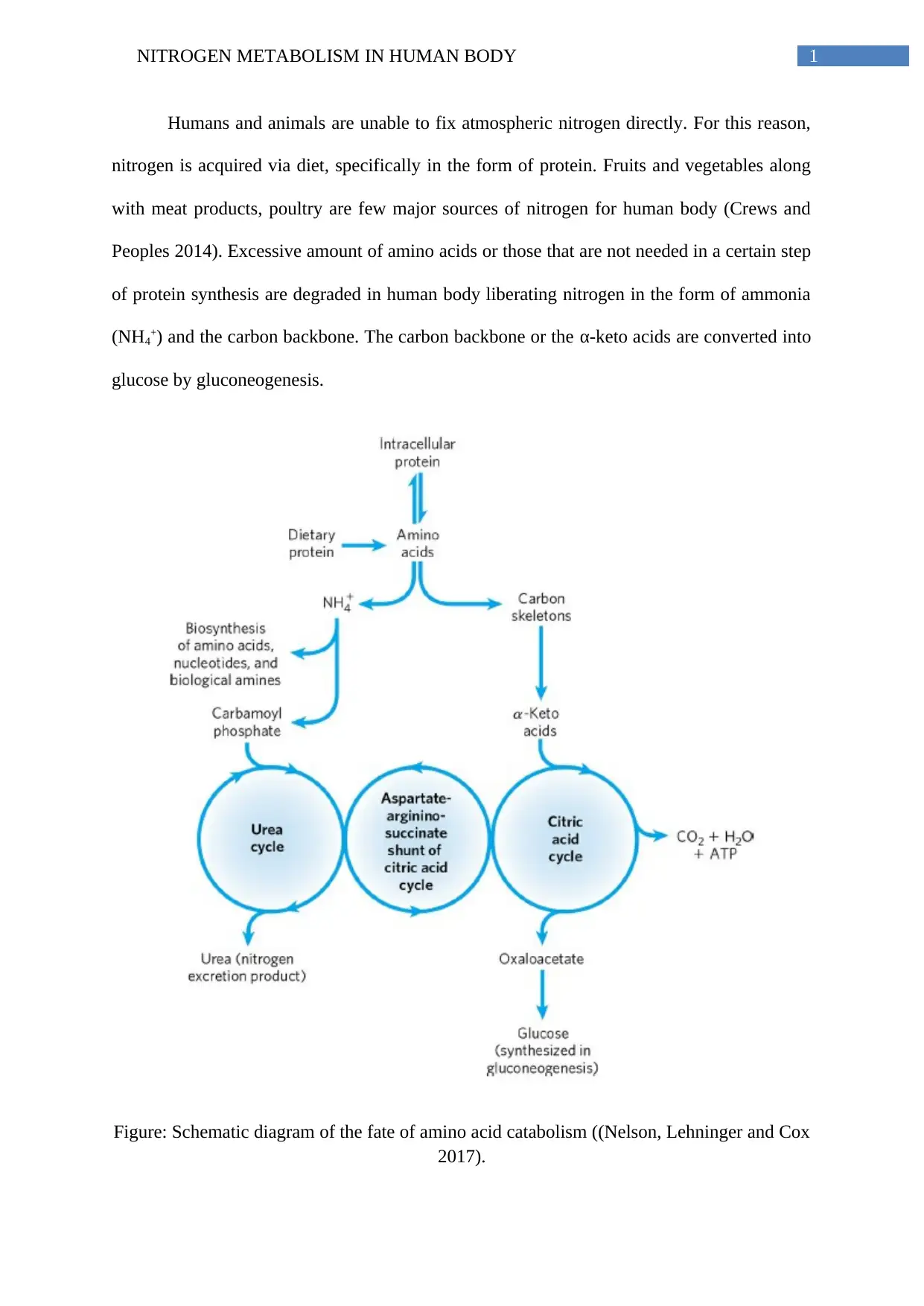
1NITROGEN METABOLISM IN HUMAN BODY
Humans and animals are unable to fix atmospheric nitrogen directly. For this reason,
nitrogen is acquired via diet, specifically in the form of protein. Fruits and vegetables along
with meat products, poultry are few major sources of nitrogen for human body (Crews and
Peoples 2014). Excessive amount of amino acids or those that are not needed in a certain step
of protein synthesis are degraded in human body liberating nitrogen in the form of ammonia
(NH4+) and the carbon backbone. The carbon backbone or the α-keto acids are converted into
glucose by gluconeogenesis.
