Hydrocarbons Essay: Alkanes, Alkenes, and Reactivity Differences
VerifiedAdded on 2021/02/20
|9
|1681
|37
Essay
AI Summary
This essay delves into the significance of hydrocarbons in human life, emphasizing their role in various applications while acknowledging their environmental impact, particularly global warming. It explores key concepts like structural formulas, chain isomerism, and homologous series, providing examples such as butane and pentane. The essay further examines chemical reactions involving hydrocarbons, including combustion, chlorination, and cracking, highlighting the differences in reactivity between alkanes and alkenes. It also covers the importance of polymerization reactions of alkenes. The essay concludes by stressing the importance of understanding these fundamental concepts for a deeper understanding of organic chemistry, emphasizing the role of bonding and reactivity in hydrocarbons and the need to understand the basic concepts like homologous series, functional groups, reactivity scales and more to gain accurate knowledge.

HYDROCARBONS
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TABLE OF CONTENTS
ESSAY.............................................................................................................................................1
REFERENCES................................................................................................................................7
ESSAY.............................................................................................................................................1
REFERENCES................................................................................................................................7

ESSAY
The role of Hydrocarbons is one of the biggest blessing to the human life. It has laid the
foundation for the survival with using the organic compounds to the maximum ways. However,
before using them in real life, one must gain an understanding to maintain the balance of life. For
example, rubber is a useful invention and has proved as a blessing to the humans for the survival
on the earth.
The importance of everyday uses of organic compounds has led to radical changes in the
evolution of human life. These are vital for the functioning of humans as these compounds are
formed from carbon and hydrogen with small amount of nitrogen or oxygen. They play role in
nutrition in the form of vitamins, proteins, lipids and carbohydrates (Parker and Tyedmers,
