Hydrogen Cyanide (HCN) Content in Cassava: Effects, Reduction Review
VerifiedAdded on 2022/04/26
|28
|9835
|45
Report
AI Summary
This report delves into the critical issue of hydrogen cyanide (HCN) content in cassava, a staple food for millions. It begins by establishing cassava's importance and the challenges posed by cyanogenic glycosides, which release HCN. The report reviews the sources and properties of HCN, highlighting its toxicity and mechanism of action, emphasizing its interference with cellular respiration. It then explores the presence of cyanogenic glycosides in various plant species, with a focus on cassava. The report discusses the roles of cyanogenic glycosides in plants, including their function as defense mechanisms. The study's aim is to review the impact of HCN consumption and the processes used to reduce its content in cassava. The report covers the effect of HCN consumption in the body and examines the methods to reduce HCN content in cassava before consumption.

CHAPTER ONE
INTRODUCTION
1.1 Background to the Study
Cassava (Manihot esculenta Crantz) is an important tropical root crop providing energy
to about 500 million people (Otusola, 2011). Almost all the cassava produced is used for human
consumption and less than 5 percent is used in industries. As a food crop, cassava fits well into
the farming systems of the smallholder farmers in Nigeria because it is available all year round,
thus providing household food security. Compared to grains, cassava is more tolerant to low soil
fertility and more resistant to drought, pests and diseases. Furthermore, its roots store well in the
ground for months after maturity. Cassava is important, not just as a food crop but even more so
as a major source of cash income for producing households. As a cash crop, cassava generates
cash income for the largest number of households, in comparison with other staples, contributing
positively to poverty alleviation. The presence of cyanogenic glycosides in cassava which when
broken down through enzymatic reaction librates hydrogen cyanide poses a great concern in
cassava utilization as food and as industrial raw material.
Cyanide, is usually found in compounds. It can interact with metals and other organic
compounds. Cyanide refers to all of the cyanide compounds that can be determined as the
cyanide ion, CN. The cyanide ion is a conjugate base of a weak acid, hydrogen cyanide, which is
an extremely poisonous gas with an almond odor. Other forms of cyanide compounds are sodium
cyanide (NaCN) and potassium cyanide (KCN). Cyanide can be produced by certain organism
(e.g bacteria, fungi and algae), and equally present in plants. Cyanide ion is one of the most
rapidly working poisons. Lethal doses taken orally act in minutes, cyanide, poisons by
asphyxiation, as does carbon monoxide, but the mechanism is different. Instead of preventing the
cells from getting oxygen, cyanide interferes with oxidative enzymes, such as cytochrome
oxidize, which is vital to every cell in use of oxygen. Oxidizes are enzymes containing metal
usually iron or copper. Cyanide binds tightly to the enzyme cytochrome C and forms stable
cyanide complexes with Fe3+ ion and inactivates the enzyme system.
1
INTRODUCTION
1.1 Background to the Study
Cassava (Manihot esculenta Crantz) is an important tropical root crop providing energy
to about 500 million people (Otusola, 2011). Almost all the cassava produced is used for human
consumption and less than 5 percent is used in industries. As a food crop, cassava fits well into
the farming systems of the smallholder farmers in Nigeria because it is available all year round,
thus providing household food security. Compared to grains, cassava is more tolerant to low soil
fertility and more resistant to drought, pests and diseases. Furthermore, its roots store well in the
ground for months after maturity. Cassava is important, not just as a food crop but even more so
as a major source of cash income for producing households. As a cash crop, cassava generates
cash income for the largest number of households, in comparison with other staples, contributing
positively to poverty alleviation. The presence of cyanogenic glycosides in cassava which when
broken down through enzymatic reaction librates hydrogen cyanide poses a great concern in
cassava utilization as food and as industrial raw material.
Cyanide, is usually found in compounds. It can interact with metals and other organic
compounds. Cyanide refers to all of the cyanide compounds that can be determined as the
cyanide ion, CN. The cyanide ion is a conjugate base of a weak acid, hydrogen cyanide, which is
an extremely poisonous gas with an almond odor. Other forms of cyanide compounds are sodium
cyanide (NaCN) and potassium cyanide (KCN). Cyanide can be produced by certain organism
(e.g bacteria, fungi and algae), and equally present in plants. Cyanide ion is one of the most
rapidly working poisons. Lethal doses taken orally act in minutes, cyanide, poisons by
asphyxiation, as does carbon monoxide, but the mechanism is different. Instead of preventing the
cells from getting oxygen, cyanide interferes with oxidative enzymes, such as cytochrome
oxidize, which is vital to every cell in use of oxygen. Oxidizes are enzymes containing metal
usually iron or copper. Cyanide binds tightly to the enzyme cytochrome C and forms stable
cyanide complexes with Fe3+ ion and inactivates the enzyme system.
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Much of the cyanide in soil, water and air comes from industrial processes gold mining,
waste waters from starch industry. The major source of cyanide in water are discharges from
metal mining processes, other sources include exhaust, release from certain chemical industries,
municipal waste burning and use of pesticides containing cyanide. Underground water can be
contaminated by cyanide present in landfills. In other body, cyanide can combines with plants
foods including almonds, millet sprouts, lima beans, soy spinach, bamboo shoots and cassava
roots, cyanide occurs as part of naturally occurring sugars or other complex organic compounds.
With respect to Cyanide levels, cassava varieties are broadly divided into two groups; the
sweet cassava known for low cyanide content and the bitter cassava with its high characteristic
content of Cyanogenic Glycosides (CGs) that is highly toxic when consumed (FSANZ, 2014).