Figure: Schematic diagram of the fate of amino acid catabolism ((Nelson, Lehninger and Cox
2017).
Humans and animals are unable to fix atmospheric nitrogen directly. For this reason,
nitrogen is acquired via diet, specifically in the form of protein. Fruits and vegetables along
with meat products, poultry are few major sources of nitrogen for human body (Crews and
Peoples 2014). Excessive amount of amino acids or those that are not needed in a certain step
of protein synthesis are degraded in human body liberating nitrogen in the form of ammonia
(NH4+) and the carbon backbone. The carbon backbone or the α-keto acids are converted into
glucose by gluconeogenesis.
Figure: Schematic diagram of the fate of amino acid catabolism ((Nelson, Lehninger and Cox
2017).

2NITROGEN METABOLISM IN HUMAN BODY
In case of humans, ingestion of dietary protein stimulates production of gastrin in
stomach. Gastrin induces release of hydrochloric acid by the parietal cells and pepsinogen
from the chief cells. The resulting gastric juice has an acidic pH, which denatures the
globular proteins and exposes the internal peptides for degradation by activated pepsin
(pepsinogen, a zymogen is converted into pepsin by autolytic cleavage). The acidic contents
of the stomach are delivered into pancreas, which stimulates secretin, a hormone responsible
for release of bi-carbonate resulting in an abrupt increase in pH (~pH 7). After reaching
duodenum, it causes release of cholecystokinin, which facilities production of pancreatic
enzymes like chymotrypsinogen, trypsinogen, procarboxypeptidase A, procarboxypeptidase
B from the exocrine cells. Intestinal enteropeptidase activates trypsinogen by converting it
into trypsin, which in turn activates proelastase, procarboxypeptidases and
chymotrypsinogen. Activated carboxypeptidases and aminopeptidases cleaves the amino acid
residues from carboxyl end and amino end respectively, along with selective cleavage by
trypsin and chymotrypsin, which produces monomeric amino acids. These amino acids are
absorbed by villi of the small intestine and transported to the liver, where they undergo
transamination (Barrett et al. 2009). Transfer of an amino group of an amino acid
(deamination) to α-keto glutarate involves specific transaminase and pyridoxal phosphate as a
cofactor, producing glutamate and the subsequent α-keto acid of the amino acid residue.
Glutamate is a key molecule, which could be utilized for nucleotide biosynthesis pathway
(anabolic) or to the excretory pathway (catabolic), according to physiological needs.
Glutamate produced in this abovementioned process travels from cytosol to the liver
mitochondria, where it undergoes oxidative deamination by glutamate dehydrogenase using
NAD+ or NADP+ as a cofactor. Variety of independent pathways contribute to the
accumulation of glutamate in the liver mitochondria. Glutamine derived from extrahepatic
tissues are converted to glutamate by glutaminase, oxaloacetate is converted to aspartate by
In case of humans, ingestion of dietary protein stimulates production of gastrin in
stomach. Gastrin induces release of hydrochloric acid by the parietal cells and pepsinogen
from the chief cells. The resulting gastric juice has an acidic pH, which denatures the
globular proteins and exposes the internal peptides for degradation by activated pepsin
(pepsinogen, a zymogen is converted into pepsin by autolytic cleavage). The acidic contents
of the stomach are delivered into pancreas, which stimulates secretin, a hormone responsible
for release of bi-carbonate resulting in an abrupt increase in pH (~pH 7). After reaching
duodenum, it causes release of cholecystokinin, which facilities production of pancreatic
enzymes like chymotrypsinogen, trypsinogen, procarboxypeptidase A, procarboxypeptidase
B from the exocrine cells. Intestinal enteropeptidase activates trypsinogen by converting it
into trypsin, which in turn activates proelastase, procarboxypeptidases and
chymotrypsinogen. Activated carboxypeptidases and aminopeptidases cleaves the amino acid
residues from carboxyl end and amino end respectively, along with selective cleavage by
trypsin and chymotrypsin, which produces monomeric amino acids. These amino acids are
absorbed by villi of the small intestine and transported to the liver, where they undergo
transamination (Barrett et al. 2009). Transfer of an amino group of an amino acid
(deamination) to α-keto glutarate involves specific transaminase and pyridoxal phosphate as a
cofactor, producing glutamate and the subsequent α-keto acid of the amino acid residue.
