Analysis of Industrial Manufacturing of Vitamin B2 Processes
VerifiedAdded on 2022/08/29
|6
|1983
|25
Essay
AI Summary
This essay provides a comprehensive overview of the industrial manufacturing of Vitamin B2, also known as riboflavin. It begins with a historical perspective, tracing the development of process techniques, from early chemical synthesis to modern fermentation methods. The essay then delves into detailed process route comparisons, analyzing the principles and industrial applications of various manufacturing techniques. A significant portion is dedicated to the bioprocess technique, describing the manufacturing steps, including upstream and downstream processing, medium preparation, and fermentation processes. The essay also includes an analysis of the inventory, detailing the components and their values within the production process. Finally, it evaluates the industrial manufacture of Vitamin B2 based on sustainability and life cycle assessment, highlighting the environmental impacts and benefits of the process, including the reduction of global warming, acidification, and ozone creation. The essay concludes by emphasizing the importance of sustainable practices in Vitamin B2 production.

Introduction:
In the literature survey, the applied methodologies show the outcomes in a significant
enhancement of vitamin B2 content as (approximate 2-3 fold enhancement). Hence, it
represents an effective and convenient food grade biotechnological usage of the production of
riboflavin or vitamin B2 (Capozzi et al., 2011).
There are a lot of methods which have used for the production of vitamin B2, some of them
from the literature survey are shown below. The SLN process or solid lipid nanoparticles are
the spherical particles from the solid fats. It can be utilized as the delivery systems by
encapsulating the bioactivities. In this process, FHCO is used for encapsulating the riboflavin
in terms of a hydrophilic bioactive (Couto, Alvarez and Temelli, 2017).
The first evaluation of riboflavin by 3D bio-printing is done in previous researches. The
proposed printing procedure deals the easy control over the printed line width. Hence, it can
confirm that the living tissue is printed with the high reliability feature (Jang et al., 2016).
Riboflavin is the group member of vitamins which occurs under the Vitamin B complex. This
property makes this vitamin a water soluble vitamin. In the application of health care, it is
prescribed by the combined formulation of other B complex vitamins. It is prescribed as a
prophylactic supplement for development prevention of its deficiency (Peechakara and
Gupta, 2019).
History of Vitamin B2:
Vitamin B2 is also recognized as Riboflavin (Bergwik and Akerstrom, 2018). It was firstly
sequestered by Blyth in 1879 by using Whey, and the yellow, water soluble, fluorescent
material known as lacto-chrome. On the basis of IUPAC rules, riboflavin or Vitamin B2 (83-
88-5) is also known as lacto-flavin. The human’s daily requirement for riboflavin is
approximate 1.7 mg. The deficiency of Vitamin B2 enhances various symptoms in human
body such as, dermatitis versions etc.
This vitamin cannot be deposited in the human body and it requires a constant consumption
for human body.
Process route comparisons in terms of process principle and industrial applications:
In the literature survey, the applied methodologies show the outcomes in a significant
enhancement of vitamin B2 content as (approximate 2-3 fold enhancement). Hence, it
represents an effective and convenient food grade biotechnological usage of the production of
riboflavin or vitamin B2 (Capozzi et al., 2011).
There are a lot of methods which have used for the production of vitamin B2, some of them
from the literature survey are shown below. The SLN process or solid lipid nanoparticles are
the spherical particles from the solid fats. It can be utilized as the delivery systems by
encapsulating the bioactivities. In this process, FHCO is used for encapsulating the riboflavin
in terms of a hydrophilic bioactive (Couto, Alvarez and Temelli, 2017).
The first evaluation of riboflavin by 3D bio-printing is done in previous researches. The
proposed printing procedure deals the easy control over the printed line width. Hence, it can
confirm that the living tissue is printed with the high reliability feature (Jang et al., 2016).
Riboflavin is the group member of vitamins which occurs under the Vitamin B complex. This
property makes this vitamin a water soluble vitamin. In the application of health care, it is
prescribed by the combined formulation of other B complex vitamins. It is prescribed as a
prophylactic supplement for development prevention of its deficiency (Peechakara and
Gupta, 2019).