2015). It also assists through formation of several compounds such as dyes, detergents, food
additives, medicines, plastics etc. that are beneficial in daily activities.
In regard to this, the environmental impact of the global hydrocarbon consumption has
resulted into global warming and fluctuations in the climatic conditions. The coral reefs are
dying and the oceanic currents are changing their directions which is impacting the whole world
adversely. The carbon footprint is high in almost all the countries which together increasing the
emission of CO2 & CH4. For instance, combustion of fossil fuels and using petroleum products
added to the global consumption. These contributed to air pollution and also led to generation of
greenhouse gases (GHG) in high levels, which has an influence on the global warming and
melting of icebergs.
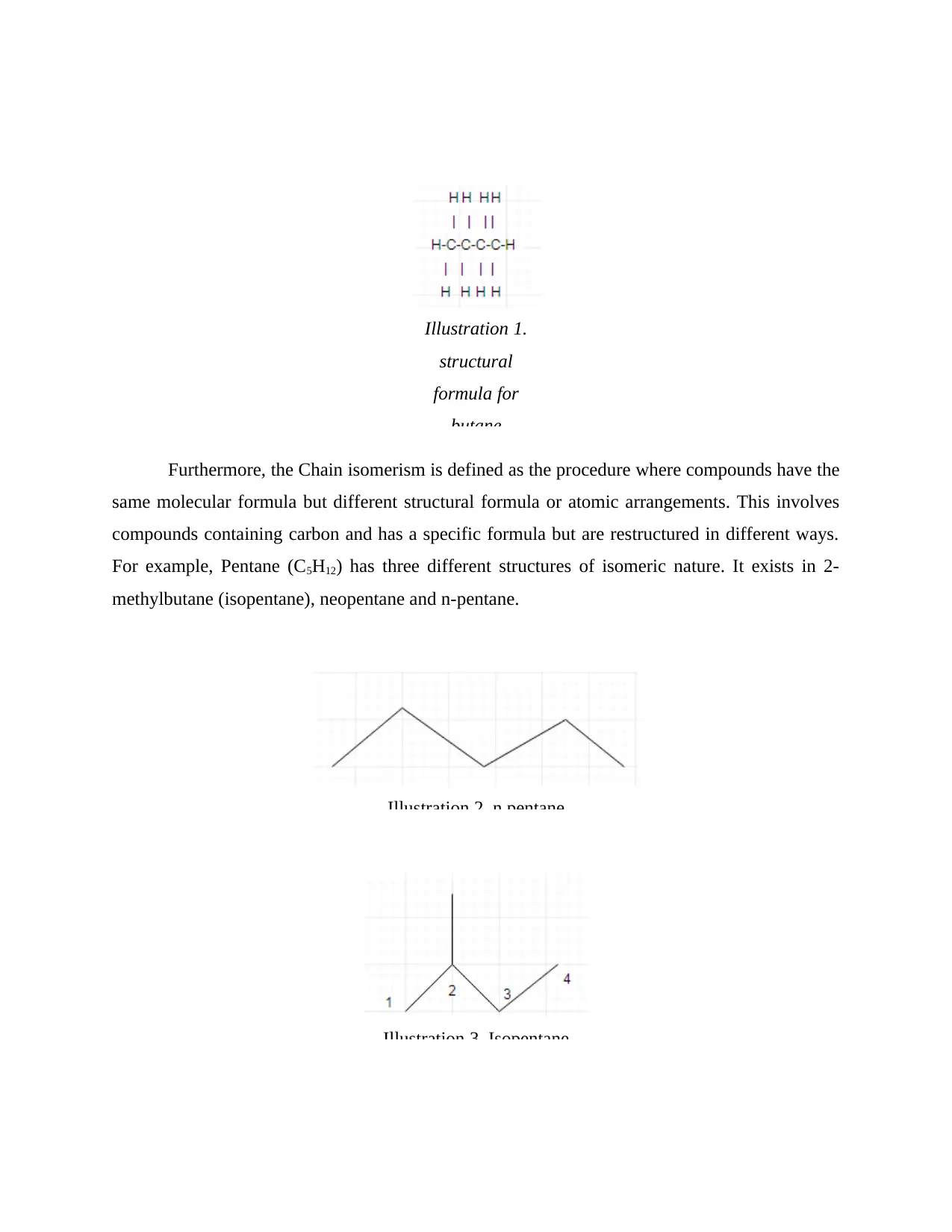
Next is the structural formula for butane with at least 4 carbons is as follows:
The role of Hydrocarbons is one of the biggest blessing to the human life. It has laid the
foundation for the survival with using the organic compounds to the maximum ways. However,
before using them in real life, one must gain an understanding to maintain the balance of life. For
example, rubber is a useful invention and has proved as a blessing to the humans for the survival
on the earth.
The importance of everyday uses of organic compounds has led to radical changes in the
evolution of human life. These are vital for the functioning of humans as these compounds are
formed from carbon and hydrogen with small amount of nitrogen or oxygen. They play role in
nutrition in the form of vitamins, proteins, lipids and carbohydrates (Parker and Tyedmers,
2015). It also assists through formation of several compounds such as dyes, detergents, food
additives, medicines, plastics etc. that are beneficial in daily activities.
In regard to this, the environmental impact of the global hydrocarbon consumption has
resulted into global warming and fluctuations in the climatic conditions. The coral reefs are
dying and the oceanic currents are changing their directions which is impacting the whole world
adversely. The carbon footprint is high in almost all the countries which together increasing the
emission of CO2 & CH4. For instance, combustion of fossil fuels and using petroleum products
added to the global consumption. These contributed to air pollution and also led to generation of
greenhouse gases (GHG) in high levels, which has an influence on the global warming and
melting of icebergs.