Total cyanide in cassava products exists in form of CGs (linamarin and lotaustralin),
cyanohydrin and free hydrocyanic acid (HCN). Notwithstanding the CGS, according to FAO,
(FAO, 2011) 172 million tons of cassava were produced world-wide in 2000 with Africa
accounting for 45%, Asia 28% and Latin America and the Caribbean 19%. The five main
producing countries are Nigeria, Brazil, Thailand, Congo (DRC) and Indonesia. The on-going
challenge is to ensure that the presence of these cyanogenic glycosides is minimized through
proper understanding and possibly control of factors that affect cyanogenic glycoside content of
cassava. Roots and leaves contain the highest amount of linamarin (Cereda, 2016).
1.2 Hydrogen Cyanide (HCN)
Hydrogen cyanide (HCN) was discovered by Scheele in 1982. He made it by heating
sulphuric acid with Prussian blue; hence the old name was prussic acid. HCN occurs in nature as
glycoside amygdalin in some plants, for almonds, cassava etc. Hydrogen cyanide together with
sodium cyanide and potassium cyanide are the most of cyanide likely to be found in the
environment as a result of industrial activities. Its presence could be found in air, water, soil, and
even in gaseous state (present in solution in cassava root), with a faint, bitter, almond like odour.
It is a potential metabolic poison present in some food crops and other plants. Hydrogen cyanide
is a small molecule composed of a carbon, hydrogen and nitrogen atom joined together by a
stable triple bond. This poison is best known for its inhibition of many enzymes that are
2
waste waters from starch industry. The major source of cyanide in water are discharges from
metal mining processes, other sources include exhaust, release from certain chemical industries,
municipal waste burning and use of pesticides containing cyanide. Underground water can be
contaminated by cyanide present in landfills. In other body, cyanide can combines with plants
foods including almonds, millet sprouts, lima beans, soy spinach, bamboo shoots and cassava
roots, cyanide occurs as part of naturally occurring sugars or other complex organic compounds.
With respect to Cyanide levels, cassava varieties are broadly divided into two groups; the
sweet cassava known for low cyanide content and the bitter cassava with its high characteristic
content of Cyanogenic Glycosides (CGs) that is highly toxic when consumed (FSANZ, 2014).
Total cyanide in cassava products exists in form of CGs (linamarin and lotaustralin),
cyanohydrin and free hydrocyanic acid (HCN). Notwithstanding the CGS, according to FAO,
(FAO, 2011) 172 million tons of cassava were produced world-wide in 2000 with Africa
accounting for 45%, Asia 28% and Latin America and the Caribbean 19%. The five main
producing countries are Nigeria, Brazil, Thailand, Congo (DRC) and Indonesia. The on-going
challenge is to ensure that the presence of these cyanogenic glycosides is minimized through
proper understanding and possibly control of factors that affect cyanogenic glycoside content of
cassava. Roots and leaves contain the highest amount of linamarin (Cereda, 2016).
1.2 Hydrogen Cyanide (HCN)
Hydrogen cyanide (HCN) was discovered by Scheele in 1982. He made it by heating
sulphuric acid with Prussian blue; hence the old name was prussic acid. HCN occurs in nature as
glycoside amygdalin in some plants, for almonds, cassava etc. Hydrogen cyanide together with
sodium cyanide and potassium cyanide are the most of cyanide likely to be found in the
environment as a result of industrial activities. Its presence could be found in air, water, soil, and
even in gaseous state (present in solution in cassava root), with a faint, bitter, almond like odour.
It is a potential metabolic poison present in some food crops and other plants. Hydrogen cyanide
is a small molecule composed of a carbon, hydrogen and nitrogen atom joined together by a
stable triple bond. This poison is best known for its inhibition of many enzymes that are
2

important in animal metabolism. Enzymes are proteins that act as catalyst in biochemical
reaction.
It could be made to act as an anti-herb ivory compound to discourage plant consumers
(pests). Most often, it attaches itself to other molecules in the form of cyanogenic glycosides. In
example of one such compound is amygdalin (from stems of cherry, apricot etc). In this form,
cyanide is non-toxic to the plant, only in the breakdown of cyanogenic glycosides, during animal
consumption or digestion, is hydrogen cyanide released. For example, cows feeding on some
species of grasses containing cyanogenic glycosides became ill as they chew on the grass, in this
fashion, it is hypothesized that cyanide in non lethal does effectively deters herbivory.
Some cyanide containing plants are listed below (plants and relative cyanide level):
Cassava (+ + + +)
Lima beans (+ + +)
Sorghum (+ +)
Millet (+ +)
Bamboo Shoots (+ +)
Sweet Potatoes (+)
Maize (+)
1.3 Cyanide in Plants
The cyanogenic glycosides are a group of nitrile-containing plant secondary compounds
that yield cyanide (cyanogenesis) following their enzymatic breakdown. The functions of
cyanogenic glycosides remain to be determined in many plants; however, in some plants they
have been implicated as herbivore deterrents and as transportable forms of reduced nitrogen
(McMahon et al., 2015). It is estimated that between 3,000 and 12,000 plant species produce
and sequester cyanogenic glycosides. The major edible plants in which cyanogenic glycosides
3
reaction.
It could be made to act as an anti-herb ivory compound to discourage plant consumers
(pests). Most often, it attaches itself to other molecules in the form of cyanogenic glycosides. In
example of one such compound is amygdalin (from stems of cherry, apricot etc). In this form,
cyanide is non-toxic to the plant, only in the breakdown of cyanogenic glycosides, during animal
consumption or digestion, is hydrogen cyanide released. For example, cows feeding on some
species of grasses containing cyanogenic glycosides became ill as they chew on the grass, in this
fashion, it is hypothesized that cyanide in non lethal does effectively deters herbivory.