History of Vitamin B2:
Vitamin B2 is also recognized as Riboflavin (Bergwik and Akerstrom, 2018). It was firstly
sequestered by Blyth in 1879 by using Whey, and the yellow, water soluble, fluorescent
material known as lacto-chrome. On the basis of IUPAC rules, riboflavin or Vitamin B2 (83-
88-5) is also known as lacto-flavin. The human’s daily requirement for riboflavin is
approximate 1.7 mg. The deficiency of Vitamin B2 enhances various symptoms in human
body such as, dermatitis versions etc.
This vitamin cannot be deposited in the human body and it requires a constant consumption
for human body.
Process route comparisons in terms of process principle and industrial applications:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
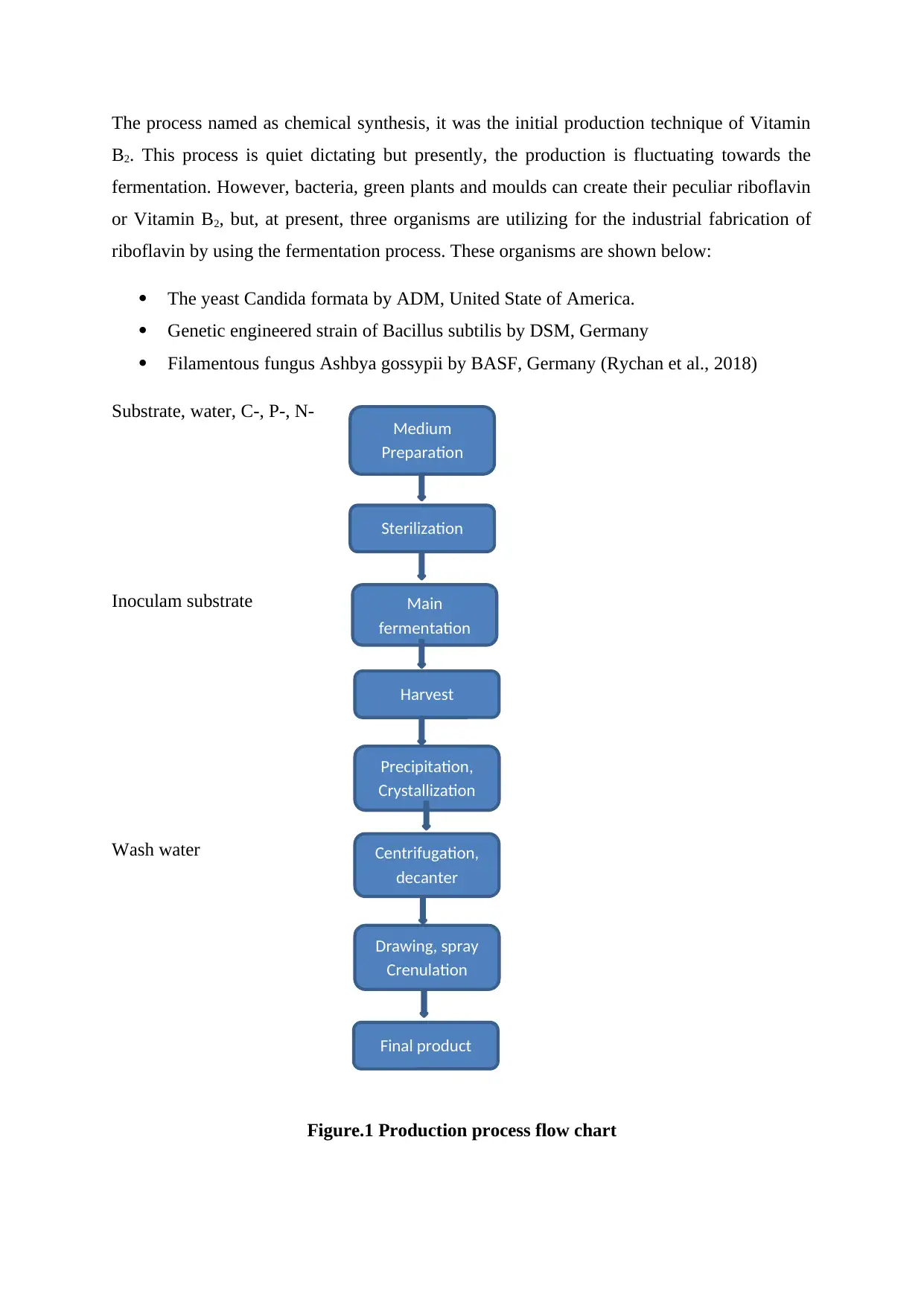
The process named as chemical synthesis, it was the initial production technique of Vitamin
B2. This process is quiet dictating but presently, the production is fluctuating towards the
fermentation. However, bacteria, green plants and moulds can create their peculiar riboflavin
or Vitamin B2, but, at present, three organisms are utilizing for the industrial fabrication of
riboflavin by using the fermentation process. These organisms are shown below:
The yeast Candida formata by ADM, United State of America.