Next is the structural formula for butane with at least 4 carbons is as follows:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
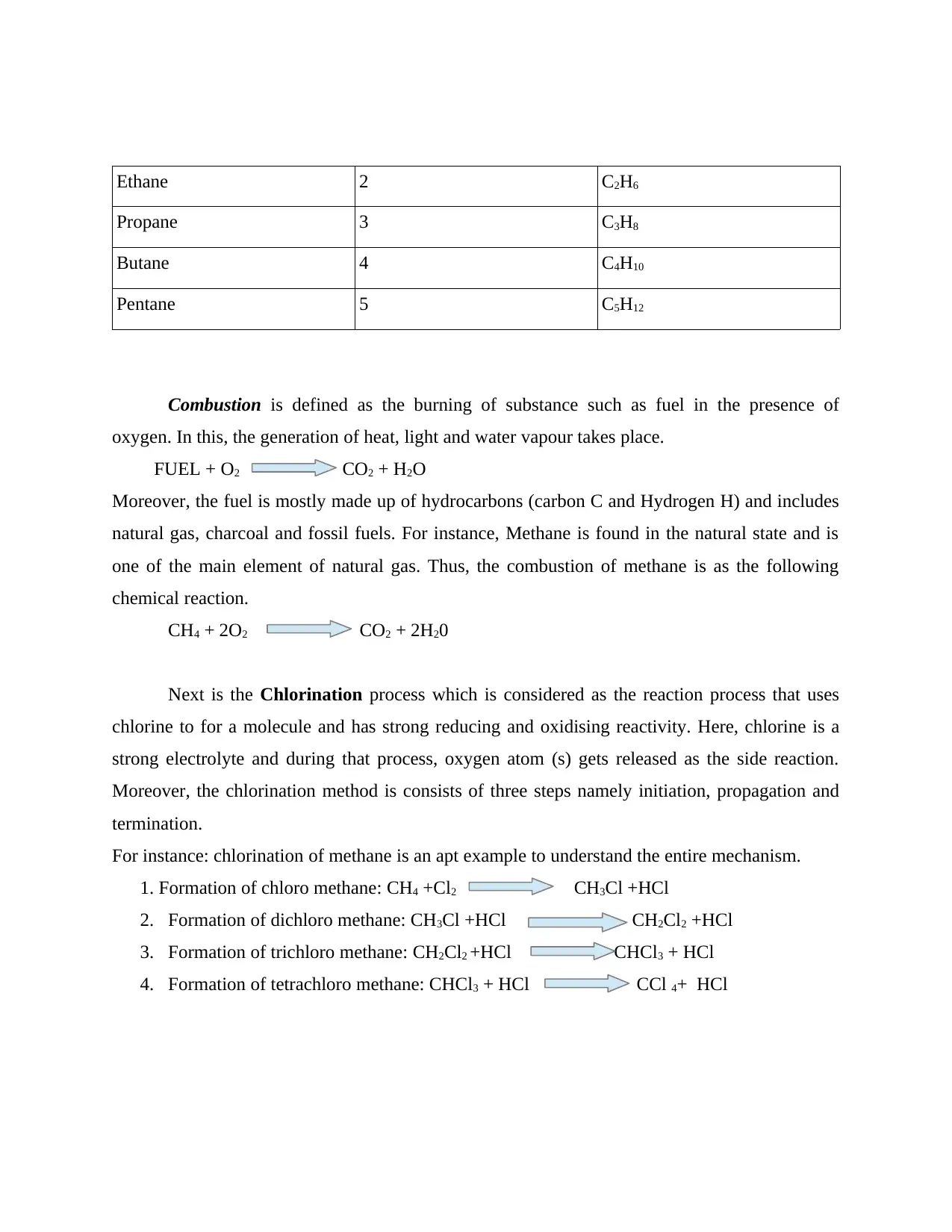
Furthermore, the Chain isomerism is defined as the procedure where compounds have the
same molecular formula but different structural formula or atomic arrangements. This involves
compounds containing carbon and has a specific formula but are restructured in different ways.
For example, Pentane (C5H12) has three different structures of isomeric nature. It exists in 2-
methylbutane (isopentane), neopentane and n-pentane.
Illustration 2. n pentane
Illustration 3. Isopentane
Illustration 1.
structural
formula for
butane
same molecular formula but different structural formula or atomic arrangements. This involves
compounds containing carbon and has a specific formula but are restructured in different ways.
For example, Pentane (C5H12) has three different structures of isomeric nature. It exists in 2-
methylbutane (isopentane), neopentane and n-pentane.
