MED3ABS Clinical Drug Development: Huntington's Trial with PBT2
VerifiedAdded on 2023/06/15
|5
|1617
|491
Project
AI Summary
This document presents a clinical trial project focused on evaluating the efficacy and safety of a combination therapy involving PBT2 and Beraxotene for treating Huntington's Disease (HD). The study employs a randomized, double-blinded, placebo-controlled design over three years, involving 1000 patients divided into three groups: a placebo control, a low-dose drug group (10mg), and a high-dose drug group (100mg). The primary objective is to assess improvements in motor function and neuron survivability, measured by changes in the Unified Huntington's Disease Rating Scale (UHDRS) from baseline to the end of the study. Secondary outcomes include cognitive assessments using verbal fluency tests and the Mini-Mental State Examination (MMSE). The study also focuses on recruitment, adherence, and retention rates, with data analysis using descriptive statistics, covariance analysis, and intention-to-treat principles to compare interventions and control groups while controlling for confounding factors like age, gender and baseline UHDRS scores. Ethical considerations are addressed, adhering to international guidelines and ethical principles for medical research involving human subjects.

Section 4
4.1 Objectives
The primary objective is the combination of drug of PBT2 and Beraxotene being an
improvement in the motor function and neuron survivability. The primary outcomes of this
study would be the feasibility assessment, effectiveness and safety of the drug therapy. Safety
factor would be accessed through review of weekly health diaries and falls diaries among the
participants. This will include prior knowledge of falls, changes in medications and hospital
admissions. Diaries for recording would be provided to participants during baseline
assessment and returned monthly basis. Another primary outcome of the study would be the
measure of completion of assigned medication to assess its tolerability.
Further the primary objective of this study is to detremeine the influcen of the drugs
therapy compared to the placebo effect. Also primary outcome of interest will be change
from the baseline assessment to end of three years gaining total functional motor capacity.
This will seek establishment on the impact progression on the change on the total functional
capacity using UHDRS between baseline and three years.
The primary objective of evaluation is the recruitment, adherence rates and retention
of patients. The recruitment phase will be assessed under the use of site recruitment logs. The
retention rate of participants will be measured using the percentages of individuals who will
have completed the intervention. Adherence rates will be assessed using the percentages of
individual’s sessions which have been completed. In this case successful intervention rate
will be defined as at least 75% of the supervised sessions and 75% of other unsupervised
sessions, (Harrison et al., 2013).
Secondary outcome measure of motor function will be assessed using modified motor
score, a subset of UHDRS TMS, this has been chosen due to the specific focus of the
voluntary impairments. This component will assess dysarthria, tongue protrusion ,
bradykinesia and luria rigidity of arms. UHDRS is an analytical tool to assess motor function,
behavioural aspects, behavioural and functional ability. Outcomes in this case will involve
motor score, behavioural frequency, functional ability assessment and independent scale.
Cognitive assessment will be assessed using verbal fluency on tests. The MMSE being a
common validated tool, will be used for general purpose of cognitive assessment, in this case
lower score will signify impairment, (Busse et al., 2013).
4.2 Data Analysis
Descriptive data will be used to asses on the evaluation, eligibility , recruitment and
retention rates at 95% confidence levels. Graphical illustrations will be used to check the
4.1 Objectives
The primary objective is the combination of drug of PBT2 and Beraxotene being an
improvement in the motor function and neuron survivability. The primary outcomes of this
study would be the feasibility assessment, effectiveness and safety of the drug therapy. Safety
factor would be accessed through review of weekly health diaries and falls diaries among the
participants. This will include prior knowledge of falls, changes in medications and hospital
admissions. Diaries for recording would be provided to participants during baseline
assessment and returned monthly basis. Another primary outcome of the study would be the
measure of completion of assigned medication to assess its tolerability.
Further the primary objective of this study is to detremeine the influcen of the drugs
therapy compared to the placebo effect. Also primary outcome of interest will be change
from the baseline assessment to end of three years gaining total functional motor capacity.