Glutamate is a key molecule, which could be utilized for nucleotide biosynthesis pathway
(anabolic) or to the excretory pathway (catabolic), according to physiological needs.
Glutamate produced in this abovementioned process travels from cytosol to the liver
mitochondria, where it undergoes oxidative deamination by glutamate dehydrogenase using
NAD+ or NADP+ as a cofactor. Variety of independent pathways contribute to the
accumulation of glutamate in the liver mitochondria. Glutamine derived from extrahepatic
tissues are converted to glutamate by glutaminase, oxaloacetate is converted to aspartate by
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
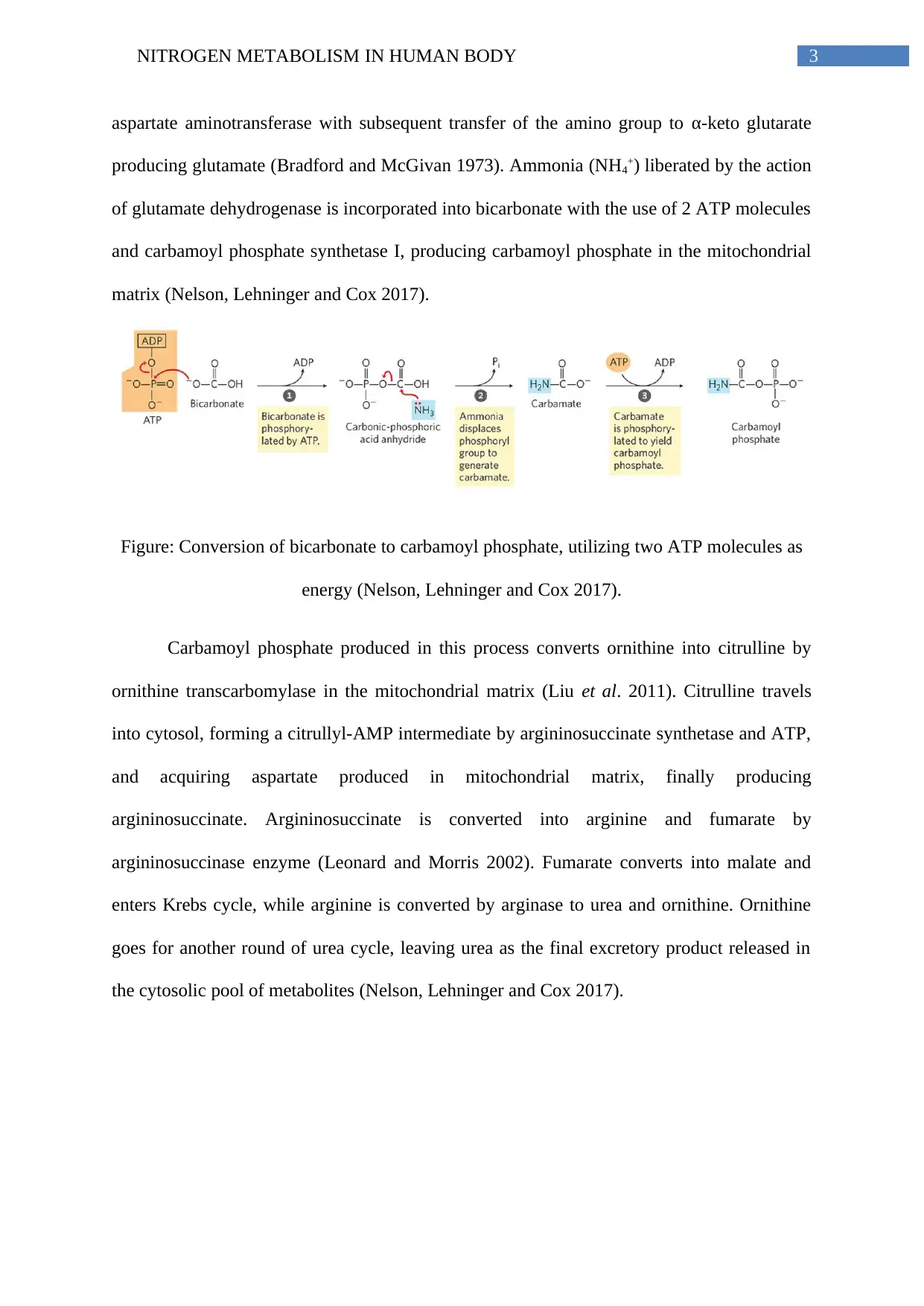
3NITROGEN METABOLISM IN HUMAN BODY
aspartate aminotransferase with subsequent transfer of the amino group to α-keto glutarate
producing glutamate (Bradford and McGivan 1973). Ammonia (NH4+) liberated by the action
of glutamate dehydrogenase is incorporated into bicarbonate with the use of 2 ATP molecules
and carbamoyl phosphate synthetase I, producing carbamoyl phosphate in the mitochondrial
matrix (Nelson, Lehninger and Cox 2017).
Figure: Conversion of bicarbonate to carbamoyl phosphate, utilizing two ATP molecules as
energy (Nelson, Lehninger and Cox 2017).