Genetic engineered strain of Bacillus subtilis by DSM, Germany
Filamentous fungus Ashbya gossypii by BASF, Germany (Rychan et al., 2018)
Substrate, water, C-, P-, N-
Inoculam substrate
Wash water
Figure.1 Production process flow chart
Medium
Preparation
Sterilization
Final product
Main
fermentation
Harvest
Precipitation,
Crystallization
Centrifugation,
decanter
Drawing, spray
Crenulation
B2. This process is quiet dictating but presently, the production is fluctuating towards the
fermentation. However, bacteria, green plants and moulds can create their peculiar riboflavin
or Vitamin B2, but, at present, three organisms are utilizing for the industrial fabrication of
riboflavin by using the fermentation process. These organisms are shown below:
The yeast Candida formata by ADM, United State of America.
Genetic engineered strain of Bacillus subtilis by DSM, Germany
Filamentous fungus Ashbya gossypii by BASF, Germany (Rychan et al., 2018)
Substrate, water, C-, P-, N-
Inoculam substrate
Wash water
Figure.1 Production process flow chart
Medium
Preparation
Sterilization
Final product
Main
fermentation
Harvest
Precipitation,
Crystallization
Centrifugation,
decanter
Drawing, spray
Crenulation

Production process:
In this production process of Vitamin B2, upstream processing contains of medium planning
and the connected continuous counter current sterilization. Various feed components at
different quantity are used in this production process such as, sulphuric acid, sunflower oil,
malt and yeast extract, 70% of glucose syrup and concentrated salt mixture at the room
temperature. The operation named as fermentation held in batch wise formation with 10% of
inoculum ratios. Downstream processing begins with the harvesting monitored by the
crystallization, decanter and final spray dryer.
In this process, the concentration of riboflavin is expected to be 70% and for the residual it is
30% by including salt and the biomass. The final product is obtained in the form of dry
powder or the Granulate.
Upstream process:
This upstream process involves sterilization and preparation of the medium. This medium’s
composition does not permit the sterilization of all the mixed components. It uses the
classical batch conditions at 1210C in 20 minutes. Hence, the medium can be divided into
various groups as shown below:
Sunflower oil and glucose
Yeast, peptone and malt extracts.
Water salts
Methionine
The latter in process is sterilized by thee filtration process. In all the required elements,
sulphuric acid does not require sterilization. There are only toe solutions which are prepared
separately such as,
Glucose at 70% rate
Other nutrient tanks or P-4.
Fermentation process:
In numerous processes, the essential seed principles are produced at various seed fermenters.
The ending seed principles are the beginning of inoculum for the chief fermentation process.
The assigned time period for seed fermentation process is approximate 50 hours, while the
major fermentation lasts at approximate 500 hours. On this time period, the strain originates
27g/L riboflavin.
Fermentation needs aeration which is consummated by the sterile filter (P-8) and gas
compressor (P-7). Exhaust gases are cleaned by using a second filter (P-10). A little amount
of the harvested broth is placed at an alternative tank and it is utilized as inoculum for the
upcoming batch (P-13).
In this production process of Vitamin B2, upstream processing contains of medium planning
and the connected continuous counter current sterilization. Various feed components at
different quantity are used in this production process such as, sulphuric acid, sunflower oil,
malt and yeast extract, 70% of glucose syrup and concentrated salt mixture at the room
temperature. The operation named as fermentation held in batch wise formation with 10% of
inoculum ratios. Downstream processing begins with the harvesting monitored by the
crystallization, decanter and final spray dryer.
