BIOCHEMISTRY AND MOLECULAR BIOLOGY Report: Protein-Ligand Interaction
VerifiedAdded on 2022/10/12
|8
|2119
|266
Report
AI Summary
This report delves into the intricate interaction between insulin, a peptide hormone, and its receptor, a transmembrane tyrosine kinase receptor. The study focuses on the significance of this protein-ligand interaction in maintaining glucose homeostasis, glycogen synthesis, and fat storage. It explores the signaling pathways activated upon insulin binding, including the PI3K and MAPK pathways, and their roles in regulating blood glucose levels and cellular processes. The report also examines the molecular mechanisms of insulin receptor activation, highlighting the structural domains and residues involved in insulin binding and receptor activation. Furthermore, it discusses the implications of disrupted insulin signaling, such as in type 2 diabetes, and the physiological consequences of impaired glucose transport and metabolism. The report concludes by emphasizing the multifaceted roles of insulin in various tissues and the importance of this interaction for overall metabolic health.

Running head: BIOCHEMISTRY AND MOLECULAR BIOLOGY
Protein-Ligand Interaction
Name of the Student
Name of the University
Author’s Note
Protein-Ligand Interaction
Name of the Student
Name of the University
Author’s Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
1
BIOCHEMISTRY AND MOLECULAR BIOLOGY
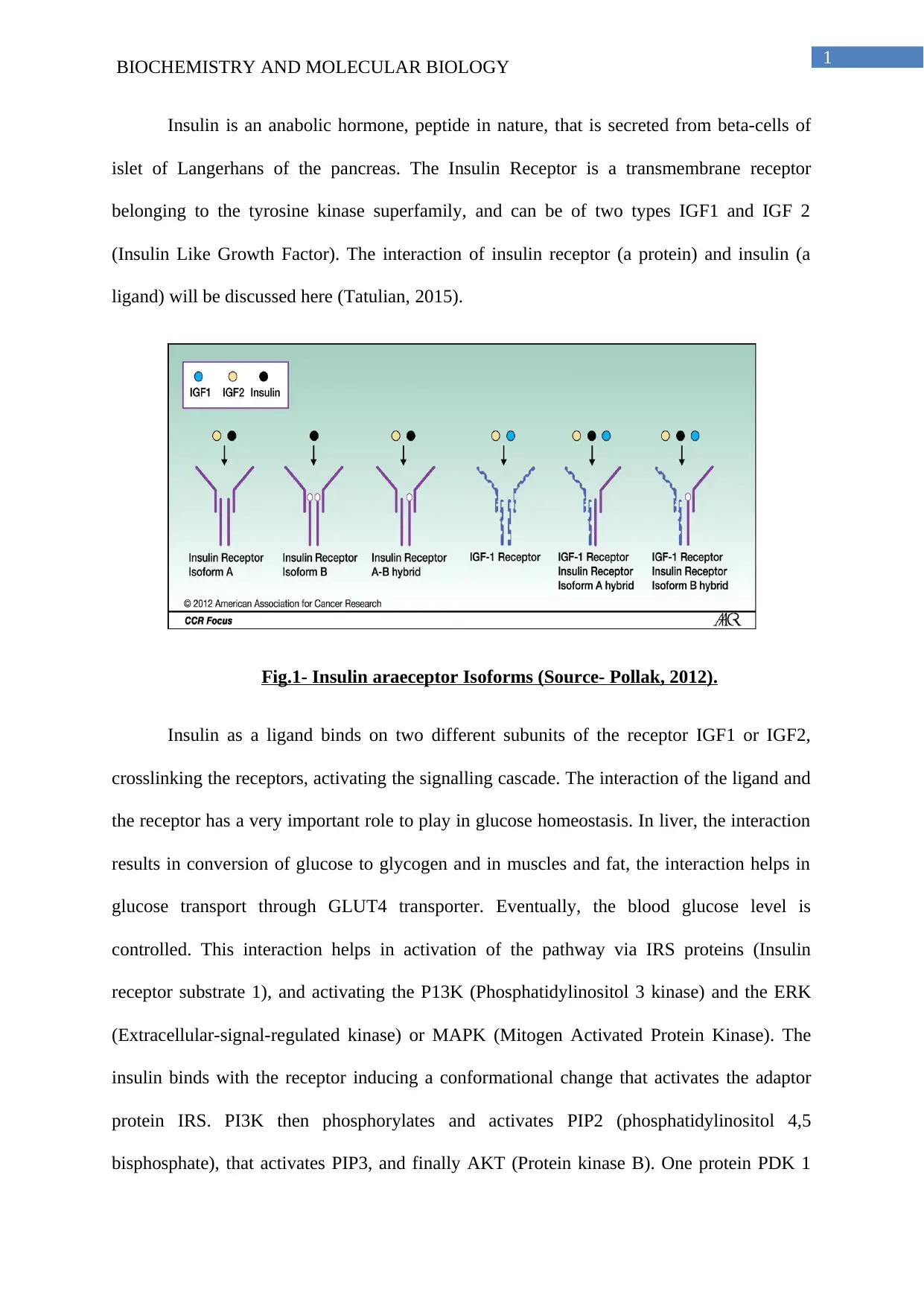
Insulin is an anabolic hormone, peptide in nature, that is secreted from beta-cells of
islet of Langerhans of the pancreas. The Insulin Receptor is a transmembrane receptor
belonging to the tyrosine kinase superfamily, and can be of two types IGF1 and IGF 2