This will seek establishment on the impact progression on the change on the total functional
capacity using UHDRS between baseline and three years.
The primary objective of evaluation is the recruitment, adherence rates and retention
of patients. The recruitment phase will be assessed under the use of site recruitment logs. The
retention rate of participants will be measured using the percentages of individuals who will
have completed the intervention. Adherence rates will be assessed using the percentages of
individual’s sessions which have been completed. In this case successful intervention rate
will be defined as at least 75% of the supervised sessions and 75% of other unsupervised
sessions, (Harrison et al., 2013).
Secondary outcome measure of motor function will be assessed using modified motor
score, a subset of UHDRS TMS, this has been chosen due to the specific focus of the
voluntary impairments. This component will assess dysarthria, tongue protrusion ,
bradykinesia and luria rigidity of arms. UHDRS is an analytical tool to assess motor function,
behavioural aspects, behavioural and functional ability. Outcomes in this case will involve
motor score, behavioural frequency, functional ability assessment and independent scale.
Cognitive assessment will be assessed using verbal fluency on tests. The MMSE being a
common validated tool, will be used for general purpose of cognitive assessment, in this case
lower score will signify impairment, (Busse et al., 2013).
4.2 Data Analysis
Descriptive data will be used to asses on the evaluation, eligibility , recruitment and
retention rates at 95% confidence levels. Graphical illustrations will be used to check the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

distribution associated with data outcome. The primary and secondary analyses will be made
comparable with among interventions and control groups. Covariance analysis will be used to
control for age factor, UHDRS, gender and baseline assessments. Analysis of the outcomes
will be assessed on the presumption of intention to treat basis.
Primary outcome measure will be measured on the change from the baseline
information to the end of the study period. This will be effected using the hunnington motor
scale on the UHDRS. Further safety measures and tolerability and adverse effects will be
assessed based on the assigned dosage. The primary tolerability will be assessed using
frequency and occurrence of adverse effects and lab results. The efficacy measures will be
measured based on the changes on baseline and monthly basis on UHDRS Mmse outcomes
will be compared on the treatment groups using repeated measures and covariance of analysis
as mentioned above. Analysis of the secondary outcome measures will be assessed on the
UHDRS tools further demographic characteristics such as medical history of the patients.
4.3 Treatment protocols
The recruitment period will be January 2017 to December 2017. Patients suffering
from Huntington disease will be enrolled in the study. Patients receiving clinical care and
attending assessments will be given trial information.
Screening of the participants will be done using screening log which records of
number of people who have been approached on the trial and eligibility. Blinding will be
conducted by blinded assessors. Site coordinators will be requested not to make any
disclosure on allocation of assessors. In order to manage confounding factors, incidence of
unbinding will be recorded.
Intervention group involve a control group who will be given placebo to imitate the
drug. Group two will be given a low dose drug while group three will be given high dose
drug. This will measure the level for drug effectiveness on managing Huntington disease
among the elderly. The three group of participants will entail administration of group one
being categorised as control group getting no treatment. Group tow will be given lose drug
therapy and group three will be given high drug therapy treatment therapy. These doses will
be administered on weekly basis and follow up visits done on monthly basis while
consultation will take place for three years. The consultation phase will entail physician
assessments using the UHDRS to measure the severity of the disease.
4.4 Study design
This study will adopt a randomised double blinded placebo controlled study. Patients
will be randomly assigned into the three groups whom they will receive placebo, low dose of
comparable with among interventions and control groups. Covariance analysis will be used to
control for age factor, UHDRS, gender and baseline assessments. Analysis of the outcomes
will be assessed on the presumption of intention to treat basis.