In this process, the concentration of riboflavin is expected to be 70% and for the residual it is
30% by including salt and the biomass. The final product is obtained in the form of dry
powder or the Granulate.
Upstream process:
This upstream process involves sterilization and preparation of the medium. This medium’s
composition does not permit the sterilization of all the mixed components. It uses the
classical batch conditions at 1210C in 20 minutes. Hence, the medium can be divided into
various groups as shown below:
Sunflower oil and glucose
Yeast, peptone and malt extracts.
Water salts
Methionine
The latter in process is sterilized by thee filtration process. In all the required elements,
sulphuric acid does not require sterilization. There are only toe solutions which are prepared
separately such as,
Glucose at 70% rate
Other nutrient tanks or P-4.
Fermentation process:
In numerous processes, the essential seed principles are produced at various seed fermenters.
The ending seed principles are the beginning of inoculum for the chief fermentation process.
The assigned time period for seed fermentation process is approximate 50 hours, while the
major fermentation lasts at approximate 500 hours. On this time period, the strain originates
27g/L riboflavin.
Fermentation needs aeration which is consummated by the sterile filter (P-8) and gas
compressor (P-7). Exhaust gases are cleaned by using a second filter (P-10). A little amount
of the harvested broth is placed at an alternative tank and it is utilized as inoculum for the
upcoming batch (P-13).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Downstream:
Afterwards the fermentation process, the broth is collected into the harvest tank named as P-
15. The product’s part crystallizes in the harvesting and fermenter tank as well.
Crystallization is accomplished in the crystallizer named as P-16 by the evaporation process
for some water. After this process, the suspension is deposited in the tank named as P-17.
From the decanter, three streams are collected, in which two streams are in liquid phase and
one is crystal suspension named as P-18. For achieving higher purity, a washing stage with
second separation is utilized.
The latter stage is drying stage. It can be done by using the spray dryer for obtaining powered
product or it can also be done by smearing a spray granulation for obtaining granulate. It can
be treated in more precise manner. (Revuelta, Ledesma-Amaro and Jiménez, 2016)
Analysis of inventory:
In this whole process, air is utilized for aeration process but solitary, little amount of oxygen
is actually obsessive which less than 1% is. Nitrogen is inactive and permits through the
system in this process and it became unchanged. Water is utilized in the fermentation process
and in the downstream processing. There are a lot of organic material which are used in this
process such as, malt, glucose and yeast extracts, peptone and sunflower oil etc. the main
products utilized in the fermentation process are biomass, riboflavin, CO2 and water etc. In
overall process, approximate 50% of the carbon, 25% CO2 and 25% biomass is used in it.
Components and their values:
In this process, the components and their input, output values are shown below: (Revuelta,
Ledesma-Amaro and Jiménez, 2016)
Biomass- 0(Kg/batch) at input - 46240(Kg/batch) at output
CO2- 0(Kg/batch) at input - 126880(Kg/batch) at output
Methionine- 2400(Kg/batch) at input -22(Kg/batch) at output
Glucose - 30000(Kg/batch) at input-22(Kg/batch) at output
K2HPO4- 1200(Kg/batch) at input - 11(Kg/batch) at output
Manganese Sulfate - 1200(Kg/batch) at input- 11(Kg/batch) at output
Malt extract-30000(Kg/batch) at input- 270(Kg/batch) at output
Nitrogen -3058100(Kg/batch) at input - 3058100(Kg/batch) at output
Oxygen- 928400(Kg/batch) at input - 9195000(Kg/batch) at output
Peptone-30000(Kg/batch) at input -270(Kg/batch) at output
Afterwards the fermentation process, the broth is collected into the harvest tank named as P-
15. The product’s part crystallizes in the harvesting and fermenter tank as well.
Crystallization is accomplished in the crystallizer named as P-16 by the evaporation process
for some water. After this process, the suspension is deposited in the tank named as P-17.
From the decanter, three streams are collected, in which two streams are in liquid phase and
one is crystal suspension named as P-18. For achieving higher purity, a washing stage with
second separation is utilized.
