Assessment of Immunization Patterns: Health Outcomes Report
VerifiedAdded on 2021/04/21
|11
|2944
|27
Report
AI Summary
This report assesses the impact of immunization patterns on children's health, comparing the outcomes of vaccinated and unvaccinated children. It examines the safety profiles of various vaccines, including HPV and MMRV, and analyzes the occurrence of adverse events. The report synthesizes findings from multiple studies, including meta-analyses and systematic reviews, to evaluate the benefits and risks associated with different immunization interventions. It also explores factors influencing parental decisions regarding vaccination, such as cultural conventions, misinformation, and social influences. The analysis highlights the importance of education and counseling to address parental concerns and promote informed decision-making. The report concludes by emphasizing the overall benefits of immunization in reducing the risk of contagious conditions and improving child health outcomes, with a focus on the limited evidence of serious adverse events. The report is designed to provide comprehensive information on the safety and efficacy of vaccines and influence public health policy.
Assessment template
Question 1
Question 1A
Question: To what extent does the immunization pattern prove to be deleterious for the growing
children, in comparison to the children who do not receive vaccination interventions?
Rationale: The research question advocates the requirement of undertaking comparative analysis
between the wellness outcomes/adverse events experienced by the immunized children and the children
whose parents do not enrol themselves in immunization programs. Indeed, the research objective
substantiates the need to conduct a systematic analysis of the safety profiles of various immunization
interventions with the objective of determining the extent of beneficial outcomes in comparison to the
patient solicited/unsolicited adversities.
Question 1B:
Source of information Type of
information
Australian Government Department of Health website
(http://www.health.nsw.gov.au/Infectious/whoopingcough/Pages/Identify-Protect-
Prevent.aspx)
Whooping
Cough
Protection
Parameters
Australian Government Department of Health Immunization Handbook
(http://immunise.health.gov.au/internet/immunise/publishing.nsf/Content
/7B28E87511E08905CA257D4D001DB1F8/$File/Aus-Imm-Handbook.pdf)
Epidemiology,
wellness
outcomes and
adverse events
following
Question 1
Question 1A
Question: To what extent does the immunization pattern prove to be deleterious for the growing
children, in comparison to the children who do not receive vaccination interventions?
Rationale: The research question advocates the requirement of undertaking comparative analysis
between the wellness outcomes/adverse events experienced by the immunized children and the children
whose parents do not enrol themselves in immunization programs. Indeed, the research objective
substantiates the need to conduct a systematic analysis of the safety profiles of various immunization
interventions with the objective of determining the extent of beneficial outcomes in comparison to the
patient solicited/unsolicited adversities.
Question 1B:
Source of information Type of
information
Australian Government Department of Health website
(http://www.health.nsw.gov.au/Infectious/whoopingcough/Pages/Identify-Protect-
Prevent.aspx)
Whooping
Cough
Protection
Parameters
Australian Government Department of Health Immunization Handbook
(http://immunise.health.gov.au/internet/immunise/publishing.nsf/Content
/7B28E87511E08905CA257D4D001DB1F8/$File/Aus-Imm-Handbook.pdf)
Epidemiology,
wellness
outcomes and
adverse events
following
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
immunization
WHO
(http://apps.who.int/iris/bitstream/handle/10665/70854/WHO_IVB_12.04_eng.pdf;jsessionid
=
F674E9DEEADAF2C3F5EDD3283F0A1949?sequence=1)
Global vaccine
safety blueprint
Question 1C:
Keyword Search term for Boolean ‘or”
1 “Immunization” AND “Barriers”
AND “Adversities” AND “Safety”
Vaccination
2 “Immunization” AND “Outcomes”
OR “Wellness”
Vaccination, Results
3 “Vaccination” AND “Safety” Immunization, Health
4 “Vaccination” AND “Adverse”
“Reactions”
Immunization, Allergy
5 “Immunization”, AND “Children”,
AND “anti-vaccination”, AND
“diseases”, AND “health”
Vaccination, Manifestations, Wellness
Question 1D:
The absence of retrieval of satisfactory quantity of qualitative articles will substantiate the