Carbamoyl phosphate produced in this process converts ornithine into citrulline by
ornithine transcarbomylase in the mitochondrial matrix (Liu et al. 2011). Citrulline travels
into cytosol, forming a citrullyl-AMP intermediate by argininosuccinate synthetase and ATP,
and acquiring aspartate produced in mitochondrial matrix, finally producing
argininosuccinate. Argininosuccinate is converted into arginine and fumarate by
argininosuccinase enzyme (Leonard and Morris 2002). Fumarate converts into malate and
enters Krebs cycle, while arginine is converted by arginase to urea and ornithine. Ornithine
goes for another round of urea cycle, leaving urea as the final excretory product released in
the cytosolic pool of metabolites (Nelson, Lehninger and Cox 2017).
aspartate aminotransferase with subsequent transfer of the amino group to α-keto glutarate
producing glutamate (Bradford and McGivan 1973). Ammonia (NH4+) liberated by the action
of glutamate dehydrogenase is incorporated into bicarbonate with the use of 2 ATP molecules
and carbamoyl phosphate synthetase I, producing carbamoyl phosphate in the mitochondrial
matrix (Nelson, Lehninger and Cox 2017).
Figure: Conversion of bicarbonate to carbamoyl phosphate, utilizing two ATP molecules as
energy (Nelson, Lehninger and Cox 2017).