(Insulin Like Growth Factor). The interaction of insulin receptor (a protein) and insulin (a
ligand) will be discussed here (Tatulian, 2015).
Fig.1- Insulin araeceptor Isoforms (Source- Pollak, 2012).
Insulin as a ligand binds on two different subunits of the receptor IGF1 or IGF2,
crosslinking the receptors, activating the signalling cascade. The interaction of the ligand and
the receptor has a very important role to play in glucose homeostasis. In liver, the interaction
results in conversion of glucose to glycogen and in muscles and fat, the interaction helps in
glucose transport through GLUT4 transporter. Eventually, the blood glucose level is
controlled. This interaction helps in activation of the pathway via IRS proteins (Insulin
receptor substrate 1), and activating the P13K (Phosphatidylinositol 3 kinase) and the ERK
(Extracellular-signal-regulated kinase) or MAPK (Mitogen Activated Protein Kinase). The
insulin binds with the receptor inducing a conformational change that activates the adaptor
protein IRS. PI3K then phosphorylates and activates PIP2 (phosphatidylinositol 4,5
bisphosphate), that activates PIP3, and finally AKT (Protein kinase B). One protein PDK 1
BIOCHEMISTRY AND MOLECULAR BIOLOGY
Insulin is an anabolic hormone, peptide in nature, that is secreted from beta-cells of
islet of Langerhans of the pancreas. The Insulin Receptor is a transmembrane receptor
belonging to the tyrosine kinase superfamily, and can be of two types IGF1 and IGF 2
(Insulin Like Growth Factor). The interaction of insulin receptor (a protein) and insulin (a
ligand) will be discussed here (Tatulian, 2015).
Fig.1- Insulin araeceptor Isoforms (Source- Pollak, 2012).