requirement of changing the search parameters for identifying the articles of interest. The utilization
of more dynamic and relevant search terms might suffice the requirement of acquiring significant
content related to the study question. Alternatively, modification in the research question or
expanding the scope of research parameters will increase the exploratory dimensions that might assist
in acquiring the relevant systematic studies related to the immunization interventions and their
associated outcomes. For example, search terms could merely focus on exploring patient adversities
that emanate from the administration of a range of immunization interventions in children of age
group 0-10 years. Similarly, another search strategy could effectively articulate facts regarding the
safety parameters related to various vaccine interventions (including HPV and hepatitis B) for the
WHO
(http://apps.who.int/iris/bitstream/handle/10665/70854/WHO_IVB_12.04_eng.pdf;jsessionid
=
F674E9DEEADAF2C3F5EDD3283F0A1949?sequence=1)
Global vaccine
safety blueprint
Question 1C:
Keyword Search term for Boolean ‘or”
1 “Immunization” AND “Barriers”
AND “Adversities” AND “Safety”
Vaccination
2 “Immunization” AND “Outcomes”
OR “Wellness”
Vaccination, Results
3 “Vaccination” AND “Safety” Immunization, Health
4 “Vaccination” AND “Adverse”
“Reactions”
Immunization, Allergy
5 “Immunization”, AND “Children”,
AND “anti-vaccination”, AND
“diseases”, AND “health”
Vaccination, Manifestations, Wellness
Question 1D:
The absence of retrieval of satisfactory quantity of qualitative articles will substantiate the
requirement of changing the search parameters for identifying the articles of interest. The utilization
of more dynamic and relevant search terms might suffice the requirement of acquiring significant
content related to the study question. Alternatively, modification in the research question or
expanding the scope of research parameters will increase the exploratory dimensions that might assist
in acquiring the relevant systematic studies related to the immunization interventions and their
associated outcomes. For example, search terms could merely focus on exploring patient adversities
that emanate from the administration of a range of immunization interventions in children of age
group 0-10 years. Similarly, another search strategy could effectively articulate facts regarding the
safety parameters related to various vaccine interventions (including HPV and hepatitis B) for the

selected patients. The exploration of disease-based articles in the context of immunization
requirement and associated outcomes will indeed widen the scope of research and lead to the
acquisition of desirable articles in the shortest timeframe.
requirement and associated outcomes will indeed widen the scope of research and lead to the
acquisition of desirable articles in the shortest timeframe.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Question 2
Table 1. Meta-analysis (+/- systematic review)
Full reference:
Nursing
Stassijns, J, Bollaerts, K, Baay, M & Verstraeten, T 2016, 'A systematic review and meta-analysis on the safety of newly adjuvanted
vaccines among children', Vaccine, vol 34, no. 6, pp. 714-722, <https://www.ncbi.nlm.nih.gov/pubmed/26740250>.
Search strategy Vaccine safety data from a multitude of published clinical studies was systematically explored by the research professionals. The
vaccines of interest included the immunostimulants AS02 and AS01.
Inclusion/exclusion criteria Inclusion Parameters
1. Children of less than 10 years of ages
2. Children with eligibility for receiving adjuvanted vaccines
3. Children with no previous record of allergy or adverse reactions in response to the administration of adjuvanted vaccines
Exclusion Criteria
1. Children with pre-existing vaccine-related allergy or toxicity profile
2. Children affected with disease pandemic against which the vaccine administration was recommended
Table 1. Meta-analysis (+/- systematic review)
Full reference:
Nursing
Stassijns, J, Bollaerts, K, Baay, M & Verstraeten, T 2016, 'A systematic review and meta-analysis on the safety of newly adjuvanted
vaccines among children', Vaccine, vol 34, no. 6, pp. 714-722, <https://www.ncbi.nlm.nih.gov/pubmed/26740250>.
Search strategy Vaccine safety data from a multitude of published clinical studies was systematically explored by the research professionals. The
vaccines of interest included the immunostimulants AS02 and AS01.