Some cyanide containing plants are listed below (plants and relative cyanide level):
Cassava (+ + + +)
Lima beans (+ + +)
Sorghum (+ +)
Millet (+ +)
Bamboo Shoots (+ +)
Sweet Potatoes (+)
Maize (+)
1.3 Cyanide in Plants
The cyanogenic glycosides are a group of nitrile-containing plant secondary compounds
that yield cyanide (cyanogenesis) following their enzymatic breakdown. The functions of
cyanogenic glycosides remain to be determined in many plants; however, in some plants they
have been implicated as herbivore deterrents and as transportable forms of reduced nitrogen
(McMahon et al., 2015). It is estimated that between 3,000 and 12,000 plant species produce
and sequester cyanogenic glycosides. The major edible plants in which cyanogenic glycosides
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

occur are almonds, sorghum, cassava, lima beans, stone fruits and bamboo shoots (Poulton,
2010). In certain sapindaceous seeds, HCN may arise during cyanolipid hydrolysis. More
frequently, HCN production in higher plants results from the catabolism of cyanogenic
glycosides. The approximately 75 documented cyanogenic glycosides are all O-β-glycosidic
derivatives of ahydroxynitriles. Depending on their precursor amino acid, they may be aromatic,
aliphatic, or cyclopentenoid in nature. Most are cyanogenic monosaccharides in which the
unstable cyanohydrin moiety is stabilized by glycosidic linkage to a single sugar residue.
Alternatively, in the cyanogenic disaccharides [e.g. (R)-amygdalin, (R)-vicianin, and
linustatin] or trisaccharides (e.g. xeranthin), two or three sugar moieties, respectively, are
involved in such stabilization. Sulfated, malonylated, and acylated derivatives of cyanogenic
glycosides are also known. are also known. Cyanogenesis is not exclusive to those plant species
accumulating cyanolipids and cyanogenic glycosides. All higher plants probably form low levels
of HCN as a coproduct of ethylene biosynthesis. This might explain why even 'acyanogenic'
plants contain significant levels of the cyanide detoxifying enzyme β-cyanoalanine synthase.
Cyanogenesis is also known in animals, but is restricted to the arthropods, notably to certain
centipedes, millipedes, and insects. In fungi and bacteria, HCN may originate via oxidative
decarboxylation of glycine.
A cyanogenic food of particular economic importance is cassava (Manihot esculenta),
which is also known by the names manioc, yuca and tapioca. Cassava is by far the most
important cyanogenic food crop for humans and is an important source of dietary energy in
tropical regions. The predominant cyanoglycoside in cassava is linamarin. It is present in leaves
and tubers, both of which are eaten. Linamarin is also present in beans of the lima or butter type.
Amygdalin is the cyanogenic glycoside responsible for the toxicity of the seeds of many species
of Rosaceae, such as bitter almonds, peaches and apricots. Sweet almonds are low in amygdalin
as a result of breeding processes. Their use in marzipan is common but the preparation procedure
should eliminate most of the cyanide. Cyanogen levels can vary widely with cultivar, climatic
conditions, plant part and degree of processing.
In areas of the world where cyanogenic plants such as cassava and lima beans comprise
the major item of the diet, chronic cyanide poisoning and associated pathological conditions still
4
2010). In certain sapindaceous seeds, HCN may arise during cyanolipid hydrolysis. More
frequently, HCN production in higher plants results from the catabolism of cyanogenic
glycosides. The approximately 75 documented cyanogenic glycosides are all O-β-glycosidic
derivatives of ahydroxynitriles. Depending on their precursor amino acid, they may be aromatic,
aliphatic, or cyclopentenoid in nature. Most are cyanogenic monosaccharides in which the
unstable cyanohydrin moiety is stabilized by glycosidic linkage to a single sugar residue.
Alternatively, in the cyanogenic disaccharides [e.g. (R)-amygdalin, (R)-vicianin, and
linustatin] or trisaccharides (e.g. xeranthin), two or three sugar moieties, respectively, are
involved in such stabilization. Sulfated, malonylated, and acylated derivatives of cyanogenic
glycosides are also known. are also known. Cyanogenesis is not exclusive to those plant species
accumulating cyanolipids and cyanogenic glycosides. All higher plants probably form low levels
of HCN as a coproduct of ethylene biosynthesis. This might explain why even 'acyanogenic'
plants contain significant levels of the cyanide detoxifying enzyme β-cyanoalanine synthase.
Cyanogenesis is also known in animals, but is restricted to the arthropods, notably to certain
centipedes, millipedes, and insects. In fungi and bacteria, HCN may originate via oxidative
decarboxylation of glycine.
A cyanogenic food of particular economic importance is cassava (Manihot esculenta),
which is also known by the names manioc, yuca and tapioca. Cassava is by far the most
important cyanogenic food crop for humans and is an important source of dietary energy in
tropical regions. The predominant cyanoglycoside in cassava is linamarin. It is present in leaves
and tubers, both of which are eaten. Linamarin is also present in beans of the lima or butter type.
Amygdalin is the cyanogenic glycoside responsible for the toxicity of the seeds of many species
of Rosaceae, such as bitter almonds, peaches and apricots. Sweet almonds are low in amygdalin
as a result of breeding processes. Their use in marzipan is common but the preparation procedure
should eliminate most of the cyanide. Cyanogen levels can vary widely with cultivar, climatic
conditions, plant part and degree of processing.
In areas of the world where cyanogenic plants such as cassava and lima beans comprise
the major item of the diet, chronic cyanide poisoning and associated pathological conditions still
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

exist (Poulton, 2009). It is highly desirable that the toxicity of cyanogenic plants to humans and
livestock be reduced. This is achievable by: (a) selective breeding to produce low-cyanogen
varieties, as was accomplished for almonds, (b) screening of natural populations for low-
cyanogen varieties, (c) mutagenesis of protoplasts or cell cultures with subsequent regeneration
of plants having desired mutant genotypes, or (d) genetic engineering.