The latter stage is drying stage. It can be done by using the spray dryer for obtaining powered
product or it can also be done by smearing a spray granulation for obtaining granulate. It can
be treated in more precise manner. (Revuelta, Ledesma-Amaro and Jiménez, 2016)
Analysis of inventory:
In this whole process, air is utilized for aeration process but solitary, little amount of oxygen
is actually obsessive which less than 1% is. Nitrogen is inactive and permits through the
system in this process and it became unchanged. Water is utilized in the fermentation process
and in the downstream processing. There are a lot of organic material which are used in this
process such as, malt, glucose and yeast extracts, peptone and sunflower oil etc. the main
products utilized in the fermentation process are biomass, riboflavin, CO2 and water etc. In
overall process, approximate 50% of the carbon, 25% CO2 and 25% biomass is used in it.
Components and their values:
In this process, the components and their input, output values are shown below: (Revuelta,
Ledesma-Amaro and Jiménez, 2016)
Biomass- 0(Kg/batch) at input - 46240(Kg/batch) at output
CO2- 0(Kg/batch) at input - 126880(Kg/batch) at output
Methionine- 2400(Kg/batch) at input -22(Kg/batch) at output
Glucose - 30000(Kg/batch) at input-22(Kg/batch) at output
K2HPO4- 1200(Kg/batch) at input - 11(Kg/batch) at output
Manganese Sulfate - 1200(Kg/batch) at input- 11(Kg/batch) at output
Malt extract-30000(Kg/batch) at input- 270(Kg/batch) at output
Nitrogen -3058100(Kg/batch) at input - 3058100(Kg/batch) at output
Oxygen- 928400(Kg/batch) at input - 9195000(Kg/batch) at output
Peptone-30000(Kg/batch) at input -270(Kg/batch) at output
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Riboflavin- 0(Kg/batch) at input - 9420(Kg/batch) at output
Riboflavin in crystalline formation- 0(Kg/batch) at input-84800(Kg/batch) at output
Sunflower oil- 90000(Kg/batch) at input- 811(Kg/batch) at output
Water-3078000(Kg/batch) at input-3112000(Kg/batch) at output
Yeast extract- 43157800(Kg/batch) at input-53157284(Kg/batch) at output
Evaluation of the industrial manufacture of Vitamin B2 on the basis of sustainability
and life cycle assessment:
Apart from the cost, sustainability is a major issue for switching towards the bioprocesses.
However, the ecological influence prediction is challenging. In the case of vitamin B2 or
riboflavin, a life cycle calculation elaborated that the fermented procedure is better than the
chemical one by scoring 4 out of 5 indicators. These indicators are evaluated on the basis of
various parameters such as, global warning potential reduction by 30% (by CO2 reduction),
reduction in acidification by 50% (by SO4 reduction), ozone creation by 60% ( by NO3
reduction) (Acevedo-Rocha et al. 2019).
Riboflavin in crystalline formation- 0(Kg/batch) at input-84800(Kg/batch) at output
Sunflower oil- 90000(Kg/batch) at input- 811(Kg/batch) at output
Water-3078000(Kg/batch) at input-3112000(Kg/batch) at output
Yeast extract- 43157800(Kg/batch) at input-53157284(Kg/batch) at output
Evaluation of the industrial manufacture of Vitamin B2 on the basis of sustainability
and life cycle assessment:
Apart from the cost, sustainability is a major issue for switching towards the bioprocesses.
However, the ecological influence prediction is challenging. In the case of vitamin B2 or
riboflavin, a life cycle calculation elaborated that the fermented procedure is better than the
chemical one by scoring 4 out of 5 indicators. These indicators are evaluated on the basis of
various parameters such as, global warning potential reduction by 30% (by CO2 reduction),
reduction in acidification by 50% (by SO4 reduction), ozone creation by 60% ( by NO3
reduction) (Acevedo-Rocha et al. 2019).

References
Acevedo-rocha, C., Gronenberg, L., Mack, M., Commichau, F. and Genee, H. (2019)
Microbial cell factories for the sustainable manufacturing of B vitamins. Current Opinion in
Biotechnology. 56(1), pp. 18-29. Available from:
https://www.sciencedirect.com/science/article/pii/S0958166918300740 [Accessed 24 march
2020].