Inclusion/exclusion criteria Inclusion Parameters
1. Children of less than 10 years of ages
2. Children with eligibility for receiving adjuvanted vaccines
3. Children with no previous record of allergy or adverse reactions in response to the administration of adjuvanted vaccines
Exclusion Criteria
1. Children with pre-existing vaccine-related allergy or toxicity profile
2. Children affected with disease pandemic against which the vaccine administration was recommended
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Issue (what was being
studied)
The systematic study and meta-analysis evaluated the pattern of adverse events, unsolicited adverse events, solicited adverse events
and serious adverse events related to the administration of newly adjuvanted vaccines in the treated children.
Context (study setting) The systematic study explored and analysed 29-trials that effectively evaluated the outcomes of newly administered adjuvanted
vaccines in 25,056 children.
Outcome (main findings) The study outcomes did not reveal the pattern of solicited adverse events following the administration of adjuvanted vaccines to the
treated children. The findings also did not reveal any potential elevation in the immunization-based risk of chronic diseases, febrile
convulsions and unsolicited adverse events. However, short-duration mild pain across the site of injection was evidently reported by
the research professionals. Limited evidence was revealed in relation to the high risk of adjuvanted malaria vaccine-based meningitis
and adjuvanted pandemic vaccine-based narcolepsy. The overall findings reveal weak evidence regarding the adverse clinical
implications in relation to the utilization of adjuvanted vaccines in the target population.
Table 2. Systematic review
Full reference
Nursing
Ogawa, Y, Takei, H, Ogawa, R & Mihara, K 2017, 'Safety of human papillomavirus vaccines in healthy young women: a
meta-analysis of 24 controlled studies', JPHCS, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5504559/>.
Search strategy The researchers explored MEDLINE, COCHRANE, and JCRM databases while systematically utilizing the following
MeSH terms.
studied)
The systematic study and meta-analysis evaluated the pattern of adverse events, unsolicited adverse events, solicited adverse events
and serious adverse events related to the administration of newly adjuvanted vaccines in the treated children.
Context (study setting) The systematic study explored and analysed 29-trials that effectively evaluated the outcomes of newly administered adjuvanted
vaccines in 25,056 children.
Outcome (main findings) The study outcomes did not reveal the pattern of solicited adverse events following the administration of adjuvanted vaccines to the
treated children. The findings also did not reveal any potential elevation in the immunization-based risk of chronic diseases, febrile
convulsions and unsolicited adverse events. However, short-duration mild pain across the site of injection was evidently reported by
the research professionals. Limited evidence was revealed in relation to the high risk of adjuvanted malaria vaccine-based meningitis
and adjuvanted pandemic vaccine-based narcolepsy. The overall findings reveal weak evidence regarding the adverse clinical
implications in relation to the utilization of adjuvanted vaccines in the target population.
Table 2. Systematic review
Full reference
Nursing
Ogawa, Y, Takei, H, Ogawa, R & Mihara, K 2017, 'Safety of human papillomavirus vaccines in healthy young women: a
meta-analysis of 24 controlled studies', JPHCS, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5504559/>.
Search strategy The researchers explored MEDLINE, COCHRANE, and JCRM databases while systematically utilizing the following
MeSH terms.