1.4 Roles of Cyanogenic Glycosides in Plants
A common feature of cyanophoric plants is that cyanogenic glycoside hydrolysis occurs
at a significant rate only after their tissues have been disrupted by herbivores, fungal attack, or
mechanical means. Although other explanations are possible, it is generally assumed that the
glycosides and their catabolic enzymes are separated in the intact plant by compartmentation at
either tissue or subcellular levels (Poulton, 2009). These possibilities have been extensively
tested in a single organism, namely the leaves of 6-day old light-grown sorghum seedlings.
Somewhat unexpectedly, the authors demonstrated that the substrate and its catabolic enzymes
were localized within different tissues. The cyanogenic glycoside dhurrin was sequestered in the
vacuoles of epidermal cells, whereas the 3- glycosidase and hydroxynitrile lyase were present
almost entirely in the underlying mesophyll cells. These two enzymes were located in the
chloroplasts and cytosol, respectively. It therefore seems likely that the large-scale hydrolysis of
dhurrin, which probably provides a defense mechanism against herbivores by liberating HCN,
occurs only after tissue disruption allows the mixing of contents of different tissues.
Available evidence from other plant species, however, favors compartmentation of
components of the 'cyanide bomb' at the subcellular level. In cassava, cells throughout the entire
root cross-section possess both cyanogens (principally linamarin) and linamarase (Kojima et al.,
2013). As in sorghum, highest glycoside levels are found in outer cell layers, again suggesting
the involvement of cyanogens in defense against herbivores or pathogens, but the subcellular
localizations of linamarin and linamarase remain unknown. In Phaseolus lunatus, the low
recoveries of linamarin, linamarase, and hydroxynitrile lyase in leaf mesophyll protoplasts
pointed to other tissues, perhaps the epidermis, as the principal site for these components.
Although these data cannot unequivocally distinguish between an epidermal or mesophyll
location, it seems certain that the P. lunatus linamarase is apoplastic. Leaf discs hydrolyzed
5
livestock be reduced. This is achievable by: (a) selective breeding to produce low-cyanogen
varieties, as was accomplished for almonds, (b) screening of natural populations for low-
cyanogen varieties, (c) mutagenesis of protoplasts or cell cultures with subsequent regeneration
of plants having desired mutant genotypes, or (d) genetic engineering.
1.4 Roles of Cyanogenic Glycosides in Plants
A common feature of cyanophoric plants is that cyanogenic glycoside hydrolysis occurs
at a significant rate only after their tissues have been disrupted by herbivores, fungal attack, or
mechanical means. Although other explanations are possible, it is generally assumed that the
glycosides and their catabolic enzymes are separated in the intact plant by compartmentation at
either tissue or subcellular levels (Poulton, 2009). These possibilities have been extensively
tested in a single organism, namely the leaves of 6-day old light-grown sorghum seedlings.
Somewhat unexpectedly, the authors demonstrated that the substrate and its catabolic enzymes
were localized within different tissues. The cyanogenic glycoside dhurrin was sequestered in the
vacuoles of epidermal cells, whereas the 3- glycosidase and hydroxynitrile lyase were present
almost entirely in the underlying mesophyll cells. These two enzymes were located in the
chloroplasts and cytosol, respectively. It therefore seems likely that the large-scale hydrolysis of
dhurrin, which probably provides a defense mechanism against herbivores by liberating HCN,
occurs only after tissue disruption allows the mixing of contents of different tissues.
Available evidence from other plant species, however, favors compartmentation of
components of the 'cyanide bomb' at the subcellular level. In cassava, cells throughout the entire
root cross-section possess both cyanogens (principally linamarin) and linamarase (Kojima et al.,
2013). As in sorghum, highest glycoside levels are found in outer cell layers, again suggesting
the involvement of cyanogens in defense against herbivores or pathogens, but the subcellular
localizations of linamarin and linamarase remain unknown. In Phaseolus lunatus, the low
recoveries of linamarin, linamarase, and hydroxynitrile lyase in leaf mesophyll protoplasts
pointed to other tissues, perhaps the epidermis, as the principal site for these components.
Although these data cannot unequivocally distinguish between an epidermal or mesophyll
location, it seems certain that the P. lunatus linamarase is apoplastic. Leaf discs hydrolyzed
5

externally supplied linamarin, and about one-third of the total linamarase activity was extractable
by multiple infiltrations of the leaves. The T. repens linamarase was detected by
immunocytofluorescence in cell walls, especially those of the epidermis, and in the cuticle. More
recently, protoplast isolation and tissue filtration experiments with Hevea endosperm showed
that linamarin and the hydroxynitrile lyase were intracellular but that linamarase occurred both
intra- and extracellularly. The apoplastic distribution of most linamarases contrasts with the
intracellular location of sorghum dhurrinase, a fact perhaps related to the nonglycoprotein
character of the latter (Poulton, 2009).
The physiological importance of cyanogenic compounds in plant metabolism is currently
receiving renewed interest. As with other secondary products, cyanogenics were originally
viewed as excretory substances, but their turnover (seasonal and even diurnal) argues strongly
against this hypothesis. Given the well documented toxicity of HCN, a role in plant protection
against herbivores, pathogens, and competitors is appealing. Much evidence, indeed, favors a
defence function for cyanogenics against certain animals including insects.
1.5 Statement of the Problem
Since high concentration of hydrogen cyanide is fatal to human and other life species
especially when consumed and is lethal to the body system or dangerous to health, the problem
lies on how to reduce this high concentration to a certain limit or how its concentration could be
eliminated.