Carbamoyl phosphate produced in this process converts ornithine into citrulline by
ornithine transcarbomylase in the mitochondrial matrix (Liu et al. 2011). Citrulline travels
into cytosol, forming a citrullyl-AMP intermediate by argininosuccinate synthetase and ATP,
and acquiring aspartate produced in mitochondrial matrix, finally producing
argininosuccinate. Argininosuccinate is converted into arginine and fumarate by
argininosuccinase enzyme (Leonard and Morris 2002). Fumarate converts into malate and
enters Krebs cycle, while arginine is converted by arginase to urea and ornithine. Ornithine
goes for another round of urea cycle, leaving urea as the final excretory product released in
the cytosolic pool of metabolites (Nelson, Lehninger and Cox 2017).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
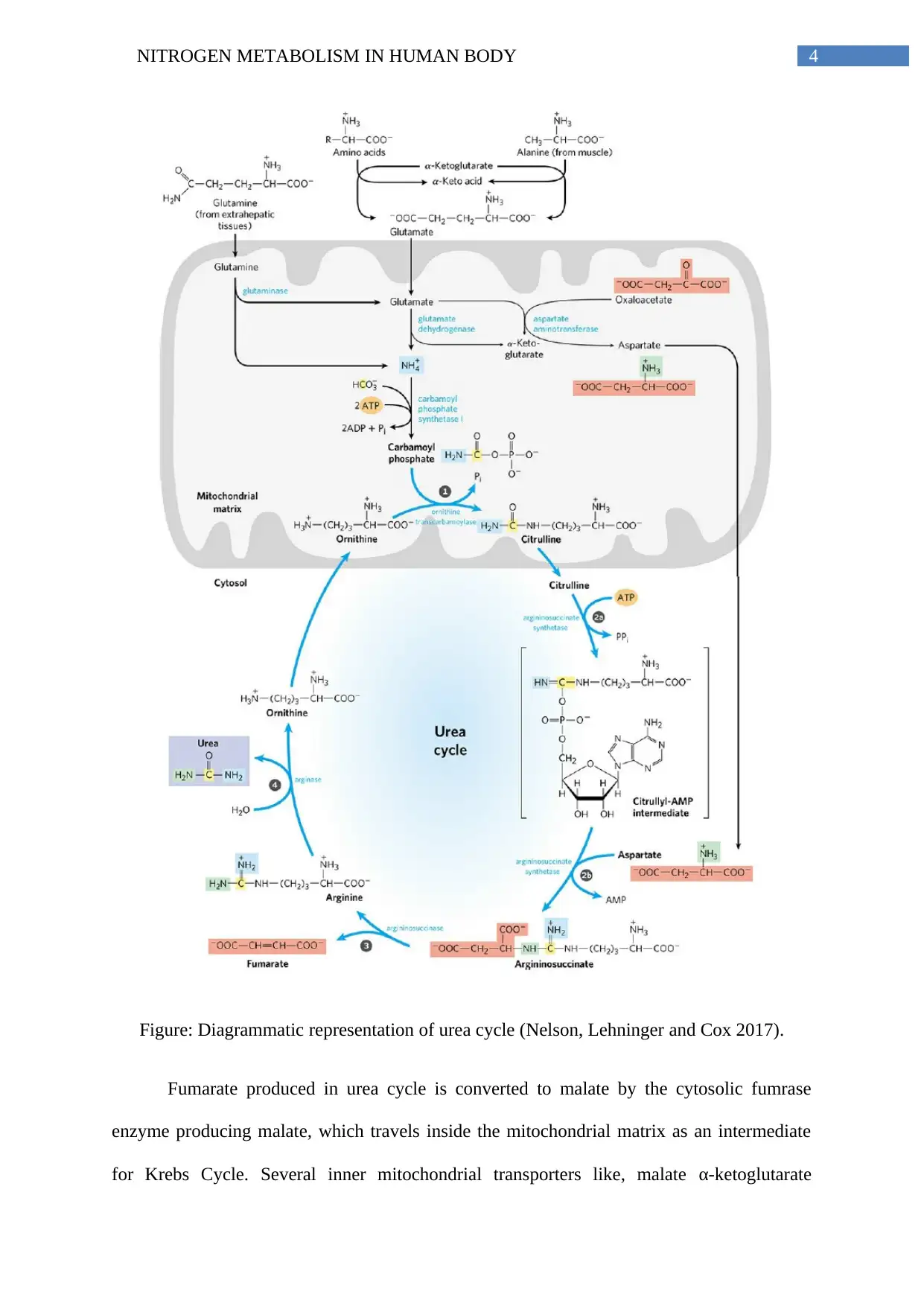
4NITROGEN METABOLISM IN HUMAN BODY
Figure: Diagrammatic representation of urea cycle (Nelson, Lehninger and Cox 2017).
Fumarate produced in urea cycle is converted to malate by the cytosolic fumrase
enzyme producing malate, which travels inside the mitochondrial matrix as an intermediate
for Krebs Cycle. Several inner mitochondrial transporters like, malate α-ketoglutarate
Figure: Diagrammatic representation of urea cycle (Nelson, Lehninger and Cox 2017).