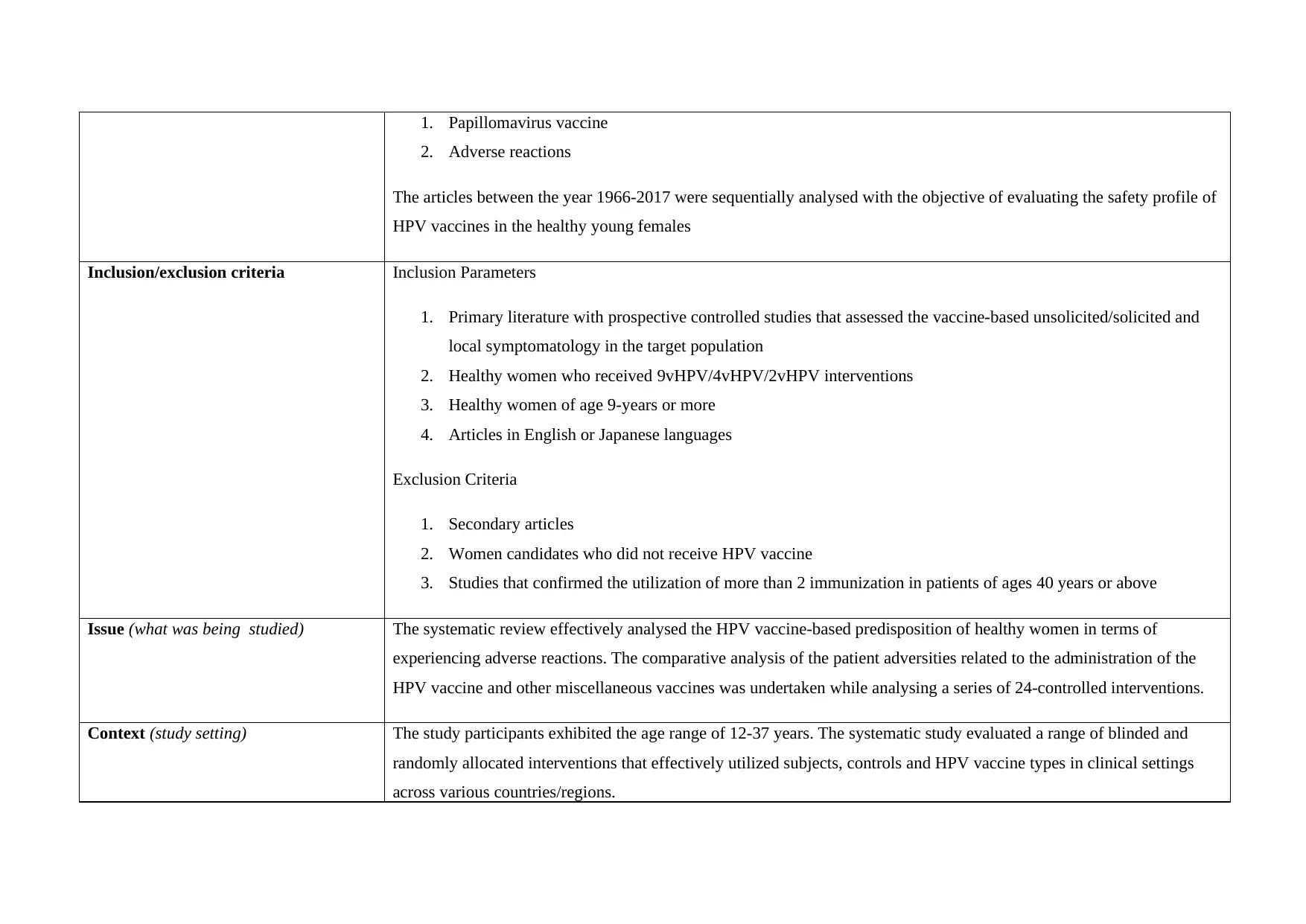
1. Papillomavirus vaccine
2. Adverse reactions
The articles between the year 1966-2017 were sequentially analysed with the objective of evaluating the safety profile of
HPV vaccines in the healthy young females
Inclusion/exclusion criteria Inclusion Parameters
1. Primary literature with prospective controlled studies that assessed the vaccine-based unsolicited/solicited and
local symptomatology in the target population
2. Healthy women who received 9vHPV/4vHPV/2vHPV interventions
3. Healthy women of age 9-years or more
4. Articles in English or Japanese languages
Exclusion Criteria
1. Secondary articles
2. Women candidates who did not receive HPV vaccine
3. Studies that confirmed the utilization of more than 2 immunization in patients of ages 40 years or above
Issue (what was being studied) The systematic review effectively analysed the HPV vaccine-based predisposition of healthy women in terms of
experiencing adverse reactions. The comparative analysis of the patient adversities related to the administration of the
HPV vaccine and other miscellaneous vaccines was undertaken while analysing a series of 24-controlled interventions.
Context (study setting) The study participants exhibited the age range of 12-37 years. The systematic study evaluated a range of blinded and
randomly allocated interventions that effectively utilized subjects, controls and HPV vaccine types in clinical settings
across various countries/regions.
