Intellectual Property Management in Chinese Pharmaceutical Companies
VerifiedAdded on 2023/01/17
|7
|1741
|46
Report
AI Summary
This report provides a comprehensive analysis of intellectual property (IP) management within the Chinese pharmaceutical industry, with a specific focus on two prominent companies: China TCM and PuraPharm. The study investigates the companies' approaches to trade secrets, the core of their IP strategies, and compares their similarities and differences. It highlights the influence of Chinese policies and legal frameworks, including the impact of Hong Kong's legal environment on PuraPharm. The report examines the uniqueness of trade secrets in the context of traditional Chinese medicine, the legal measures in place, and other forms of IP protection, such as patents and trademarks. It also addresses the challenges posed by globalization and the increasing difficulty of maintaining trade secrets in the face of evolving legal landscapes. The report concludes by emphasizing the importance of a comprehensive approach to IP protection, incorporating various strategies to safeguard proprietary information and adapt to changes in the industry.

Running head: INTELLECTUAL PROPERTY MANAGEMENT 0
INTELLECTUAL PROPERTY MANAGEMENT
Name of Student
Institution affiliation
0
INTELLECTUAL PROPERTY MANAGEMENT
Name of Student
Institution affiliation
0
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INTELLECTUAL PROPERTY MANAGEMENT 1
INTRODUCTION
Secrecy and strict guarding of the pharmaceutical formulas used in coming up with the
various medicines and supplements manufactured in China and by the major Chinese
corporations has always been part of the Chinese policy. The case study looks at two of the most
successful pharmaceutical companies in China, China TCM and PuraPharm, the write-up will
critically assess their trade secrets. The companies’ trade secrets are similar and also different in
some ways despite the foundational philosophy behind them being similar. The trade secrets are
based on the inherent Chinese-rooted need for protecting their national resources and on modern
laws that grant Chinese inventors monopoly for inventions.
DIFFERENCES
PuraPharm being a Hong Kong based company depicts some kind of openness in the way
they go about business. The company, by virtue of being based in Hong Kong where democracy
is more welcome and the need for cooperation with the outside world, tends to be less secretive
(Effron, 2016). Nonetheless, PuraPharm still maintains the original formulas for most of the base
pharmaceutical fundamental concoctions and will not gladly share those openly. However,
unlike China TCM, the company offers these concoctions as by-products to other pharmaceutical
manufacturers with strict secrecy undertakings and agreements on their use and applications.
The company, according to their vision statement admits that cooperation is very vital
and the goal of every pharmaceutical products manufacturer is dedicating themselves to
humanity's health (PuraPharm Corporation Limited, 2018). The company also envisions a
situation whereby it will be a leading and one of the most admired company in global
pharmaceutical products arena through it success in modernization of traditional medicine
globally (Schuman, 2017). In attaining the goal, the company vowed to ensure that it will involve
investors, business partners and the global community in fighting some of the deadliest ailments
affecting humanity. The endeavor has therefore soften their resolve in upholding total secrecy
but instead has only left PuraPharm with only the need to protect the base trade secrets of only
restriction the base pharmaceutical concoctions (Staff and Sandeen, 2017). The partial openness
has currently led to integration and sharing of research and development data with many global
pharmaceutical giants. As of April 2019, the company had active collaborations with companies
in USA, Canada, India and Australia.
INTRODUCTION
Secrecy and strict guarding of the pharmaceutical formulas used in coming up with the
various medicines and supplements manufactured in China and by the major Chinese
corporations has always been part of the Chinese policy. The case study looks at two of the most
successful pharmaceutical companies in China, China TCM and PuraPharm, the write-up will
critically assess their trade secrets. The companies’ trade secrets are similar and also different in
some ways despite the foundational philosophy behind them being similar. The trade secrets are
based on the inherent Chinese-rooted need for protecting their national resources and on modern
laws that grant Chinese inventors monopoly for inventions.