Fumarate produced in urea cycle is converted to malate by the cytosolic fumrase
enzyme producing malate, which travels inside the mitochondrial matrix as an intermediate
for Krebs Cycle. Several inner mitochondrial transporters like, malate α-ketoglutarate

5NITROGEN METABOLISM IN HUMAN BODY
transporter, glutamate OH transporter, glutamate aspartate transporter facilitate transport of
malate and glutamate inside the mitochondrial matrix, along with movement of aspartate and
α-ketoglutarate into the cytosol. Aspartate produced by amination of oxaloacetate is
transported into the cytosol to produce argininosuccinate by glutamate aspartate transporter
and is linked via aspartate-argininosuccinate shunt, with the anabolic process of ATP
formation (Electron Transport Chain), as oxaloacetate is one of the key intermediates of
Krebs Cycle (Saheki et al. 2005). The NADH pool in the mitochondrial matrix is also
maintained by malate-aspartate shuttle pathway (LaNoue and Williamson 1971). Aspartate
produced in the mitochondrial matrix travels into the cytosol and undergoes deamination,
producing cytosolic oxaloacetate, which is reduced into malate with the subsequent oxidation
of NADH to NAD+. Malate is transported back in the mitochondrial matrix to feed the TCA
cycle, which is oxidized to oxaloacetate, with subsequent reduction of NAD+ to NADH
(Kornberg 2000). In this way, urea cycle aids in mitochondrial NADH concentration, which
is further utilized to produce ATP via Electron Transport Chain (Nelson, Lehninger and Cox
2017).
Figure: Inter-link between TCA/Krebs cycle and urea cycle (Nelson and Cox 2017)
transporter, glutamate OH transporter, glutamate aspartate transporter facilitate transport of
malate and glutamate inside the mitochondrial matrix, along with movement of aspartate and
α-ketoglutarate into the cytosol. Aspartate produced by amination of oxaloacetate is
transported into the cytosol to produce argininosuccinate by glutamate aspartate transporter
and is linked via aspartate-argininosuccinate shunt, with the anabolic process of ATP
formation (Electron Transport Chain), as oxaloacetate is one of the key intermediates of
Krebs Cycle (Saheki et al. 2005). The NADH pool in the mitochondrial matrix is also