2. Adverse reactions
The articles between the year 1966-2017 were sequentially analysed with the objective of evaluating the safety profile of
HPV vaccines in the healthy young females
Inclusion/exclusion criteria Inclusion Parameters
1. Primary literature with prospective controlled studies that assessed the vaccine-based unsolicited/solicited and
local symptomatology in the target population
2. Healthy women who received 9vHPV/4vHPV/2vHPV interventions
3. Healthy women of age 9-years or more
4. Articles in English or Japanese languages
Exclusion Criteria
1. Secondary articles
2. Women candidates who did not receive HPV vaccine
3. Studies that confirmed the utilization of more than 2 immunization in patients of ages 40 years or above
Issue (what was being studied) The systematic review effectively analysed the HPV vaccine-based predisposition of healthy women in terms of
experiencing adverse reactions. The comparative analysis of the patient adversities related to the administration of the
HPV vaccine and other miscellaneous vaccines was undertaken while analysing a series of 24-controlled interventions.
Context (study setting) The study participants exhibited the age range of 12-37 years. The systematic study evaluated a range of blinded and
randomly allocated interventions that effectively utilized subjects, controls and HPV vaccine types in clinical settings
across various countries/regions.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Outcome (main findings) Most of the HPV vaccine-based adverse reactions appeared transiently in the treated subjects. HPV vaccine
administration elevated the risk of injection location symptoms in the healthy young women.
Table 3. Experimental quantitative studies
Full reference:
Nursing
Ma, SJ, Li, X, Xiong, YQ, Yao, AL & Chen, Q 2015, 'Combination Measles-Mumps-Rubella-Varicella Vaccine in Healthy Children
- A Systematic Review and Meta-analysis of Immunogenicity and Safety', Medicine, vol 94, no. 44,
<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4915870/>.
Study Design (e.g.: cohort,
RCT,)
The systematic analysis of the randomized controlled trials (RCTs)
Population (how many
participants, age, gender,
disease, etc)
Indeed, 2190 research studies along with 99 clinical trials were tracked through online exploration. Furthermore, 19 RCTs that
evaluated 21098 candidates became an integral part of the quantitative analysis.
Intervention (what was
being implemented)
The immune response of the study subjects against the administration of Measles-Mumps-Rubella-Varicella Vaccine was
systematically evaluated in the healthy children of age groups 9-24 months.
Comparison (was the
intervention being compared
The researchers effectively compared and analysed the safety and immunogenicity outcomes between MMR+V (measles-mumps-
rubella-varicella) and MMR (measles-mumps-rubella) groups.
administration elevated the risk of injection location symptoms in the healthy young women.
Table 3. Experimental quantitative studies
Full reference:
Nursing
Ma, SJ, Li, X, Xiong, YQ, Yao, AL & Chen, Q 2015, 'Combination Measles-Mumps-Rubella-Varicella Vaccine in Healthy Children
- A Systematic Review and Meta-analysis of Immunogenicity and Safety', Medicine, vol 94, no. 44,
<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4915870/>.
Study Design (e.g.: cohort,
RCT,)
The systematic analysis of the randomized controlled trials (RCTs)
Population (how many
participants, age, gender,
disease, etc)
Indeed, 2190 research studies along with 99 clinical trials were tracked through online exploration. Furthermore, 19 RCTs that
evaluated 21098 candidates became an integral part of the quantitative analysis.
Intervention (what was
being implemented)
The immune response of the study subjects against the administration of Measles-Mumps-Rubella-Varicella Vaccine was
systematically evaluated in the healthy children of age groups 9-24 months.
Comparison (was the
intervention being compared
The researchers effectively compared and analysed the safety and immunogenicity outcomes between MMR+V (measles-mumps-
rubella-varicella) and MMR (measles-mumps-rubella) groups.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
to another strategy, e.g.
placebo?)
Outcome (main findings) The study outcomes revealed the occurrence of vaccination-based serious adverse events in 1% of the entire study groups. MMRV
and MMR groups exhibited comparable safety and immunogenicity pattern. These findings indicated that the benefits of MMRV
vaccination outweighed the associated risks and patient adversities.