DIFFERENCES
PuraPharm being a Hong Kong based company depicts some kind of openness in the way
they go about business. The company, by virtue of being based in Hong Kong where democracy
is more welcome and the need for cooperation with the outside world, tends to be less secretive
(Effron, 2016). Nonetheless, PuraPharm still maintains the original formulas for most of the base
pharmaceutical fundamental concoctions and will not gladly share those openly. However,
unlike China TCM, the company offers these concoctions as by-products to other pharmaceutical
manufacturers with strict secrecy undertakings and agreements on their use and applications.
The company, according to their vision statement admits that cooperation is very vital
and the goal of every pharmaceutical products manufacturer is dedicating themselves to
humanity's health (PuraPharm Corporation Limited, 2018). The company also envisions a
situation whereby it will be a leading and one of the most admired company in global
pharmaceutical products arena through it success in modernization of traditional medicine
globally (Schuman, 2017). In attaining the goal, the company vowed to ensure that it will involve
investors, business partners and the global community in fighting some of the deadliest ailments
affecting humanity. The endeavor has therefore soften their resolve in upholding total secrecy
but instead has only left PuraPharm with only the need to protect the base trade secrets of only
restriction the base pharmaceutical concoctions (Staff and Sandeen, 2017). The partial openness
has currently led to integration and sharing of research and development data with many global
pharmaceutical giants. As of April 2019, the company had active collaborations with companies
in USA, Canada, India and Australia.

INTELLECTUAL PROPERTY MANAGEMENT 2
Unlike PuraPharm, China TCM tends to concentrate more on the Chinese market. Of
course the Chinese market currently holds more than a billion people. The Chinese patriotism
and policy favors Chinese medicine and makes the company virtually unopposed when it comes
to competition with foreign pharmaceutical manufacturers (Menell, 2017). In a bid to also ensure
that they fully dominate the market, China TCM has gone an extra step and acquired or managed
to form alliances with over 10 different subsidiaries scattered all over China. The mergers and
partnerships can be considered as very important and they are indicative of a very important
trade secret because they ensure a wider reach of their pharmaceutical products.
UNIQUNESS AND CHARACTERISTICS
The holders of the traditional Chinese medical knowledge have a strict adhere to
confidentiality. Furthermore, the government also maintains the same policy and uses strict laws
and policies in ensurong that this knowledge does not go to the outside world. According to
Effron (2016), the efforts invloved in keeping the pharmaceutical formulations as a national and
proffessional trade secrets makes China as one of the nations with very exceptional medicinal
formulations. However, as more and more foreign companies set up in China, intellectual
property cases in China are increasing in number as shown on table 1.1. The other uniqueness
of the trade secret is that the information related to medical formulations is that they ensure that
the base formulations are not even known to the regulatory bodies but a few operatives.
Therefore the medicine that comes out of China and most of the ones listed for sale by
PuraPahram and China TCM have ingredients that are unknown to the public. In many other
jurisdcitions, trade sectrets are kept but declared to the governments of the day. In China these
trade secrets are so unique that even the government has to rely on the confidentiality of the
holders of these information and they are only left to manage the end product, which is the
medicine.
Unlike PuraPharm, China TCM tends to concentrate more on the Chinese market. Of
course the Chinese market currently holds more than a billion people. The Chinese patriotism
and policy favors Chinese medicine and makes the company virtually unopposed when it comes
to competition with foreign pharmaceutical manufacturers (Menell, 2017). In a bid to also ensure
that they fully dominate the market, China TCM has gone an extra step and acquired or managed
to form alliances with over 10 different subsidiaries scattered all over China. The mergers and
partnerships can be considered as very important and they are indicative of a very important
trade secret because they ensure a wider reach of their pharmaceutical products.
UNIQUNESS AND CHARACTERISTICS
The holders of the traditional Chinese medical knowledge have a strict adhere to
confidentiality. Furthermore, the government also maintains the same policy and uses strict laws
and policies in ensurong that this knowledge does not go to the outside world. According to
Effron (2016), the efforts invloved in keeping the pharmaceutical formulations as a national and
proffessional trade secrets makes China as one of the nations with very exceptional medicinal
formulations. However, as more and more foreign companies set up in China, intellectual
property cases in China are increasing in number as shown on table 1.1. The other uniqueness
of the trade secret is that the information related to medical formulations is that they ensure that
the base formulations are not even known to the regulatory bodies but a few operatives.