Primary outcome measure will be measured on the change from the baseline
information to the end of the study period. This will be effected using the hunnington motor
scale on the UHDRS. Further safety measures and tolerability and adverse effects will be
assessed based on the assigned dosage. The primary tolerability will be assessed using
frequency and occurrence of adverse effects and lab results. The efficacy measures will be
measured based on the changes on baseline and monthly basis on UHDRS Mmse outcomes
will be compared on the treatment groups using repeated measures and covariance of analysis
as mentioned above. Analysis of the secondary outcome measures will be assessed on the
UHDRS tools further demographic characteristics such as medical history of the patients.
4.3 Treatment protocols
The recruitment period will be January 2017 to December 2017. Patients suffering
from Huntington disease will be enrolled in the study. Patients receiving clinical care and
attending assessments will be given trial information.
Screening of the participants will be done using screening log which records of
number of people who have been approached on the trial and eligibility. Blinding will be
conducted by blinded assessors. Site coordinators will be requested not to make any
disclosure on allocation of assessors. In order to manage confounding factors, incidence of
unbinding will be recorded.
Intervention group involve a control group who will be given placebo to imitate the
drug. Group two will be given a low dose drug while group three will be given high dose
drug. This will measure the level for drug effectiveness on managing Huntington disease
among the elderly. The three group of participants will entail administration of group one
being categorised as control group getting no treatment. Group tow will be given lose drug
therapy and group three will be given high drug therapy treatment therapy. These doses will
be administered on weekly basis and follow up visits done on monthly basis while
consultation will take place for three years. The consultation phase will entail physician
assessments using the UHDRS to measure the severity of the disease.
4.4 Study design
This study will adopt a randomised double blinded placebo controlled study. Patients
will be randomly assigned into the three groups whom they will receive placebo, low dose of
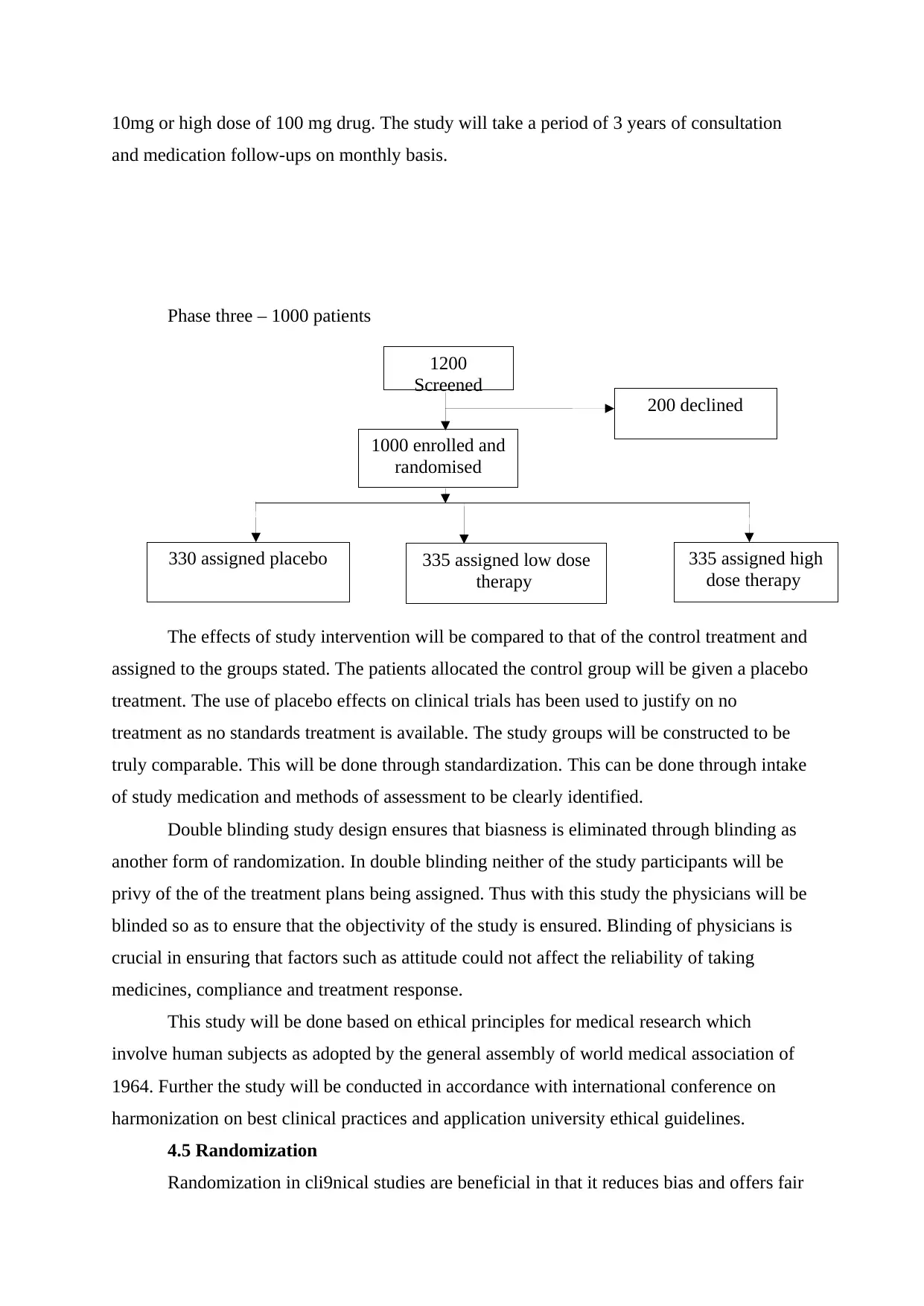
1200
Screened
1000 enrolled and
randomised
200 declined
330 assigned placebo 335 assigned low dose
therapy
335 assigned high
dose therapy
10mg or high dose of 100 mg drug. The study will take a period of 3 years of consultation
and medication follow-ups on monthly basis.
Phase three – 1000 patients
The effects of study intervention will be compared to that of the control treatment and