Insulin as a ligand binds on two different subunits of the receptor IGF1 or IGF2,
crosslinking the receptors, activating the signalling cascade. The interaction of the ligand and
the receptor has a very important role to play in glucose homeostasis. In liver, the interaction
results in conversion of glucose to glycogen and in muscles and fat, the interaction helps in
glucose transport through GLUT4 transporter. Eventually, the blood glucose level is
controlled. This interaction helps in activation of the pathway via IRS proteins (Insulin
receptor substrate 1), and activating the P13K (Phosphatidylinositol 3 kinase) and the ERK
(Extracellular-signal-regulated kinase) or MAPK (Mitogen Activated Protein Kinase). The
insulin binds with the receptor inducing a conformational change that activates the adaptor
protein IRS. PI3K then phosphorylates and activates PIP2 (phosphatidylinositol 4,5
bisphosphate), that activates PIP3, and finally AKT (Protein kinase B). One protein PDK 1
2
BIOCHEMISTRY AND MOLECULAR BIOLOGY
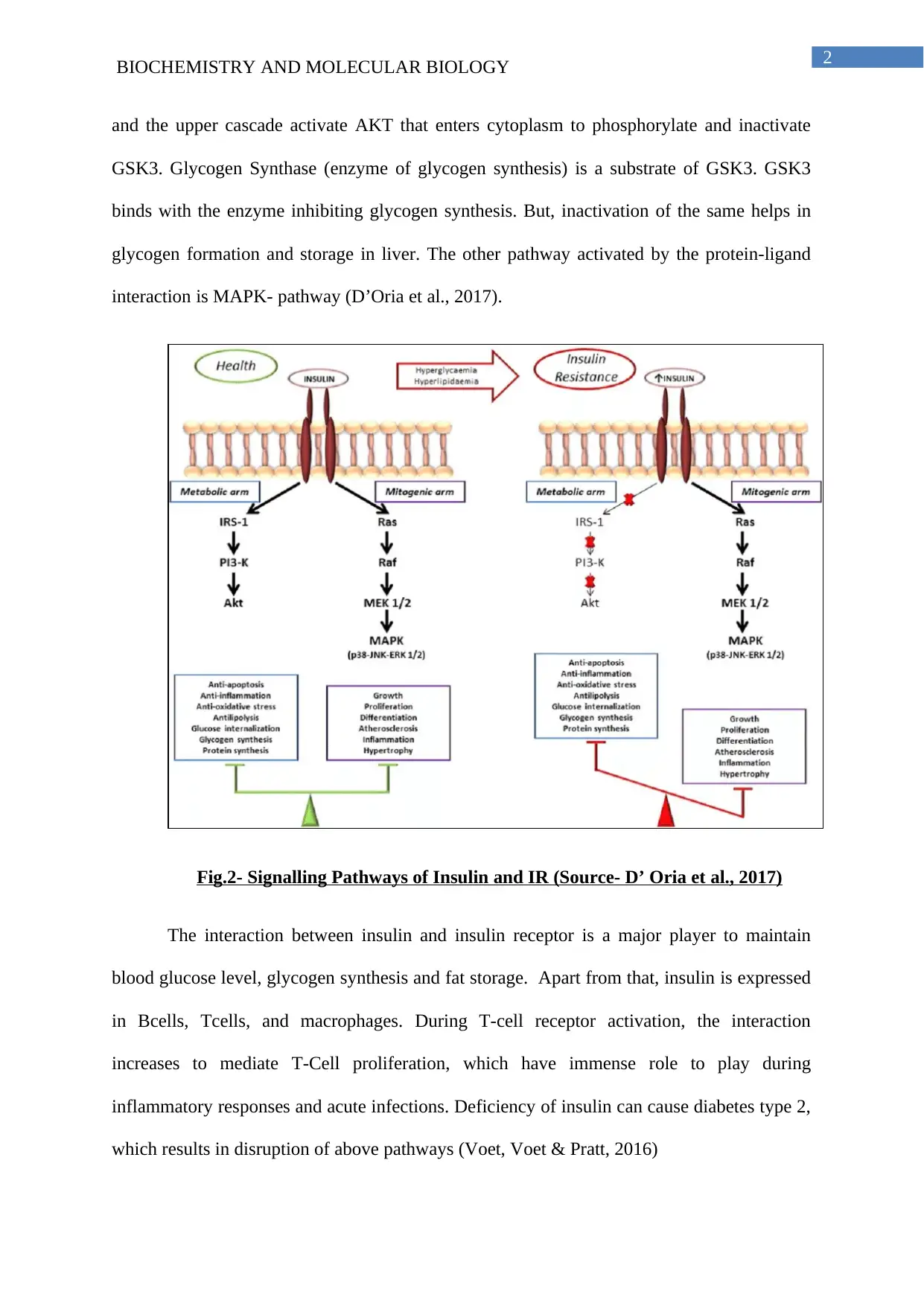
and the upper cascade activate AKT that enters cytoplasm to phosphorylate and inactivate
GSK3. Glycogen Synthase (enzyme of glycogen synthesis) is a substrate of GSK3. GSK3
binds with the enzyme inhibiting glycogen synthesis. But, inactivation of the same helps in
glycogen formation and storage in liver. The other pathway activated by the protein-ligand
interaction is MAPK- pathway (D’Oria et al., 2017).