Table 4. Qualitative study
Full reference
Nursing
Forster, AS, Rockliffe, L, Chorley, AJ, Marlow, LAV, Bedford, H, Smith, SG & Waller, J 2016, 'A qualitative systematic review of
factors influencing parents’ vaccination decision-making in the United Kingdom', SSM-Population Health, pp. 603-612,
<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5165048/>.
Study Design (ie: cohort) Qualitative systematic analysis
Population (how many
participants, age, gender,
disease, etc)
The qualitative study intervention effectively utilized 34 peer-reviewed papers related to the child immunization program.
Behavioural outcomes of the parents of vaccination candidates were recorded through survey responses, focus groups and interview
sessions.
Issue (what was being
studied)
The study intervention subjectively evaluated the influential factors that potentially impact the parental decisions related to the
vaccination of their children.
Context (study setting) The qualitative study executed thematic analysis while reviewing immunization-decision-related research papers across the databases
like Web of Science, Social Policy and Practice, Embase, CINAHL Plus, MEDLINE and PsycINFO.
Outcome (main findings) The findings revealed that vaccination attitude of parents was influenced by the following factors.
placebo?)
Outcome (main findings) The study outcomes revealed the occurrence of vaccination-based serious adverse events in 1% of the entire study groups. MMRV
and MMR groups exhibited comparable safety and immunogenicity pattern. These findings indicated that the benefits of MMRV
vaccination outweighed the associated risks and patient adversities.
Table 4. Qualitative study
Full reference
Nursing
Forster, AS, Rockliffe, L, Chorley, AJ, Marlow, LAV, Bedford, H, Smith, SG & Waller, J 2016, 'A qualitative systematic review of
factors influencing parents’ vaccination decision-making in the United Kingdom', SSM-Population Health, pp. 603-612,
<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5165048/>.
Study Design (ie: cohort) Qualitative systematic analysis
Population (how many
participants, age, gender,
disease, etc)
The qualitative study intervention effectively utilized 34 peer-reviewed papers related to the child immunization program.
Behavioural outcomes of the parents of vaccination candidates were recorded through survey responses, focus groups and interview
sessions.
Issue (what was being
studied)
The study intervention subjectively evaluated the influential factors that potentially impact the parental decisions related to the
vaccination of their children.
Context (study setting) The qualitative study executed thematic analysis while reviewing immunization-decision-related research papers across the databases
like Web of Science, Social Policy and Practice, Embase, CINAHL Plus, MEDLINE and PsycINFO.
Outcome (main findings) The findings revealed that vaccination attitude of parents was influenced by the following factors.

1. Non-deliberative decisions that advocated the non-utilization of vaccination intervention in accordance with the social
conventions.
2. Deliberative decisions that advocated the vaccination requirement after evaluating the minimum risks and potential benefits
of a multitude of immunization interventions.
conventions.
2. Deliberative decisions that advocated the vaccination requirement after evaluating the minimum risks and potential benefits
of a multitude of immunization interventions.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Question 3 – 800 words
The assessment by (Stassijns, Bollaerts, Baay & Verstraeten, 2016) reveals the absence of any
potential risk of febrile convulsions or other adverse events in relation to the administration of
adjuvanted vaccines to the children of various age groups. Indeed, limited research interventions
regarding adjuvanted vaccines resulted in the lack of any strong evidence of patient adversities or
allergic reactions in relation to the utilization of adjuvanted vaccines in the target population.
However, research literature provides substantial evidence regarding the enhancement of vaccine
immunogenicity following the systematic administration of adjuvant vaccines (Petrovsky 2015). The
findings by (Stassijns et al. 2016) exhibit a moderate relative value in comparison to the outcomes
emphasized by evidence-based literature in relation to the safety profile of adjuvant vaccines. Lack of
appropriate data regarding the tolerability and reactogenicity of adjuvant vaccines substantiates the
requirement of conducting prospective research interventions for effectively improving the awareness
and knowledge of healthcare professionals and caretakers regarding the administration of adjuvant
vaccines to the eligible children.