Therefore the medicine that comes out of China and most of the ones listed for sale by
PuraPahram and China TCM have ingredients that are unknown to the public. In many other
jurisdcitions, trade sectrets are kept but declared to the governments of the day. In China these
trade secrets are so unique that even the government has to rely on the confidentiality of the
holders of these information and they are only left to manage the end product, which is the
medicine.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
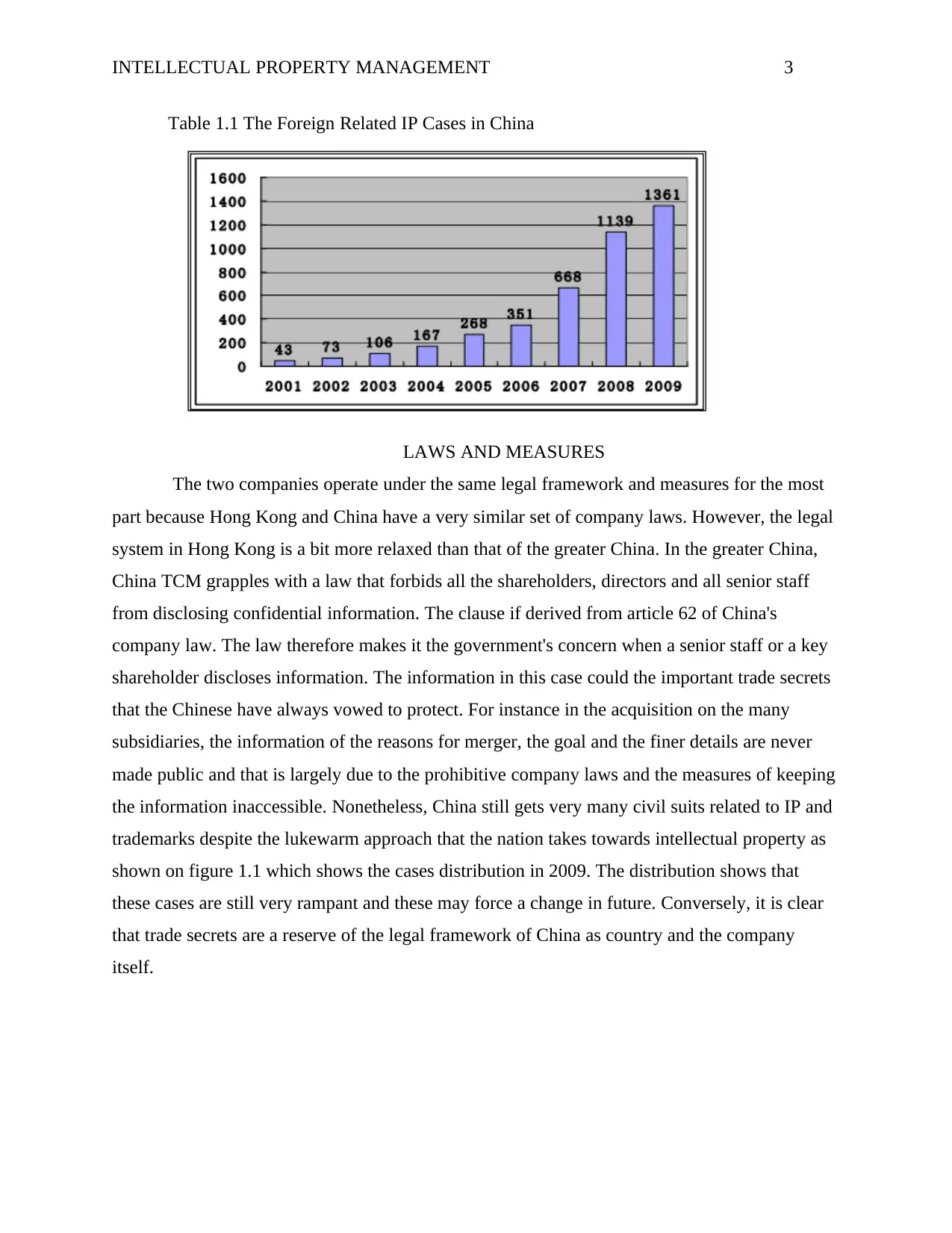
INTELLECTUAL PROPERTY MANAGEMENT 3
Table 1.1 The Foreign Related IP Cases in China
LAWS AND MEASURES
The two companies operate under the same legal framework and measures for the most
part because Hong Kong and China have a very similar set of company laws. However, the legal
system in Hong Kong is a bit more relaxed than that of the greater China. In the greater China,
China TCM grapples with a law that forbids all the shareholders, directors and all senior staff
from disclosing confidential information. The clause if derived from article 62 of China's
company law. The law therefore makes it the government's concern when a senior staff or a key
shareholder discloses information. The information in this case could the important trade secrets
that the Chinese have always vowed to protect. For instance in the acquisition on the many
subsidiaries, the information of the reasons for merger, the goal and the finer details are never
made public and that is largely due to the prohibitive company laws and the measures of keeping
the information inaccessible. Nonetheless, China still gets very many civil suits related to IP and
trademarks despite the lukewarm approach that the nation takes towards intellectual property as
shown on figure 1.1 which shows the cases distribution in 2009. The distribution shows that
these cases are still very rampant and these may force a change in future. Conversely, it is clear