Illustration 2. n pentane
Illustration 3. Isopentane
Illustration 1.
structural
formula for
butane
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Homologous series is refereed as the list of compounds having same physical properties,
chemical properties and functional group. The characteristics include a common general formula
which is different due to a CH2 group. The significance of the term ‘homologous series’ is to
understand about the organic compounds to be examined. The functional groups are same in this
series which indicates towards the similar nature of physical and chemical properties. Moreover,
it predicts the nature, reactive status, state of matter etc. of the organic compounds to be studied
(Schwarzenbach and Gschwend, 2016). This also emphasis on gaining knowledge about the rate
of reaction to find the intermediate states and also reflect upon the reactivity levels. In addition,
the dependency is also determined through knowing the values for the physical properties of
compounds. This involves MP (melting point), BP (boiling point), Solubility etc. that further
support in preparation methods. Nevertheless, it also gives basic teaching about the chemistry of
compounds by using the functional group. Along with this, the alkyl group reflects upon the
physical properties of compounds to understand the opposing nature and restricted properties.
The following table is an example for homologous series for ALKANE with molecular formula
CnH2n+2
Name of Alkane Number of carbon atoms Chemical Formula
Methane 1 CH4
Illustration 4.
Neopentane
chemical properties and functional group. The characteristics include a common general formula
which is different due to a CH2 group. The significance of the term ‘homologous series’ is to
understand about the organic compounds to be examined. The functional groups are same in this
series which indicates towards the similar nature of physical and chemical properties. Moreover,
it predicts the nature, reactive status, state of matter etc. of the organic compounds to be studied
(Schwarzenbach and Gschwend, 2016). This also emphasis on gaining knowledge about the rate
of reaction to find the intermediate states and also reflect upon the reactivity levels. In addition,
the dependency is also determined through knowing the values for the physical properties of
compounds. This involves MP (melting point), BP (boiling point), Solubility etc. that further
support in preparation methods. Nevertheless, it also gives basic teaching about the chemistry of
compounds by using the functional group. Along with this, the alkyl group reflects upon the
physical properties of compounds to understand the opposing nature and restricted properties.
The following table is an example for homologous series for ALKANE with molecular formula
CnH2n+2
Name of Alkane Number of carbon atoms Chemical Formula
Methane 1 CH4
Illustration 4.
Neopentane
Ethane 2 C2H6
Propane 3 C3H8
Butane 4 C4H10
Pentane 5 C5H12
Combustion is defined as the burning of substance such as fuel in the presence of
oxygen. In this, the generation of heat, light and water vapour takes place.
FUEL + O2 CO2 + H2O
Moreover, the fuel is mostly made up of hydrocarbons (carbon C and Hydrogen H) and includes
natural gas, charcoal and fossil fuels. For instance, Methane is found in the natural state and is
one of the main element of natural gas. Thus, the combustion of methane is as the following
chemical reaction.
CH4 + 2O2 CO2 + 2H20
Next is the Chlorination process which is considered as the reaction process that uses
chlorine to for a molecule and has strong reducing and oxidising reactivity. Here, chlorine is a
strong electrolyte and during that process, oxygen atom (s) gets released as the side reaction.
Moreover, the chlorination method is consists of three steps namely initiation, propagation and
termination.
For instance: chlorination of methane is an apt example to understand the entire mechanism.
1. Formation of chloro methane: CH4 +Cl2 CH3Cl +HCl
2. Formation of dichloro methane: CH3Cl +HCl CH2Cl2 +HCl
3. Formation of trichloro methane: CH2Cl2 +HCl CHCl3 + HCl
4. Formation of tetrachloro methane: CHCl3 + HCl CCl 4+ HCl
Propane 3 C3H8
Butane 4 C4H10
Pentane 5 C5H12
Combustion is defined as the burning of substance such as fuel in the presence of
oxygen. In this, the generation of heat, light and water vapour takes place.