Fig.2- Signalling Pathways of Insulin and IR (Source- D’ Oria et al., 2017)
The interaction between insulin and insulin receptor is a major player to maintain
blood glucose level, glycogen synthesis and fat storage. Apart from that, insulin is expressed
in Bcells, Tcells, and macrophages. During T-cell receptor activation, the interaction
increases to mediate T-Cell proliferation, which have immense role to play during
inflammatory responses and acute infections. Deficiency of insulin can cause diabetes type 2,
which results in disruption of above pathways (Voet, Voet & Pratt, 2016)
BIOCHEMISTRY AND MOLECULAR BIOLOGY
and the upper cascade activate AKT that enters cytoplasm to phosphorylate and inactivate
GSK3. Glycogen Synthase (enzyme of glycogen synthesis) is a substrate of GSK3. GSK3
binds with the enzyme inhibiting glycogen synthesis. But, inactivation of the same helps in
glycogen formation and storage in liver. The other pathway activated by the protein-ligand
interaction is MAPK- pathway (D’Oria et al., 2017).
Fig.2- Signalling Pathways of Insulin and IR (Source- D’ Oria et al., 2017)
The interaction between insulin and insulin receptor is a major player to maintain
blood glucose level, glycogen synthesis and fat storage. Apart from that, insulin is expressed
in Bcells, Tcells, and macrophages. During T-cell receptor activation, the interaction
increases to mediate T-Cell proliferation, which have immense role to play during
inflammatory responses and acute infections. Deficiency of insulin can cause diabetes type 2,
which results in disruption of above pathways (Voet, Voet & Pratt, 2016)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3
BIOCHEMISTRY AND MOLECULAR BIOLOGY
According to Xu et al., (2018), the Insulin receptor is heterotetramer in nature. Each
subunit comprises of an alpha-subunit, along with leucine-rich repeat domain (beta sheet),
and a cysteine-rich domain (CR). It also contains an alpha-subunit C-terminal segment, and
together known as microreceptor. There are also three fibronectin type 3 domains, which
along with the microreceptor forms holoreceptor. An insert domain along with a C-terminal
peptide domain is also present here. The domains of beta sheet and alpha-CT together bind an
insulin monomer, which modifies the conformation of the insulin and the IR holoreceptor
which initiates the signalling pathway. Before binding the microreceptor, insulin has to alter
its conformation. The active conformation of the insulin takes place when there is a rotation
between Glycine B20 and B27 residues (hinge-like rotations). After this rotation, the 705-714
residues present in the alpha-CT helix, prevail in the position of B25-B30 residues. The B26
turn containing the Tyrosine residues, is stabilized by the hydrogen bonds present there.