1.6 Aim and Objectives of the Study
The aim of the study is to review hydrogen cyanide content in cassava. The specific
objectives are;
To determine the effect of HCN consumption in the body.
To examine the process of reducing HCN content in cassava before consumption.
6
by multiple infiltrations of the leaves. The T. repens linamarase was detected by
immunocytofluorescence in cell walls, especially those of the epidermis, and in the cuticle. More
recently, protoplast isolation and tissue filtration experiments with Hevea endosperm showed
that linamarin and the hydroxynitrile lyase were intracellular but that linamarase occurred both
intra- and extracellularly. The apoplastic distribution of most linamarases contrasts with the
intracellular location of sorghum dhurrinase, a fact perhaps related to the nonglycoprotein
character of the latter (Poulton, 2009).
The physiological importance of cyanogenic compounds in plant metabolism is currently
receiving renewed interest. As with other secondary products, cyanogenics were originally
viewed as excretory substances, but their turnover (seasonal and even diurnal) argues strongly
against this hypothesis. Given the well documented toxicity of HCN, a role in plant protection
against herbivores, pathogens, and competitors is appealing. Much evidence, indeed, favors a
defence function for cyanogenics against certain animals including insects.
1.5 Statement of the Problem
Since high concentration of hydrogen cyanide is fatal to human and other life species
especially when consumed and is lethal to the body system or dangerous to health, the problem
lies on how to reduce this high concentration to a certain limit or how its concentration could be
eliminated.
1.6 Aim and Objectives of the Study
The aim of the study is to review hydrogen cyanide content in cassava. The specific
objectives are;
To determine the effect of HCN consumption in the body.
To examine the process of reducing HCN content in cassava before consumption.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CHAPTER TWO
LITERATURE REVIEW
2.1 Cassava (Manihot esculenta)
Manihot esculenta, commonly called cassava is a woody shrub native to South America
of the spurge family, Euphorbiaceae. Although a perennial plant, cassava is extensively
cultivated as an annual crop in tropical and subtropical regions for its edible starchy tuberous
root, a major source of carbohydrates. Though it is often called yuca in parts of Spanish America
and in the United States, it is not related to yucca, a shrub in the family Asparagaceae. Cassava is
predominantly consumed in boiled form, but substantial quantities are used to extract cassava
starch, called tapioca, which is used for food, animal feed, and industrial purposes. The Brazilian
farinha, and the related garri of West Africa, is edible coarse flour obtained by grating cassava
roots, pressing moisture off the obtained grated pulp, and finally drying it (and roasting in the
case of farinha) (Fauquet, 2010).
Cassava is the third-largest source of food carbohydrates in the tropics,
after rice and maize (Fauquet et al., 2010). Cassava is a major staple food in the developing
world, providing a basic diet for over half a billion people (FAO, 2015). It is one of the most
drought-tolerant crops, capable of growing on marginal soils. Nigeria is the world's largest
producer of cassava, while Thailand is the largest exporter of cassava starch. Cassava is
classified as either sweet or bitter. Like other roots and tubers, both bitter and sweet varieties of
cassava contain antinutritional factors and toxins, with the bitter varieties containing much larger
amounts. It must be properly prepared before consumption, as improper preparation of cassava
can leave enough residual cyanide to cause acute cyanide intoxication, goiters, and even ataxia,
partial paralysis, or death. The more toxic varieties of cassava are a fall-back resource (a "food
security crop") in times of famine or food insecurity in some places. Farmers often prefer the
bitter varieties because they deter pests, animals, and thieves (Chiwona-karltun, 2012).
The oldest direct evidence of cassava cultivation comes from a 1,400-year-
old Maya site, Joya de Cerén, in El Salvador. With its high food potential, it had become a staple
food of the native populations of northern South America, southern Mesoamerica, and
7
LITERATURE REVIEW
2.1 Cassava (Manihot esculenta)
Manihot esculenta, commonly called cassava is a woody shrub native to South America
of the spurge family, Euphorbiaceae. Although a perennial plant, cassava is extensively
cultivated as an annual crop in tropical and subtropical regions for its edible starchy tuberous
root, a major source of carbohydrates. Though it is often called yuca in parts of Spanish America
and in the United States, it is not related to yucca, a shrub in the family Asparagaceae. Cassava is
predominantly consumed in boiled form, but substantial quantities are used to extract cassava
starch, called tapioca, which is used for food, animal feed, and industrial purposes. The Brazilian
farinha, and the related garri of West Africa, is edible coarse flour obtained by grating cassava
roots, pressing moisture off the obtained grated pulp, and finally drying it (and roasting in the
case of farinha) (Fauquet, 2010).
Cassava is the third-largest source of food carbohydrates in the tropics,
after rice and maize (Fauquet et al., 2010). Cassava is a major staple food in the developing
world, providing a basic diet for over half a billion people (FAO, 2015). It is one of the most
drought-tolerant crops, capable of growing on marginal soils. Nigeria is the world's largest
producer of cassava, while Thailand is the largest exporter of cassava starch. Cassava is
classified as either sweet or bitter. Like other roots and tubers, both bitter and sweet varieties of
cassava contain antinutritional factors and toxins, with the bitter varieties containing much larger
amounts. It must be properly prepared before consumption, as improper preparation of cassava
can leave enough residual cyanide to cause acute cyanide intoxication, goiters, and even ataxia,
partial paralysis, or death. The more toxic varieties of cassava are a fall-back resource (a "food
security crop") in times of famine or food insecurity in some places. Farmers often prefer the
bitter varieties because they deter pests, animals, and thieves (Chiwona-karltun, 2012).