FUEL + O2 CO2 + H2O
Moreover, the fuel is mostly made up of hydrocarbons (carbon C and Hydrogen H) and includes
natural gas, charcoal and fossil fuels. For instance, Methane is found in the natural state and is
one of the main element of natural gas. Thus, the combustion of methane is as the following
chemical reaction.
CH4 + 2O2 CO2 + 2H20
Next is the Chlorination process which is considered as the reaction process that uses
chlorine to for a molecule and has strong reducing and oxidising reactivity. Here, chlorine is a
strong electrolyte and during that process, oxygen atom (s) gets released as the side reaction.
Moreover, the chlorination method is consists of three steps namely initiation, propagation and
termination.
For instance: chlorination of methane is an apt example to understand the entire mechanism.
1. Formation of chloro methane: CH4 +Cl2 CH3Cl +HCl
2. Formation of dichloro methane: CH3Cl +HCl CH2Cl2 +HCl
3. Formation of trichloro methane: CH2Cl2 +HCl CHCl3 + HCl
4. Formation of tetrachloro methane: CHCl3 + HCl CCl 4+ HCl
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

In addition to this, cracking is the procedure where the breakage of long and complicated
chains of hydrocarbons are transformed into simpler molecules. The focus is on breakage of
carbon-carbon (C-C) bonds. For example, Hexane can be cracked into simpler substances named
ethene and butane.
C6H14 C4H10 + C2H4
In regard to the above, the alkanes are relatively unreactive and the reason behind this is
the presence of single bond which is stronger in nature. It also gets difficult to break and the
electronegativity is non polar in nature. The carbon and hydrogen both gives molecules with non
polarity. For instance, C-C bond is unpolar and has no charges that indicated towards high levels
of saturation across the carbon atoms. However, the activation energies are high and also C-H
bond is covalent in nature and having low dipole moment.
The nature of the double bond is that chemical bond which demonstrate presence of two
electron pairs and are reactive in nature. These are shorter and are stronger than the single bonds.
Primarily, it is found in the homologous series of alkenes where the double bond is between two
carbon atoms. From this, it is clearly inferred that energy requirement to break the bonded atoms
is more and the rotation is also restricted. For e.g. C2H4 is called as ethylene.
Furthermore, the differences in reactivity between an alkane and an alkene depends on
the bonds sharing between the two carbon atoms. The alkane consists of C-C π bond that is weak
in nature and are less reactive than alkenes and immediately form single bonds only. On the
other hand, alkenes have sigma bondage where the electrons are half way and share small space
which makes it difficult to break the bond.
Alkenes undergo addition reactions because of the presence of two or more reactants to
give a single product and takes place in the unsaturated compounds. It is understandable that
alkenes are unstable in structure when compared to alkanes and thus, it undergoes breakage off
chains of hydrocarbons are transformed into simpler molecules. The focus is on breakage of
carbon-carbon (C-C) bonds. For example, Hexane can be cracked into simpler substances named
ethene and butane.
C6H14 C4H10 + C2H4
In regard to the above, the alkanes are relatively unreactive and the reason behind this is
the presence of single bond which is stronger in nature. It also gets difficult to break and the
electronegativity is non polar in nature. The carbon and hydrogen both gives molecules with non
polarity. For instance, C-C bond is unpolar and has no charges that indicated towards high levels
of saturation across the carbon atoms. However, the activation energies are high and also C-H
bond is covalent in nature and having low dipole moment.
The nature of the double bond is that chemical bond which demonstrate presence of two
electron pairs and are reactive in nature. These are shorter and are stronger than the single bonds.
Primarily, it is found in the homologous series of alkenes where the double bond is between two
carbon atoms. From this, it is clearly inferred that energy requirement to break the bonded atoms
is more and the rotation is also restricted. For e.g. C2H4 is called as ethylene.
Furthermore, the differences in reactivity between an alkane and an alkene depends on
the bonds sharing between the two carbon atoms. The alkane consists of C-C π bond that is weak
in nature and are less reactive than alkenes and immediately form single bonds only. On the
other hand, alkenes have sigma bondage where the electrons are half way and share small space
which makes it difficult to break the bond.