These help in rotation of the insulin hormone and attaining its active state. In addition to the
rotation for the stability of the insulin monomer to bind to the receptor, the residues A1-A8 of
the N-terminal A chain alpha-helix must be stable. The stable structure is brought about by
ValA3, IleA2 tight packaging and intramolecular disulphide bonds (De Meyts, 2015). The
interaction of the insulin and insulin receptor is a matter of further research. Some portions
and residues of Site 1 are crucial for the binding of the insulin and insulin receptor. After the
first two hinge rotations of the insulin, the residues of GlyA1, IleA2, ValA3, GlnA5, Tyr
A19, present in Chain A and ValB12, LeuB11, PheB24, and PheB25 on chain B of Site 1, are
exposed. This site 1 along with the residues are inserted within the alpha CT and L1 beta two
sheet and can interact with them via Vander Waal’s interaction. Many such interactions can
happen when the site 1 residues prepare non-polar pockets, and the IR residues are inserted
there. Hydrophobic interactions in this site can help to bind the insulin with the insulin
receptor. Again, some residues show aromatic nature, for example the PheB25 side chain can
BIOCHEMISTRY AND MOLECULAR BIOLOGY
According to Xu et al., (2018), the Insulin receptor is heterotetramer in nature. Each
subunit comprises of an alpha-subunit, along with leucine-rich repeat domain (beta sheet),
and a cysteine-rich domain (CR). It also contains an alpha-subunit C-terminal segment, and
together known as microreceptor. There are also three fibronectin type 3 domains, which
along with the microreceptor forms holoreceptor. An insert domain along with a C-terminal
peptide domain is also present here. The domains of beta sheet and alpha-CT together bind an
insulin monomer, which modifies the conformation of the insulin and the IR holoreceptor
which initiates the signalling pathway. Before binding the microreceptor, insulin has to alter
its conformation. The active conformation of the insulin takes place when there is a rotation
between Glycine B20 and B27 residues (hinge-like rotations). After this rotation, the 705-714
residues present in the alpha-CT helix, prevail in the position of B25-B30 residues. The B26
turn containing the Tyrosine residues, is stabilized by the hydrogen bonds present there.
These help in rotation of the insulin hormone and attaining its active state. In addition to the
rotation for the stability of the insulin monomer to bind to the receptor, the residues A1-A8 of
the N-terminal A chain alpha-helix must be stable. The stable structure is brought about by
ValA3, IleA2 tight packaging and intramolecular disulphide bonds (De Meyts, 2015). The
interaction of the insulin and insulin receptor is a matter of further research. Some portions
and residues of Site 1 are crucial for the binding of the insulin and insulin receptor. After the
first two hinge rotations of the insulin, the residues of GlyA1, IleA2, ValA3, GlnA5, Tyr
A19, present in Chain A and ValB12, LeuB11, PheB24, and PheB25 on chain B of Site 1, are
exposed. This site 1 along with the residues are inserted within the alpha CT and L1 beta two
sheet and can interact with them via Vander Waal’s interaction. Many such interactions can
happen when the site 1 residues prepare non-polar pockets, and the IR residues are inserted
there. Hydrophobic interactions in this site can help to bind the insulin with the insulin
receptor. Again, some residues show aromatic nature, for example the PheB25 side chain can
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4
BIOCHEMISTRY AND MOLECULAR BIOLOGY
move apart from beta two sheets, that help in insertion of the sheet inside the alpha CT
residues of Val and Pro at position 15 and 18. The Tyr residue (aromatic in nature) at 19 th
position is also helpful for the interaction, along with the Phe at 24th position. The aromatic
ring of Phe facilitates its insertion in a hydrophobic pocket, where it can interact with
Vanderwaal’s force with the residue of Phe714, and with the residues of Beta-chain that
include ValB12, LeuB15 and Tyr B26. Some non-aromatic portions are also significant for
the process of insulin-binding. That also includes the residue of Val A3 which has a major
role to play in the process of stabilization of insulin (Susec et al., 2019). One unique
interaction of IGF1 with IGFR is initiated by an alternative binding pocket in the cysteine
rich domain of IGFR. The intracellular tyrosine kinase domains of IR containing 250 amino
acids, is bounded with a juxtamembrane domain containing 45 amino acids, and a 100 amino-
acids containing CT domain, as discussed above. The residues of IR 1158, 62, 63 and IGFR
1131, 35, 36 of the regulatory zone of TK domain can help in conformational change of the
active site. It also creates the binding pocket for downstream regulatory adaptor proteins of
Grb10/14, and APS/SH2-B. This helps in activation of the pathway. The tyrosine kinases are
phosphorylated, resulting in the activation of the signal transduction pathway, activating
downstream molecules, and causing the necessary physiological action. Any discrepancy in
this interaction, can lead to interrupted signalling leading to the disorder diabetes mellitus
(Gustafson, Hedzajifar, Gogg, Hammarstedt & Smith, 2015).