The oldest direct evidence of cassava cultivation comes from a 1,400-year-
old Maya site, Joya de Cerén, in El Salvador. With its high food potential, it had become a staple
food of the native populations of northern South America, southern Mesoamerica, and
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

the Taino people in the Caribbean islands, who grew it using a high-yielding form of shifting
agriculture by the time of European contact in 1492. Cassava was a staple food of pre-
Columbian peoples in the Americas and is often portrayed in indigenous art. Cassava was
introduced to Africa by Portuguese traders from Brazil in the 16th century. Around the same
period, it was also introduced to Asia through Columbian Exchange by Portuguese and Spanish
traders, planted in their colonies in Goa, Malacca, Eastern Indonesia, Timor and the
Philippines. Maize and cassava are now important staple foods, replacing native African crops in
places such as Tanzania (Nweke, 2005). Cassava has also become an important crop in Asia.
While it is a valued food staple in parts of eastern Indonesia, it is primarily cultivated for starch
extraction and bio-fuel production in Thailand, Cambodia or Vietnam. Cassava is sometimes
described as the "bread of the tropics" but should not be confused with the tropical and
equatorial bread tree (Encephalartos), the breadfruit (Artocarpus altilis) or the African
breadfruit (Treculia africana). This description definitely holds in Africa and parts of South
America; in Asian countries such as Vietnam fresh cassava barely features in human diets
(Adams, 2009).
There is a legend that cassava was introduced in 1880-1885 C.E. to the South Indian state
of Kerala by the King of Travancore, Vishakham Thirunal Maharaja, after a great famine hit the
kingdom, as a substitute for rice. However, there are documented cases of cassava cultivation in
parts of the state before the time of Vishakham Thirunal Maharaja. Cassava is called kappa or
maricheeni in Malayalam. It is also referred to as tapioca in Indian English usage (Adams, 2009).
2.2 Taxonomic Classification of Cassava (Manihot esculenta)
Domain: Eukaryota
Kingdom: Plantae
Phylum: Spermatophyta
Subphylum: Angiospermae
Class: Dicotyledonae
Order: Euphorbiales
8
agriculture by the time of European contact in 1492. Cassava was a staple food of pre-
Columbian peoples in the Americas and is often portrayed in indigenous art. Cassava was
introduced to Africa by Portuguese traders from Brazil in the 16th century. Around the same
period, it was also introduced to Asia through Columbian Exchange by Portuguese and Spanish
traders, planted in their colonies in Goa, Malacca, Eastern Indonesia, Timor and the
Philippines. Maize and cassava are now important staple foods, replacing native African crops in
places such as Tanzania (Nweke, 2005). Cassava has also become an important crop in Asia.
While it is a valued food staple in parts of eastern Indonesia, it is primarily cultivated for starch
extraction and bio-fuel production in Thailand, Cambodia or Vietnam. Cassava is sometimes
described as the "bread of the tropics" but should not be confused with the tropical and
equatorial bread tree (Encephalartos), the breadfruit (Artocarpus altilis) or the African
breadfruit (Treculia africana). This description definitely holds in Africa and parts of South
America; in Asian countries such as Vietnam fresh cassava barely features in human diets
(Adams, 2009).
There is a legend that cassava was introduced in 1880-1885 C.E. to the South Indian state
of Kerala by the King of Travancore, Vishakham Thirunal Maharaja, after a great famine hit the
kingdom, as a substitute for rice. However, there are documented cases of cassava cultivation in
parts of the state before the time of Vishakham Thirunal Maharaja. Cassava is called kappa or
maricheeni in Malayalam. It is also referred to as tapioca in Indian English usage (Adams, 2009).
2.2 Taxonomic Classification of Cassava (Manihot esculenta)
Domain: Eukaryota
Kingdom: Plantae
Phylum: Spermatophyta
Subphylum: Angiospermae
Class: Dicotyledonae
Order: Euphorbiales
8

Family: Euphorbiaceae
Genus: Manihot
Species: Manihot esculenta (USDA, 2021)
2.3 General Description and Morphology of Cassava (Manihot esculenta)
The cassava root is long and tapered, with a firm; homogeneous flesh encased in a
detachable rind, about 1 mm thick, rough and brown on the outside. Commercial cultivars can be
5 to 10 centimetres (2 to 4 inches) in diameter at the top, and around 15 to 30 cm (6 to 12 in)
long. A woody vascular bundle runs along the root's axis. The flesh can be chalk-white or
yellowish. Cassava roots are very rich in starch and contain small amounts of calcium
(16 mg/100 g), phosphorus (27 mg/100 g), and vitamin C (20.6 mg/100 g). However, they are
poor in protein and other nutrients. In contrast, cassava leaves are a good source of protein (rich
in lysine), but deficient in the amino acid methionine and possibly tryptophan (US Department of
Agriculture USDA, 2016)
Plant: This is a tall semi-woody perennial shrub or tree, which can grow up to 7 m high, having
single to few stems, sparingly branching. The outer bark is smooth, light brown to yellowish grey
in colour while inner bark is cream-green in colour and wood is soft in consistency (Fasuyi,
2015).
Leaves: Petiole light greenish to red in colour. Leaves are dark green above and pale light
greenish grayish underneath, sometimes variegated and pedicels are light green to red.
Fruit: Somewhat subglobose, green (to light yellow, white, dark brown), smooth, and with 6
longitudinal wings (Fasuyi, 2015).
Roots: Grows in clusters of 4-8 at the stem base. Roots are from 1-4 inches in diameter and 8-15
inches long. The pure white interior is firmer than potatoes and contains high starch content. The
roots are covered with a thin reddish brown fibrous bark that is removed by scraping and peeling.