maintained by malate-aspartate shuttle pathway (LaNoue and Williamson 1971). Aspartate
produced in the mitochondrial matrix travels into the cytosol and undergoes deamination,
producing cytosolic oxaloacetate, which is reduced into malate with the subsequent oxidation
of NADH to NAD+. Malate is transported back in the mitochondrial matrix to feed the TCA
cycle, which is oxidized to oxaloacetate, with subsequent reduction of NAD+ to NADH
(Kornberg 2000). In this way, urea cycle aids in mitochondrial NADH concentration, which
is further utilized to produce ATP via Electron Transport Chain (Nelson, Lehninger and Cox
2017).
Figure: Inter-link between TCA/Krebs cycle and urea cycle (Nelson and Cox 2017)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
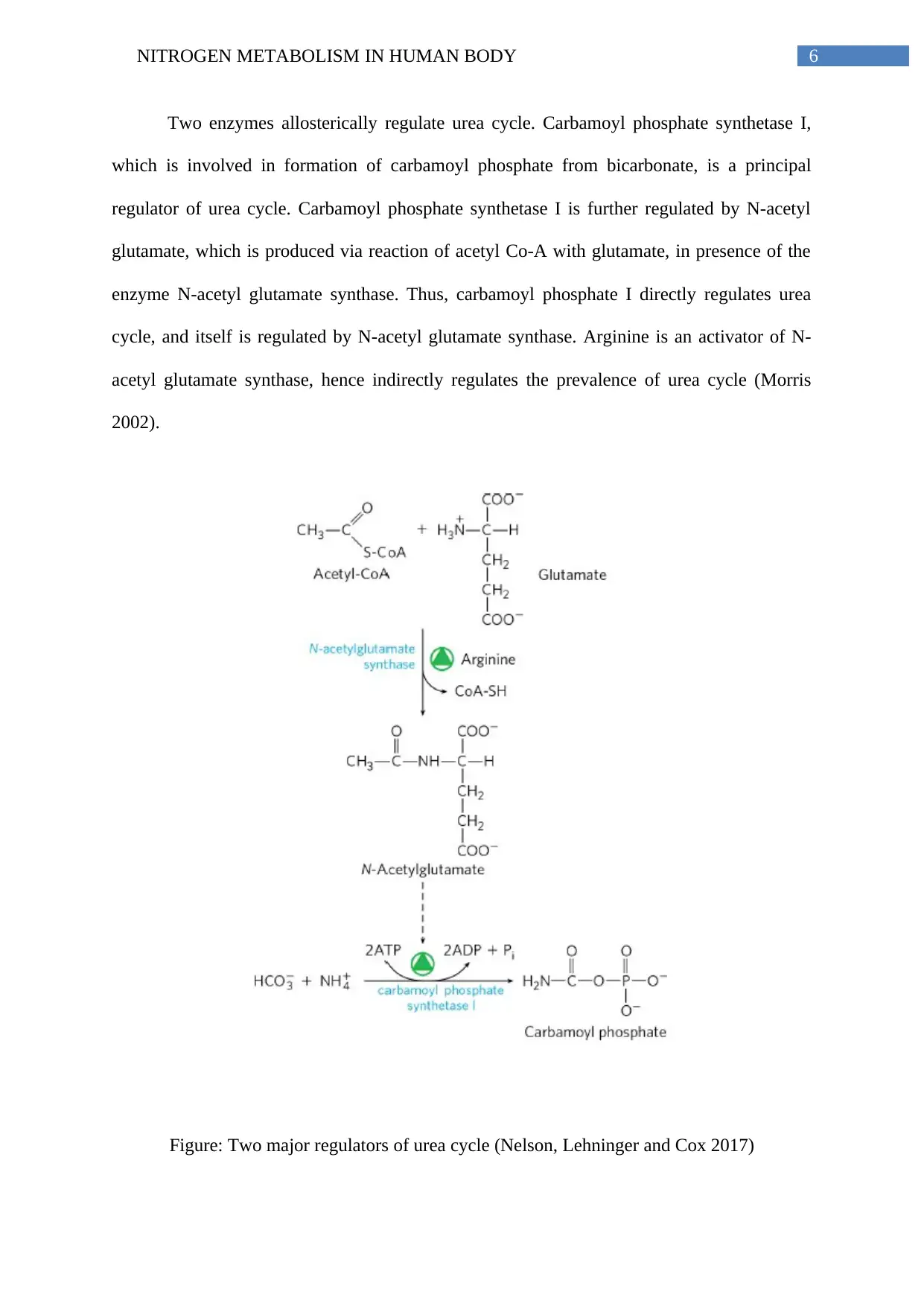
6NITROGEN METABOLISM IN HUMAN BODY
Two enzymes allosterically regulate urea cycle. Carbamoyl phosphate synthetase I,
which is involved in formation of carbamoyl phosphate from bicarbonate, is a principal
regulator of urea cycle. Carbamoyl phosphate synthetase I is further regulated by N-acetyl
glutamate, which is produced via reaction of acetyl Co-A with glutamate, in presence of the
enzyme N-acetyl glutamate synthase. Thus, carbamoyl phosphate I directly regulates urea
cycle, and itself is regulated by N-acetyl glutamate synthase. Arginine is an activator of N-
acetyl glutamate synthase, hence indirectly regulates the prevalence of urea cycle (Morris
2002).
Figure: Two major regulators of urea cycle (Nelson, Lehninger and Cox 2017)
Two enzymes allosterically regulate urea cycle. Carbamoyl phosphate synthetase I,
which is involved in formation of carbamoyl phosphate from bicarbonate, is a principal
regulator of urea cycle. Carbamoyl phosphate synthetase I is further regulated by N-acetyl
glutamate, which is produced via reaction of acetyl Co-A with glutamate, in presence of the
enzyme N-acetyl glutamate synthase. Thus, carbamoyl phosphate I directly regulates urea
cycle, and itself is regulated by N-acetyl glutamate synthase. Arginine is an activator of N-
acetyl glutamate synthase, hence indirectly regulates the prevalence of urea cycle (Morris
2002).