Alkenes undergo addition reactions because of the presence of two or more reactants to
give a single product and takes place in the unsaturated compounds. It is understandable that
alkenes are unstable in structure when compared to alkanes and thus, it undergoes breakage off
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

bonds by using large amount of energy. Henceforth, the alkenes are undergoing the addition
reaction in the presence of hydrogen and with the help of a catalyst to form a compound of lower
bond (alkane). For example, hydrohalogenation is the process where alkene reacts with hydrogen
atom and halogen atom as well.
CH2=CH2 + HCl C2H5Cl
Here, bromine water can be used to test for an alkene due to the reaction between them.
The bromine has the capacity to break the double bonds and leads towards the breakage of
unsaturated hydrocarbons. The end result is the de-colourisation from a yellowish to colourless
when reacted with bromine at the room temperature. This is done using a test tube and comes
under the qualitative test when unsaturated compounds are involved. Here more unsaturated an
unknown compound is, more the reactive reaction takes place.
The importance of polymerisation reactions of alkenes help in successive addition of
monomers to form polymers that proved beneficial and led to the formation of substances. Its
advantages involves several uses that shed light on manufacturing of polythene, styrene,
synthetic rubber and more. Such diverse forms are helpful in the manufacturing sector to utilise
these products effectively. In addition, the low concentration levels of ethene that are used for
fruits ripening and help the humans in more wholesome ways. These are adding significant
changes to use the organic compounds and produce good products or materials.
To sum up, the organic chemistry is too large to understand the deeper meaning behind
the carbon bonds and the relationship. Moreover, it is important to know about the basic
concepts like homologous series, functional groups, reactivity scales and more to gain accurate
knowledge. The best way to understand the conceptual frameworks of organic chemistry is to
practice and understand the role of bonding and reactivity regarding the hydrocarbons for better
clarity.
reaction in the presence of hydrogen and with the help of a catalyst to form a compound of lower
bond (alkane). For example, hydrohalogenation is the process where alkene reacts with hydrogen
atom and halogen atom as well.
CH2=CH2 + HCl C2H5Cl
Here, bromine water can be used to test for an alkene due to the reaction between them.
The bromine has the capacity to break the double bonds and leads towards the breakage of
unsaturated hydrocarbons. The end result is the de-colourisation from a yellowish to colourless
when reacted with bromine at the room temperature. This is done using a test tube and comes
under the qualitative test when unsaturated compounds are involved. Here more unsaturated an
unknown compound is, more the reactive reaction takes place.
The importance of polymerisation reactions of alkenes help in successive addition of
monomers to form polymers that proved beneficial and led to the formation of substances. Its
advantages involves several uses that shed light on manufacturing of polythene, styrene,
synthetic rubber and more. Such diverse forms are helpful in the manufacturing sector to utilise
these products effectively. In addition, the low concentration levels of ethene that are used for
fruits ripening and help the humans in more wholesome ways. These are adding significant
changes to use the organic compounds and produce good products or materials.
To sum up, the organic chemistry is too large to understand the deeper meaning behind
the carbon bonds and the relationship. Moreover, it is important to know about the basic
concepts like homologous series, functional groups, reactivity scales and more to gain accurate
knowledge. The best way to understand the conceptual frameworks of organic chemistry is to
practice and understand the role of bonding and reactivity regarding the hydrocarbons for better
clarity.

REFERENCES
Books and Journals
Parker, R.W. and Tyedmers, P.H., 2015. Fuel consumption of global fishing fleets: current
understanding and knowledge gaps. Fish and Fisheries. 16(4), pp.684-696.
Schwarzenbach, R.P. and Gschwend, P.M., 2016. Environmental organic chemistry. John Wiley
& Sons.
Books and Journals
Parker, R.W. and Tyedmers, P.H., 2015. Fuel consumption of global fishing fleets: current
understanding and knowledge gaps. Fish and Fisheries. 16(4), pp.684-696.
Schwarzenbach, R.P. and Gschwend, P.M., 2016. Environmental organic chemistry. John Wiley
& Sons.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