9
Genus: Manihot
Species: Manihot esculenta (USDA, 2021)
2.3 General Description and Morphology of Cassava (Manihot esculenta)
The cassava root is long and tapered, with a firm; homogeneous flesh encased in a
detachable rind, about 1 mm thick, rough and brown on the outside. Commercial cultivars can be
5 to 10 centimetres (2 to 4 inches) in diameter at the top, and around 15 to 30 cm (6 to 12 in)
long. A woody vascular bundle runs along the root's axis. The flesh can be chalk-white or
yellowish. Cassava roots are very rich in starch and contain small amounts of calcium
(16 mg/100 g), phosphorus (27 mg/100 g), and vitamin C (20.6 mg/100 g). However, they are
poor in protein and other nutrients. In contrast, cassava leaves are a good source of protein (rich
in lysine), but deficient in the amino acid methionine and possibly tryptophan (US Department of
Agriculture USDA, 2016)
Plant: This is a tall semi-woody perennial shrub or tree, which can grow up to 7 m high, having
single to few stems, sparingly branching. The outer bark is smooth, light brown to yellowish grey
in colour while inner bark is cream-green in colour and wood is soft in consistency (Fasuyi,
2015).
Leaves: Petiole light greenish to red in colour. Leaves are dark green above and pale light
greenish grayish underneath, sometimes variegated and pedicels are light green to red.
Fruit: Somewhat subglobose, green (to light yellow, white, dark brown), smooth, and with 6
longitudinal wings (Fasuyi, 2015).
Roots: Grows in clusters of 4-8 at the stem base. Roots are from 1-4 inches in diameter and 8-15
inches long. The pure white interior is firmer than potatoes and contains high starch content. The
roots are covered with a thin reddish brown fibrous bark that is removed by scraping and peeling.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Stem: Single to few stems, sparingly branching; branchlets light green to tinged reddish, nodes
reddish. The outer bark is smooth, light brown to yellowish grey & inner bark is cream-green in
colour (Fasuyi, 2015)
Figure 1: Cassava Plant
2.4 Nutritional Profile of Cassava (Manihot esculenta)
Cassava is a calorie-rich vegetable that contains plenty of carbohydrates and
key vitamins and minerals. Cassava is a good source of vitamin C, thiamine, riboflavin,
and niacin. The leaves, which are also edible if a person cooks them or dries them in the sun, can
contain up to 25% protein. However, the cassava root does not deliver the same nutritional value
as other tuber vegetables. Tapioca starch is gaining attention as a source of gluten-free flour to
make bread and other baked products that are suitable for people with an intolerance to gluten
(Olumide, 2004).
Cassava is a source of resistant starch, which scientists suggest can boost a person’s gut
health by helping nurture beneficial gut bacteria. Resistant starches remain relatively unchanged
as they pass through the digestive tract. Raw cassava is 60% water, 38% carbohydrates,
10
reddish. The outer bark is smooth, light brown to yellowish grey & inner bark is cream-green in
colour (Fasuyi, 2015)
Figure 1: Cassava Plant
2.4 Nutritional Profile of Cassava (Manihot esculenta)
Cassava is a calorie-rich vegetable that contains plenty of carbohydrates and
key vitamins and minerals. Cassava is a good source of vitamin C, thiamine, riboflavin,
and niacin. The leaves, which are also edible if a person cooks them or dries them in the sun, can
contain up to 25% protein. However, the cassava root does not deliver the same nutritional value
as other tuber vegetables. Tapioca starch is gaining attention as a source of gluten-free flour to
make bread and other baked products that are suitable for people with an intolerance to gluten
(Olumide, 2004).
Cassava is a source of resistant starch, which scientists suggest can boost a person’s gut
health by helping nurture beneficial gut bacteria. Resistant starches remain relatively unchanged
as they pass through the digestive tract. Raw cassava is 60% water, 38% carbohydrates,
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1% protein, and has negligible fat (Olumide, 2004). In a 100-gram (3 1⁄2-ounce) reference
serving, raw cassava provides 670 kilojoules (160 kilocalories) of food energy and 25% of
the Daily Value (DV) of vitamin C, but otherwise has no micronutrients in significant content
(i.e. above 10% of the relevant DV). Cooked cassava starch has a digestibility of over 75%
(Olumide, 2004).
Cassava, like other foods, also has antinutritional and toxic factors. Of particular concern
are the cyanogenic glucosides of cassava (linamarin and lotaustralin). On hydrolysis, these
release hydrogen cyanide (HCN). The presence of cyanide in cassava is of concern for human
and for animal consumption. The concentration of these antinutritional and unsafe glycosides
varies considerably between varieties and also with climatic and cultural conditions. Selection of
cassava species to be grown, therefore, is quite important. Once harvested, bitter cassava must be
treated and prepared properly prior to human or animal consumption, while sweet cassava can be
used after boiling.
11
serving, raw cassava provides 670 kilojoules (160 kilocalories) of food energy and 25% of
the Daily Value (DV) of vitamin C, but otherwise has no micronutrients in significant content
(i.e. above 10% of the relevant DV). Cooked cassava starch has a digestibility of over 75%
(Olumide, 2004).
Cassava, like other foods, also has antinutritional and toxic factors. Of particular concern
are the cyanogenic glucosides of cassava (linamarin and lotaustralin). On hydrolysis, these
release hydrogen cyanide (HCN). The presence of cyanide in cassava is of concern for human
and for animal consumption. The concentration of these antinutritional and unsafe glycosides
varies considerably between varieties and also with climatic and cultural conditions. Selection of
cassava species to be grown, therefore, is quite important. Once harvested, bitter cassava must be
treated and prepared properly prior to human or animal consumption, while sweet cassava can be
used after boiling.