Bergwik, J. and Åkerström, B., 2018. Riboflavin/vitamin B2 radicals-a novel target for the
radical scavenger α1-microglobulin. Free Radical Biology and Medicine, 120, p.S99.
Capozzi, V., Menga, V., Digesu, A.M., De Vita, P., van Sinderen, D., Cattivelli, L., Fares, C.
and Spano, G., 2011. Biotechnological production of vitamin B2-enriched bread and
pasta. Journal of agricultural and food chemistry, 59(14), pp.8013-8020.
Couto, R., Alvarez, V. and Temelli, F., 2017. Encapsulation of Vitamin B2 in solid lipid
nanoparticles using supercritical CO2. The Journal of Supercritical Fluids, 120, pp.432-442.
EFSA Panel on Additives and Products or Substances used in Animal Feed (EFSA FEEDAP
Panel), Rychen, G., Aquilina, G., Azimonti, G., Bampidis, V., Bastos, M.D.L., Bories, G.,
Chesson, A., Cocconcelli, P.S., Flachowsky, G. and Gropp, J., 2018. Safety and efficacy of
vitamin B2 (riboflavin) produced by Ashbya gossypii■■■■■ for all animal species based on
a dossier submitted by BASF SE. EFSA Journal, 16(7), p.e05337.
Jang, J., Kim, T.G., Kim, B.S., Kim, S.W., Kwon, S.M. and Cho, D.W., 2016. Tailoring
mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced
photo-crosslinking. Acta biomaterialia, 33, pp.88-95.
Peechakara, B.V. and Gupta, M., 2019. Vitamin B2 (riboflavin). In StatPearls [Internet].
StatPearls Publishing.
Revuelta, J.L., Ledesma-Amaro, R. and Jiménez, A., 2016. Industrial Production of Vitamin
B2 by Microbial Fermentation. Industrial Biotechnology of Vitamins, Biopigments, and
Antioxidants, pp.17-40.
Acevedo-rocha, C., Gronenberg, L., Mack, M., Commichau, F. and Genee, H. (2019)
Microbial cell factories for the sustainable manufacturing of B vitamins. Current Opinion in
Biotechnology. 56(1), pp. 18-29. Available from:
https://www.sciencedirect.com/science/article/pii/S0958166918300740 [Accessed 24 march
2020].
Bergwik, J. and Åkerström, B., 2018. Riboflavin/vitamin B2 radicals-a novel target for the
radical scavenger α1-microglobulin. Free Radical Biology and Medicine, 120, p.S99.
Capozzi, V., Menga, V., Digesu, A.M., De Vita, P., van Sinderen, D., Cattivelli, L., Fares, C.
and Spano, G., 2011. Biotechnological production of vitamin B2-enriched bread and
pasta. Journal of agricultural and food chemistry, 59(14), pp.8013-8020.
Couto, R., Alvarez, V. and Temelli, F., 2017. Encapsulation of Vitamin B2 in solid lipid
nanoparticles using supercritical CO2. The Journal of Supercritical Fluids, 120, pp.432-442.
EFSA Panel on Additives and Products or Substances used in Animal Feed (EFSA FEEDAP
Panel), Rychen, G., Aquilina, G., Azimonti, G., Bampidis, V., Bastos, M.D.L., Bories, G.,
Chesson, A., Cocconcelli, P.S., Flachowsky, G. and Gropp, J., 2018. Safety and efficacy of
vitamin B2 (riboflavin) produced by Ashbya gossypii■■■■■ for all animal species based on
a dossier submitted by BASF SE. EFSA Journal, 16(7), p.e05337.
Jang, J., Kim, T.G., Kim, B.S., Kim, S.W., Kwon, S.M. and Cho, D.W., 2016. Tailoring
mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced
photo-crosslinking. Acta biomaterialia, 33, pp.88-95.
Peechakara, B.V. and Gupta, M., 2019. Vitamin B2 (riboflavin). In StatPearls [Internet].
StatPearls Publishing.
Revuelta, J.L., Ledesma-Amaro, R. and Jiménez, A., 2016. Industrial Production of Vitamin
B2 by Microbial Fermentation. Industrial Biotechnology of Vitamins, Biopigments, and
Antioxidants, pp.17-40.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.