The evaluation by (Ogawa et al. 2017) emphasized the occurrence of transient adverse reactions and
injection site symptoms after the administration of HPV vaccines in healthy young women. The
relative significance of the findings of this research study is advocated by the evidence-based research
literature. For example, the assessment by (Basu et al. 2013) affirms the high significance of HPV
vaccination in terms of reducing the risk of cervical cancer in adolescent girls. The administration of
one million HPV dosages in Australia leads to the occurrence of anaphylaxis in 1.7 cases. This fact
affirms the safety profile of HPV vaccine advocates the requirement of its administration to the target
population.
The findings by (Ma et al. 2015) affirm the immunogenicity and safety profile of MMRV vaccine for
the target population. The MMRV vaccine-related solicited symptoms include fever and rash that
rarely lead to any potential clinical complication in the treated patients. However, unsolicited
symptoms including rhinitis, vomiting, cough, otitis media, irritability and urinary tract infections
manifestations were recorded in 5%-10% subjects who receive MMRV vaccine. Furthermore, the rare
occurrence of serious adverse events and benefits related to the prevention of measles, mumps, rubella
and varicella overweigh the associated risks of MMRV utilization by the target population. Similar
findings were revealed by the evidence-based literature that confirms the limited MMRV vaccine-
The assessment by (Stassijns, Bollaerts, Baay & Verstraeten, 2016) reveals the absence of any
potential risk of febrile convulsions or other adverse events in relation to the administration of
adjuvanted vaccines to the children of various age groups. Indeed, limited research interventions
regarding adjuvanted vaccines resulted in the lack of any strong evidence of patient adversities or
allergic reactions in relation to the utilization of adjuvanted vaccines in the target population.
However, research literature provides substantial evidence regarding the enhancement of vaccine
immunogenicity following the systematic administration of adjuvant vaccines (Petrovsky 2015). The
findings by (Stassijns et al. 2016) exhibit a moderate relative value in comparison to the outcomes
emphasized by evidence-based literature in relation to the safety profile of adjuvant vaccines. Lack of
appropriate data regarding the tolerability and reactogenicity of adjuvant vaccines substantiates the
requirement of conducting prospective research interventions for effectively improving the awareness
and knowledge of healthcare professionals and caretakers regarding the administration of adjuvant
vaccines to the eligible children.
The evaluation by (Ogawa et al. 2017) emphasized the occurrence of transient adverse reactions and
injection site symptoms after the administration of HPV vaccines in healthy young women. The
relative significance of the findings of this research study is advocated by the evidence-based research
literature. For example, the assessment by (Basu et al. 2013) affirms the high significance of HPV
vaccination in terms of reducing the risk of cervical cancer in adolescent girls. The administration of
one million HPV dosages in Australia leads to the occurrence of anaphylaxis in 1.7 cases. This fact
affirms the safety profile of HPV vaccine advocates the requirement of its administration to the target
population.
The findings by (Ma et al. 2015) affirm the immunogenicity and safety profile of MMRV vaccine for
the target population. The MMRV vaccine-related solicited symptoms include fever and rash that
rarely lead to any potential clinical complication in the treated patients. However, unsolicited
symptoms including rhinitis, vomiting, cough, otitis media, irritability and urinary tract infections
manifestations were recorded in 5%-10% subjects who receive MMRV vaccine. Furthermore, the rare
occurrence of serious adverse events and benefits related to the prevention of measles, mumps, rubella
and varicella overweigh the associated risks of MMRV utilization by the target population. Similar
findings were revealed by the evidence-based literature that confirms the limited MMRV vaccine-
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

based febrile seizure adversity in comparison to potential clinical complications that emanate after the
establishment of measles in the predisposed children (Top & MacDonald 2014). Hence, MMRV-
vaccine utilization is highly recommended for children with the objective of reducing their risk of
measles-based adverse clinical complications.
The assessment by (Forster et al. 2016) presented different parental approaches regarding the
utilization of vaccines for children and adolescents. These approaches are radically governed by
cultural conventions, apprehension, pseudo beliefs regarding vaccines, emotions, individual
experience and social judgement of the care takers and parents. However, the parents who give due
importance to the benefits of vaccines deliberately provide unconditional consent for vaccine
administration in accordance with the prescribed administration schedule. The findings by (Meleo-
Erwin et al. 2017) reveal the dissemination of anti-vaccination myths by social media and parenting
blogs. These myths regarding the safety profile of vaccines substantially impact the vaccine utilization
decision of parents and caretakers in the society.