Insulin signalling is mainly concerned with glucose homeostasis, resulting in
maintenance of blood glucose level in the body. Other functions of the hormone include lipid
and energy homeostasis. So, the predominant cells where the effect occurs are in the cells of
liver, adipose tissue, and skeletal muscles. Insulin is primarily synthesized by the beta cells of
pancreas. When blood glucose level of the blood increases, the insulin is secreted. Depending
on the type of the tissue, glucose enters the tissue via facilitated or passive diffusion. In
BIOCHEMISTRY AND MOLECULAR BIOLOGY
move apart from beta two sheets, that help in insertion of the sheet inside the alpha CT
residues of Val and Pro at position 15 and 18. The Tyr residue (aromatic in nature) at 19 th
position is also helpful for the interaction, along with the Phe at 24th position. The aromatic
ring of Phe facilitates its insertion in a hydrophobic pocket, where it can interact with
Vanderwaal’s force with the residue of Phe714, and with the residues of Beta-chain that
include ValB12, LeuB15 and Tyr B26. Some non-aromatic portions are also significant for
the process of insulin-binding. That also includes the residue of Val A3 which has a major
role to play in the process of stabilization of insulin (Susec et al., 2019). One unique
interaction of IGF1 with IGFR is initiated by an alternative binding pocket in the cysteine
rich domain of IGFR. The intracellular tyrosine kinase domains of IR containing 250 amino
acids, is bounded with a juxtamembrane domain containing 45 amino acids, and a 100 amino-
acids containing CT domain, as discussed above. The residues of IR 1158, 62, 63 and IGFR
1131, 35, 36 of the regulatory zone of TK domain can help in conformational change of the
active site. It also creates the binding pocket for downstream regulatory adaptor proteins of
Grb10/14, and APS/SH2-B. This helps in activation of the pathway. The tyrosine kinases are
phosphorylated, resulting in the activation of the signal transduction pathway, activating
downstream molecules, and causing the necessary physiological action. Any discrepancy in
this interaction, can lead to interrupted signalling leading to the disorder diabetes mellitus
(Gustafson, Hedzajifar, Gogg, Hammarstedt & Smith, 2015).
Insulin signalling is mainly concerned with glucose homeostasis, resulting in
maintenance of blood glucose level in the body. Other functions of the hormone include lipid
and energy homeostasis. So, the predominant cells where the effect occurs are in the cells of
liver, adipose tissue, and skeletal muscles. Insulin is primarily synthesized by the beta cells of
pancreas. When blood glucose level of the blood increases, the insulin is secreted. Depending
on the type of the tissue, glucose enters the tissue via facilitated or passive diffusion. In

5
BIOCHEMISTRY AND MOLECULAR BIOLOGY
skeletal muscles or in adipose tissues, the glucose enters via GLUT4 receptor, as discussed
previously. In brain, kidney and retina it enters via passive diffusion. In the pancreas itself,
glucose enters via GLUT2 receptors. GLUT1 and GLUT4 glucose transport proteins are
facilitated by insulin in the cells (Lehninger, Nelson & Cox, 2013).
According to Peterson et al., (2016), if there is no interaction between the insulin and
the insulin receptors, the normal functions of the hormone will be hampered. It includes
anomaly in glucose homeostasis, and glycogen synthesis, along with defects in lipid
metabolism in muscles and adipose tissues. This can cause a variety of disorders that include
problems in glucose transport, defective glycogen metabolism, hence increased glucose in
blood which can lead to pathological conditions such as diabetes mellitus. Type 2 diabetes,
the most prevalent form of diabetes, can actually be caused by such interaction. It is generally
caused due to deficiency of insulin secretion in the blood or increased amount of glucose in
blood. Any defects in insulin receptor can hamper the binding of the insulin and IR, and can
cause insulin resistance leading to diabetes. In normal conditions, when in fasting condition
with no food, the insulin does not bind to IR,and only does so in presence of glucose
(Belfiore et al., 2017).
BIOCHEMISTRY AND MOLECULAR BIOLOGY
skeletal muscles or in adipose tissues, the glucose enters via GLUT4 receptor, as discussed
previously. In brain, kidney and retina it enters via passive diffusion. In the pancreas itself,
glucose enters via GLUT2 receptors. GLUT1 and GLUT4 glucose transport proteins are
facilitated by insulin in the cells (Lehninger, Nelson & Cox, 2013).