assigned to the groups stated. The patients allocated the control group will be given a placebo
treatment. The use of placebo effects on clinical trials has been used to justify on no
treatment as no standards treatment is available. The study groups will be constructed to be
truly comparable. This will be done through standardization. This can be done through intake
of study medication and methods of assessment to be clearly identified.
Double blinding study design ensures that biasness is eliminated through blinding as
another form of randomization. In double blinding neither of the study participants will be
privy of the of the treatment plans being assigned. Thus with this study the physicians will be
blinded so as to ensure that the objectivity of the study is ensured. Blinding of physicians is
crucial in ensuring that factors such as attitude could not affect the reliability of taking
medicines, compliance and treatment response.
This study will be done based on ethical principles for medical research which
involve human subjects as adopted by the general assembly of world medical association of
1964. Further the study will be conducted in accordance with international conference on
harmonization on best clinical practices and application university ethical guidelines.
4.5 Randomization
Randomization in cli9nical studies are beneficial in that it reduces bias and offers fair
Screened
1000 enrolled and
randomised
200 declined
330 assigned placebo 335 assigned low dose
therapy
335 assigned high
dose therapy
10mg or high dose of 100 mg drug. The study will take a period of 3 years of consultation
and medication follow-ups on monthly basis.
Phase three – 1000 patients
The effects of study intervention will be compared to that of the control treatment and
assigned to the groups stated. The patients allocated the control group will be given a placebo
treatment. The use of placebo effects on clinical trials has been used to justify on no
treatment as no standards treatment is available. The study groups will be constructed to be
truly comparable. This will be done through standardization. This can be done through intake
of study medication and methods of assessment to be clearly identified.
Double blinding study design ensures that biasness is eliminated through blinding as
another form of randomization. In double blinding neither of the study participants will be
privy of the of the treatment plans being assigned. Thus with this study the physicians will be
blinded so as to ensure that the objectivity of the study is ensured. Blinding of physicians is
crucial in ensuring that factors such as attitude could not affect the reliability of taking
medicines, compliance and treatment response.