Figure: Two major regulators of urea cycle (Nelson, Lehninger and Cox 2017)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7NITROGEN METABOLISM IN HUMAN BODY
References
Barrett, K.E., Barman, S.M., Boitano, S. and Brooks, H., 2009. Ganong’s review of medical
physiology. 23. NY: McGraw-Hill Medical.
Bradford, N.M. and McGivan, J.D., 1973. Quantitative characteristics of glutamate transport
in rat liver mitochondria. Biochemical Journal, 134(4), pp.1023-1029.
Crews, T.E. and Peoples, M.B., 2004. Legume versus fertilizer sources of nitrogen:
ecological tradeoffs and human needs. Agriculture, ecosystems & environment, 102(3),
pp.279-297.
Kornberg, H., 2000. Krebs and his trinity of cycles. Nature Reviews Molecular Cell
Biology, 1(3), p.225.
LaNoue, K.F. and Williamson, J.R., 1971. Interrelationships between malate-aspartate shuttle
and citric acid cycle in rat heart mitochondria. Metabolism, 20(2), pp.119-140.
Leonard, J.V. and Morris, A.A.M., 2002, February. Urea cycle disorders. In Seminars in
neonatology (Vol. 7, No. 1, pp. 27-35). WB Saunders.
Liu, H., Dong, H., Robertson, K. and Liu, C., 2011. DNA methylation suppresses expression
of the urea cycle enzyme carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular
carcinoma. The American journal of pathology, 178(2), pp.652-661.
Morris Jr, S.M., 2002. Regulation of enzymes of the urea cycle and arginine
metabolism. Annual review of nutrition, 22(1), pp.87-105.
References
Barrett, K.E., Barman, S.M., Boitano, S. and Brooks, H., 2009. Ganong’s review of medical
physiology. 23. NY: McGraw-Hill Medical.
Bradford, N.M. and McGivan, J.D., 1973. Quantitative characteristics of glutamate transport
in rat liver mitochondria. Biochemical Journal, 134(4), pp.1023-1029.
Crews, T.E. and Peoples, M.B., 2004. Legume versus fertilizer sources of nitrogen:
ecological tradeoffs and human needs. Agriculture, ecosystems & environment, 102(3),
pp.279-297.
Kornberg, H., 2000. Krebs and his trinity of cycles. Nature Reviews Molecular Cell
Biology, 1(3), p.225.
LaNoue, K.F. and Williamson, J.R., 1971. Interrelationships between malate-aspartate shuttle
and citric acid cycle in rat heart mitochondria. Metabolism, 20(2), pp.119-140.
Leonard, J.V. and Morris, A.A.M., 2002, February. Urea cycle disorders. In Seminars in
neonatology (Vol. 7, No. 1, pp. 27-35). WB Saunders.
Liu, H., Dong, H., Robertson, K. and Liu, C., 2011. DNA methylation suppresses expression
of the urea cycle enzyme carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular
carcinoma. The American journal of pathology, 178(2), pp.652-661.
Morris Jr, S.M., 2002. Regulation of enzymes of the urea cycle and arginine
metabolism. Annual review of nutrition, 22(1), pp.87-105.

8NITROGEN METABOLISM IN HUMAN BODY
Nelson, D.L., Lehninger, A.L. and Cox, M.M., 2017. Lehninger principles of biochemistry.
Macmillan.
Saheki, T., Kobayashi, K., Iijima, M., Moriyama, M., Yazaki, M., Takei, Y.I. and Ikeda, S.I.,
2005. Metabolic derangements in deficiency of citrin, a liver-type mitochondrial aspartate–
glutamate carrier. Hepatology research, 33(2), pp.181-184.
Nelson, D.L., Lehninger, A.L. and Cox, M.M., 2017. Lehninger principles of biochemistry.
Macmillan.
Saheki, T., Kobayashi, K., Iijima, M., Moriyama, M., Yazaki, M., Takei, Y.I. and Ikeda, S.I.,
2005. Metabolic derangements in deficiency of citrin, a liver-type mitochondrial aspartate–
glutamate carrier. Hepatology research, 33(2), pp.181-184.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