that trade secrets are a reserve of the legal framework of China as country and the company
itself.
Table 1.1 The Foreign Related IP Cases in China
LAWS AND MEASURES
The two companies operate under the same legal framework and measures for the most
part because Hong Kong and China have a very similar set of company laws. However, the legal
system in Hong Kong is a bit more relaxed than that of the greater China. In the greater China,
China TCM grapples with a law that forbids all the shareholders, directors and all senior staff
from disclosing confidential information. The clause if derived from article 62 of China's
company law. The law therefore makes it the government's concern when a senior staff or a key
shareholder discloses information. The information in this case could the important trade secrets
that the Chinese have always vowed to protect. For instance in the acquisition on the many
subsidiaries, the information of the reasons for merger, the goal and the finer details are never
made public and that is largely due to the prohibitive company laws and the measures of keeping
the information inaccessible. Nonetheless, China still gets very many civil suits related to IP and
trademarks despite the lukewarm approach that the nation takes towards intellectual property as
shown on figure 1.1 which shows the cases distribution in 2009. The distribution shows that
these cases are still very rampant and these may force a change in future. Conversely, it is clear
that trade secrets are a reserve of the legal framework of China as country and the company
itself.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
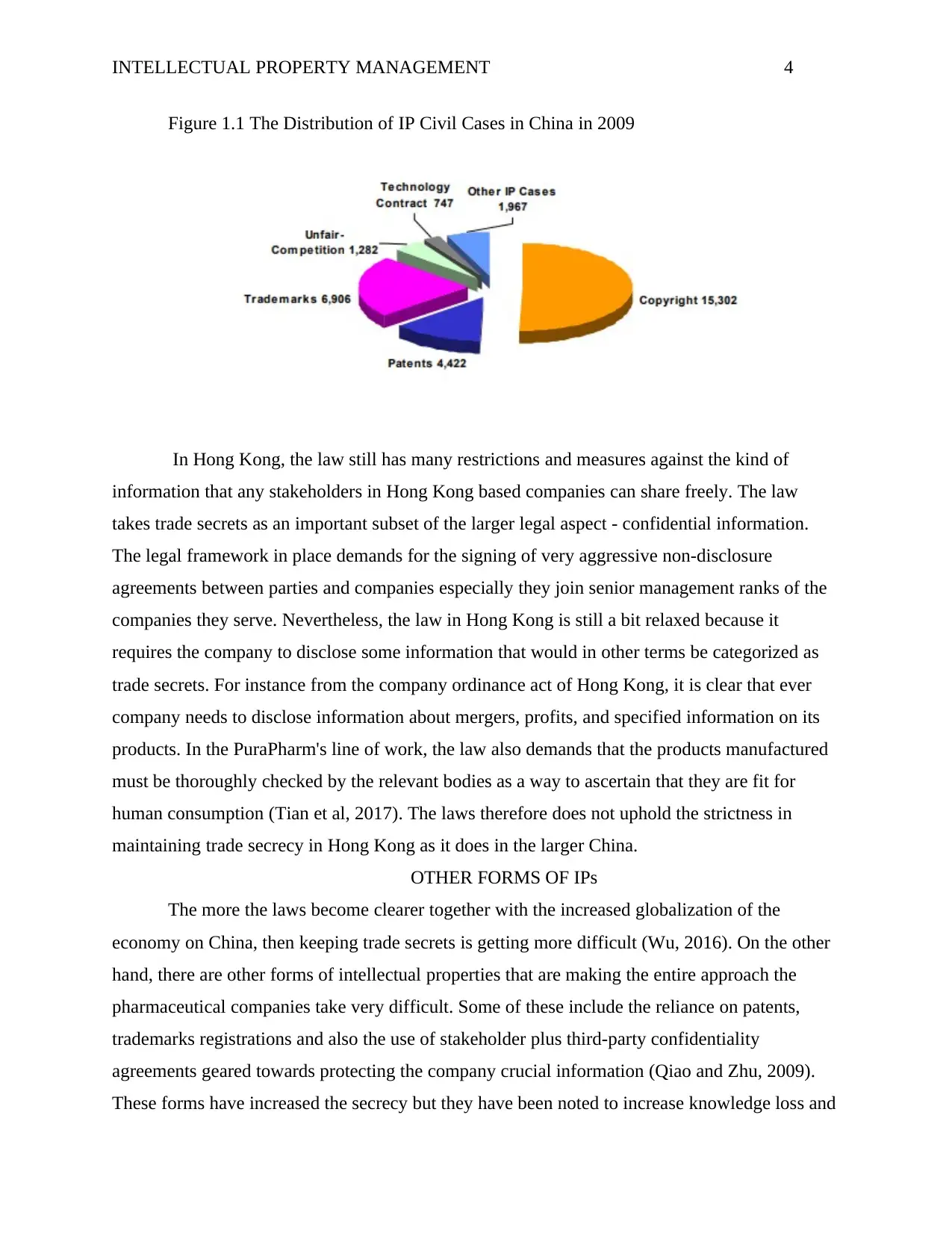
INTELLECTUAL PROPERTY MANAGEMENT 4
Figure 1.1 The Distribution of IP Civil Cases in China in 2009
In Hong Kong, the law still has many restrictions and measures against the kind of
information that any stakeholders in Hong Kong based companies can share freely. The law