11
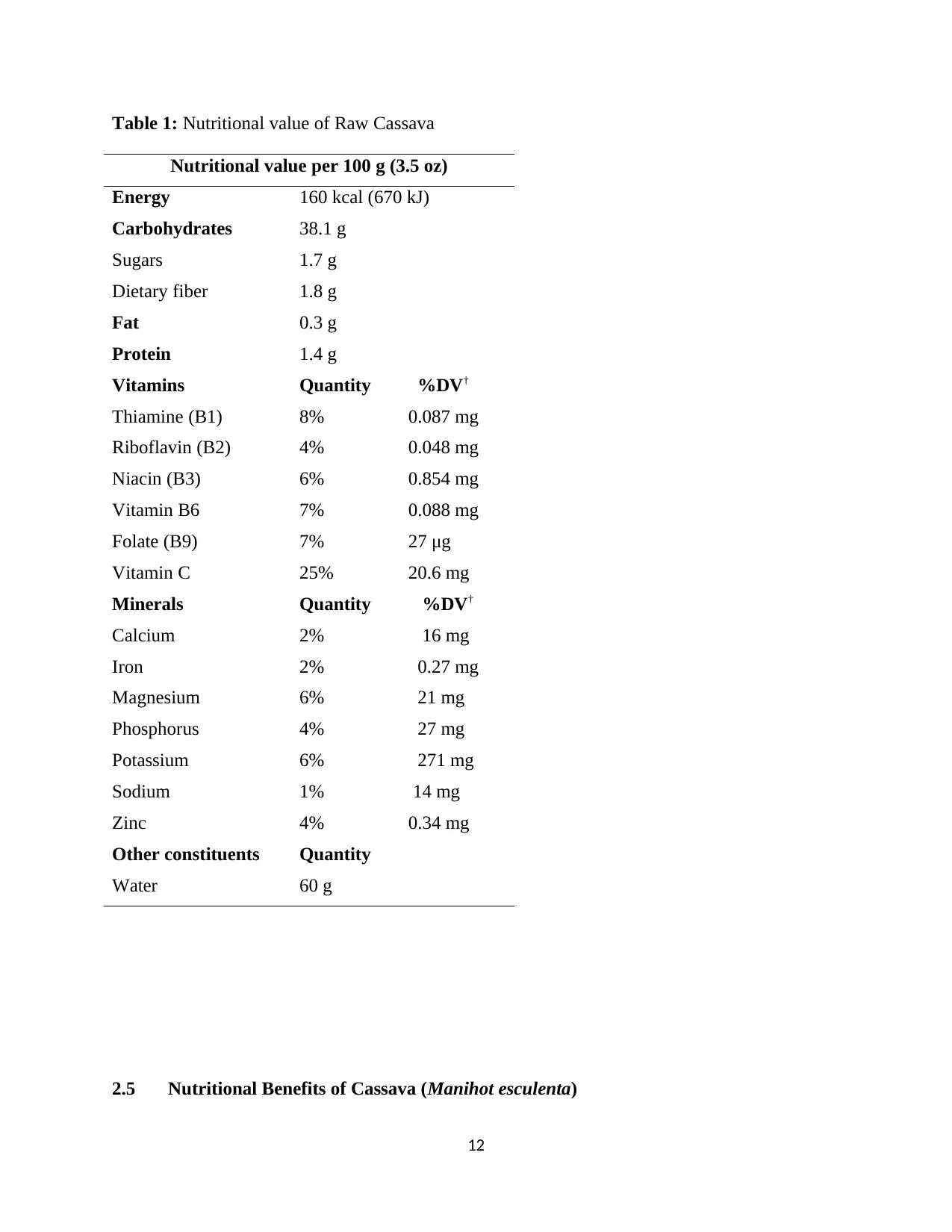
Table 1: Nutritional value of Raw Cassava
Nutritional value per 100 g (3.5 oz)
Energy 160 kcal (670 kJ)
Carbohydrates 38.1 g
Sugars 1.7 g
Dietary fiber 1.8 g
Fat 0.3 g
Protein 1.4 g
Vitamins Quantity %DV†
Thiamine (B1) 8% 0.087 mg
Riboflavin (B2) 4% 0.048 mg
Niacin (B3) 6% 0.854 mg
Vitamin B6 7% 0.088 mg
Folate (B9) 7% 27 μg
Vitamin C 25% 20.6 mg
Minerals Quantity %DV†
Calcium 2% 16 mg
Iron 2% 0.27 mg
Magnesium 6% 21 mg
Phosphorus 4% 27 mg
Potassium 6% 271 mg
Sodium 1% 14 mg
Zinc 4% 0.34 mg
Other constituents Quantity
Water 60 g
2.5 Nutritional Benefits of Cassava (Manihot esculenta)
12
Nutritional value per 100 g (3.5 oz)
Energy 160 kcal (670 kJ)
Carbohydrates 38.1 g
Sugars 1.7 g
Dietary fiber 1.8 g
Fat 0.3 g
Protein 1.4 g
Vitamins Quantity %DV†
Thiamine (B1) 8% 0.087 mg
Riboflavin (B2) 4% 0.048 mg
Niacin (B3) 6% 0.854 mg
Vitamin B6 7% 0.088 mg
Folate (B9) 7% 27 μg
Vitamin C 25% 20.6 mg
Minerals Quantity %DV†
Calcium 2% 16 mg
Iron 2% 0.27 mg
Magnesium 6% 21 mg
Phosphorus 4% 27 mg
Potassium 6% 271 mg
Sodium 1% 14 mg
Zinc 4% 0.34 mg
Other constituents Quantity
Water 60 g
2.5 Nutritional Benefits of Cassava (Manihot esculenta)
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 28
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