The evidence-based findings substantiate the requirement of cultural and social reforms to rectify the
knowledge base of parents and caretakers regarding the beneficial outcomes associated with vaccine
administration to the children of various age groups. The findings of selected systematic interventions
and their comparative analysis with the evidence-based literature outcomes radically reveal the high
potential of immunization interventions in terms of reducing the risk of contagious conditions in the
predisposed children. Evidence-based literature presents limited findings related to the vaccination-
based serious adverse events in the treated individuals. The findings rationally emphasize the need for
conducting education and counselling sessions with the parents and pregnant women with the
objective of making them understanding the elevated requirement of vaccine utilization at the national
scale. This will undoubtedly reduce the frequency of a multitude of life threatening disease conditions
in children and adolescents of various age groups across the community environment. Indeed, the
cultural variations among societies crease a lot of misconception and apprehensions regarding the
utilization of vaccinations for preventing infectious conditions. The non-utilization of vaccination
products under the sustained influence of social norms substantially increases the risk of children in
terms of acquiring a range of chronic disease conditions across the community environment. Indeed,
counselling sessions must focus on improving the knowledge of the parents and caretakers regarding
the mandatory need of using vaccines to reduce the burden of various avoidable diseases and their
debilitating manifestations in the target population. Informed discussions regarding immunization
requirements (based on the findings from the selected studies) will assist the healthcare professionals
in configuring mutual consensus within the parent community regarding systematic use of vaccines
for the prevention and prophylaxis of various disease complication in children and adolescents.
establishment of measles in the predisposed children (Top & MacDonald 2014). Hence, MMRV-
vaccine utilization is highly recommended for children with the objective of reducing their risk of
measles-based adverse clinical complications.
The assessment by (Forster et al. 2016) presented different parental approaches regarding the
utilization of vaccines for children and adolescents. These approaches are radically governed by
cultural conventions, apprehension, pseudo beliefs regarding vaccines, emotions, individual
experience and social judgement of the care takers and parents. However, the parents who give due
importance to the benefits of vaccines deliberately provide unconditional consent for vaccine
administration in accordance with the prescribed administration schedule. The findings by (Meleo-
Erwin et al. 2017) reveal the dissemination of anti-vaccination myths by social media and parenting
blogs. These myths regarding the safety profile of vaccines substantially impact the vaccine utilization
decision of parents and caretakers in the society.
The evidence-based findings substantiate the requirement of cultural and social reforms to rectify the
knowledge base of parents and caretakers regarding the beneficial outcomes associated with vaccine
administration to the children of various age groups. The findings of selected systematic interventions
and their comparative analysis with the evidence-based literature outcomes radically reveal the high
potential of immunization interventions in terms of reducing the risk of contagious conditions in the
predisposed children. Evidence-based literature presents limited findings related to the vaccination-
based serious adverse events in the treated individuals. The findings rationally emphasize the need for
conducting education and counselling sessions with the parents and pregnant women with the
objective of making them understanding the elevated requirement of vaccine utilization at the national
scale. This will undoubtedly reduce the frequency of a multitude of life threatening disease conditions
in children and adolescents of various age groups across the community environment. Indeed, the
cultural variations among societies crease a lot of misconception and apprehensions regarding the
utilization of vaccinations for preventing infectious conditions. The non-utilization of vaccination
products under the sustained influence of social norms substantially increases the risk of children in
terms of acquiring a range of chronic disease conditions across the community environment. Indeed,
counselling sessions must focus on improving the knowledge of the parents and caretakers regarding
the mandatory need of using vaccines to reduce the burden of various avoidable diseases and their
debilitating manifestations in the target population. Informed discussions regarding immunization
requirements (based on the findings from the selected studies) will assist the healthcare professionals
in configuring mutual consensus within the parent community regarding systematic use of vaccines
for the prevention and prophylaxis of various disease complication in children and adolescents.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