This study will be done based on ethical principles for medical research which
involve human subjects as adopted by the general assembly of world medical association of
1964. Further the study will be conducted in accordance with international conference on
harmonization on best clinical practices and application university ethical guidelines.
4.5 Randomization
Randomization in cli9nical studies are beneficial in that it reduces bias and offers fair
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

treatment of prognosis and not based on individual prognostic patients characteristics. Further
in this study randomization will reduce the effects of confounding factors. In this study
randomisation of the study participants will be done the three balanced groups by offering
treatment and having control group. In RCT designs patients are often randomised and
assigned different treatment groups. This is to ensure that confounding factors are equally
divided among the different groups in the study. Randomization of study participants’ trial
randomization will be performed during the baseline assessment. Group assignment will be
relayed to the respective site coordinators, who will be able to communicate and inform the
participant on the complete ion of the baseline survey. Randomization ratio will be used in
the ration of 1:1 on eligible participants. This will be aided by computer generated block
randomization plan. Minimization procedure will be effective as a way of achieving balance
amongst the groups. Variables which will be used are age, site, gender and UHDRS TMS. A
masked randomization code will be utilised so as to hide exposure and privy knowledge of
the study. These confounding factors must be controlled as they can affect the patient
treatment phase. Thus randomization in this case will be of essence in ensuring that statistical
analysis of covariance is done.
Participants will be stratified in form of gender so as to ensure an equal number of
male and female participants. Equal balancing of the groups will be done so as to ensure that
there is equal treatment of participants in control and experimental group.
Reference
Harrison, D.J., Busse, M., Openshaw, R., Rosser, A.E., Dunnett, S.B. and Brooks, S.P., 2013.
Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in
the R6/1 Huntington's disease mouse model. Experimental neurology, 248, pp.457-469.
Busse, M., Quinn, L., Debono, K., Jones, K., Collett, J., Playle, R., Kelly, M., Simpson, S.,
Backx, K., Wasley, D. and Dawes, H., 2013. A randomized feasibility study of a 12-week
community-based exercise program for people with Huntington's disease. Journal of
Neurologic Physical Therapy, 37(4), pp.149-158.
in this study randomization will reduce the effects of confounding factors. In this study
randomisation of the study participants will be done the three balanced groups by offering
treatment and having control group. In RCT designs patients are often randomised and
assigned different treatment groups. This is to ensure that confounding factors are equally
divided among the different groups in the study. Randomization of study participants’ trial
randomization will be performed during the baseline assessment. Group assignment will be
relayed to the respective site coordinators, who will be able to communicate and inform the
participant on the complete ion of the baseline survey. Randomization ratio will be used in
the ration of 1:1 on eligible participants. This will be aided by computer generated block
randomization plan. Minimization procedure will be effective as a way of achieving balance
amongst the groups. Variables which will be used are age, site, gender and UHDRS TMS. A
masked randomization code will be utilised so as to hide exposure and privy knowledge of
the study. These confounding factors must be controlled as they can affect the patient
treatment phase. Thus randomization in this case will be of essence in ensuring that statistical
analysis of covariance is done.
Participants will be stratified in form of gender so as to ensure an equal number of
male and female participants. Equal balancing of the groups will be done so as to ensure that
there is equal treatment of participants in control and experimental group.
Reference
Harrison, D.J., Busse, M., Openshaw, R., Rosser, A.E., Dunnett, S.B. and Brooks, S.P., 2013.
Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in
the R6/1 Huntington's disease mouse model. Experimental neurology, 248, pp.457-469.
Busse, M., Quinn, L., Debono, K., Jones, K., Collett, J., Playle, R., Kelly, M., Simpson, S.,
Backx, K., Wasley, D. and Dawes, H., 2013. A randomized feasibility study of a 12-week
community-based exercise program for people with Huntington's disease. Journal of
Neurologic Physical Therapy, 37(4), pp.149-158.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1 out of 5
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