According to Peterson et al., (2016), if there is no interaction between the insulin and
the insulin receptors, the normal functions of the hormone will be hampered. It includes
anomaly in glucose homeostasis, and glycogen synthesis, along with defects in lipid
metabolism in muscles and adipose tissues. This can cause a variety of disorders that include
problems in glucose transport, defective glycogen metabolism, hence increased glucose in
blood which can lead to pathological conditions such as diabetes mellitus. Type 2 diabetes,
the most prevalent form of diabetes, can actually be caused by such interaction. It is generally
caused due to deficiency of insulin secretion in the blood or increased amount of glucose in
blood. Any defects in insulin receptor can hamper the binding of the insulin and IR, and can
cause insulin resistance leading to diabetes. In normal conditions, when in fasting condition
with no food, the insulin does not bind to IR,and only does so in presence of glucose
(Belfiore et al., 2017).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6
BIOCHEMISTRY AND MOLECULAR BIOLOGY
References-
Belfiore, A., Malaguarnera, R., Vella, V., Lawrence, M. C., Sciacca, L., Frasca, F., ... &
Vigneri, R. (2017). Insulin receptor isoforms in physiology and disease: an updated
view. Endocrine reviews, 38(5), 379-431. https://doi.org/10.1210/er.2017-00073
De Meyts, P. (2015). Insulin/receptor binding: the last piece of the puzzle? What recent
progress on the structure of the insulin/receptor complex tells us (or not) about
negative cooperativity and activation. Bioessays, 37(4), 389-397.
https://doi.org/10.1002/bies.201400190
D'Oria, R., Laviola, L., Giorgino, F., Unfer, V., Bettocchi, S., & Scioscia, M. (2017).
PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: Novel
potential insights in endothelial dysfunction in preeclampsia. Pregnancy
hypertension, 10, 107-112. DOI: 10.1016/j.preghy.2017.07.001
Gustafson, B., Hedjazifar, S., Gogg, S., Hammarstedt, A., & Smith, U. (2015). Insulin
resistance and impaired adipogenesis. Trends in Endocrinology & Metabolism, 26(4),
193-200. https://doi.org/10.1016/j.tem.2015.01.006
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2013). Lehninger principles of biochemistry
New York. ISBN-13: 978-0-7167-7108-1.ISBN-10: 0-7167-7108-X
Petersen, M. C., Madiraju, A. K., Gassaway, B. M., Marcel, M., Nasiri, A. R., Butrico, G., ...
& Philbrick, W. (2016). Insulin receptor Thr 1160 phosphorylation mediates lipid-
induced hepatic insulin resistance. The Journal of clinical investigation, 126(11),
4361-4371. Free access | 10.1172/JCI86013
BIOCHEMISTRY AND MOLECULAR BIOLOGY
References-
Belfiore, A., Malaguarnera, R., Vella, V., Lawrence, M. C., Sciacca, L., Frasca, F., ... &
Vigneri, R. (2017). Insulin receptor isoforms in physiology and disease: an updated
view. Endocrine reviews, 38(5), 379-431. https://doi.org/10.1210/er.2017-00073
De Meyts, P. (2015). Insulin/receptor binding: the last piece of the puzzle? What recent
progress on the structure of the insulin/receptor complex tells us (or not) about
negative cooperativity and activation. Bioessays, 37(4), 389-397.
https://doi.org/10.1002/bies.201400190
D'Oria, R., Laviola, L., Giorgino, F., Unfer, V., Bettocchi, S., & Scioscia, M. (2017).
PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: Novel
potential insights in endothelial dysfunction in preeclampsia. Pregnancy
hypertension, 10, 107-112. DOI: 10.1016/j.preghy.2017.07.001
Gustafson, B., Hedjazifar, S., Gogg, S., Hammarstedt, A., & Smith, U. (2015). Insulin
resistance and impaired adipogenesis. Trends in Endocrinology & Metabolism, 26(4),
193-200. https://doi.org/10.1016/j.tem.2015.01.006
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2013). Lehninger principles of biochemistry
New York. ISBN-13: 978-0-7167-7108-1.ISBN-10: 0-7167-7108-X
Petersen, M. C., Madiraju, A. K., Gassaway, B. M., Marcel, M., Nasiri, A. R., Butrico, G., ...
& Philbrick, W. (2016). Insulin receptor Thr 1160 phosphorylation mediates lipid-
induced hepatic insulin resistance. The Journal of clinical investigation, 126(11),
4361-4371. Free access | 10.1172/JCI86013
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7
BIOCHEMISTRY AND MOLECULAR BIOLOGY
Pollak, M. (2012). The insulin receptor/insulin-like growth factor receptor family as a
therapeutic target in oncology. Clinical cancer research, 18(1), 40-50. 10.1158/1078-
0432.CCR-11-0998
Susec, M., Sencanski, M., Glisic, S., Veljkovic, N., Pedersen, C., Drinovec, L., ... & Vrecl,
M. (2019). Functional characterization of β2-adrenergic and insulin receptor
heteromers. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2019.01.025
Tatulian, S. A. (2015). Structural dynamics of insulin receptor and transmembrane
signaling. Biochemistry, 54(36), 5523-5532.
https://doi.org/10.1021/acs.biochem.5b00805
Voet, D., Voet, J. G., & Pratt, C. W. (2016). Fundamentals of biochemistry: life at the
molecular level. John Wiley & Sons. [Online]. Retrieved from:
https://books.google.co.in/books?
hl=en&lr=&id=9T7hCgAAQBAJ&oi=fnd&pg=PA1&dq=Voet+and+voet+biochemis
try&ots=L6LMPTcZGg&sig=Pd_FKWdk-
SWCewpAH1uTBitzS7M&redir_esc=y#v=onepage&q=Voet%20and%20voet
%20biochemistry&f=false
Xu, Y., Kong, G. K. W., Menting, J. G., Margetts, M. B., Delaine, C. A., Jenkin, L. M., ... &
Lawrence, M. C. (2018). How ligand binds to the type 1 insulin-like growth factor
receptor. Nature communications, 9(1), 821. [Online]. Retrieved from:
https://www.nature.com/articles/s41467-018-03219-7
BIOCHEMISTRY AND MOLECULAR BIOLOGY
Pollak, M. (2012). The insulin receptor/insulin-like growth factor receptor family as a
therapeutic target in oncology. Clinical cancer research, 18(1), 40-50. 10.1158/1078-
0432.CCR-11-0998
Susec, M., Sencanski, M., Glisic, S., Veljkovic, N., Pedersen, C., Drinovec, L., ... & Vrecl,
M. (2019). Functional characterization of β2-adrenergic and insulin receptor
heteromers. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2019.01.025
Tatulian, S. A. (2015). Structural dynamics of insulin receptor and transmembrane
signaling. Biochemistry, 54(36), 5523-5532.
https://doi.org/10.1021/acs.biochem.5b00805
Voet, D., Voet, J. G., & Pratt, C. W. (2016). Fundamentals of biochemistry: life at the
molecular level. John Wiley & Sons. [Online]. Retrieved from:
https://books.google.co.in/books?
hl=en&lr=&id=9T7hCgAAQBAJ&oi=fnd&pg=PA1&dq=Voet+and+voet+biochemis
try&ots=L6LMPTcZGg&sig=Pd_FKWdk-
SWCewpAH1uTBitzS7M&redir_esc=y#v=onepage&q=Voet%20and%20voet
%20biochemistry&f=false
Xu, Y., Kong, G. K. W., Menting, J. G., Margetts, M. B., Delaine, C. A., Jenkin, L. M., ... &
Lawrence, M. C. (2018). How ligand binds to the type 1 insulin-like growth factor
receptor. Nature communications, 9(1), 821. [Online]. Retrieved from:
https://www.nature.com/articles/s41467-018-03219-7
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