takes trade secrets as an important subset of the larger legal aspect - confidential information.
The legal framework in place demands for the signing of very aggressive non-disclosure
agreements between parties and companies especially they join senior management ranks of the
companies they serve. Nevertheless, the law in Hong Kong is still a bit relaxed because it
requires the company to disclose some information that would in other terms be categorized as
trade secrets. For instance from the company ordinance act of Hong Kong, it is clear that ever
company needs to disclose information about mergers, profits, and specified information on its
products. In the PuraPharm's line of work, the law also demands that the products manufactured
must be thoroughly checked by the relevant bodies as a way to ascertain that they are fit for
human consumption (Tian et al, 2017). The laws therefore does not uphold the strictness in
maintaining trade secrecy in Hong Kong as it does in the larger China.
OTHER FORMS OF IPs
The more the laws become clearer together with the increased globalization of the
economy on China, then keeping trade secrets is getting more difficult (Wu, 2016). On the other
hand, there are other forms of intellectual properties that are making the entire approach the
pharmaceutical companies take very difficult. Some of these include the reliance on patents,
trademarks registrations and also the use of stakeholder plus third-party confidentiality
agreements geared towards protecting the company crucial information (Qiao and Zhu, 2009).
These forms have increased the secrecy but they have been noted to increase knowledge loss and
Figure 1.1 The Distribution of IP Civil Cases in China in 2009
In Hong Kong, the law still has many restrictions and measures against the kind of
information that any stakeholders in Hong Kong based companies can share freely. The law
takes trade secrets as an important subset of the larger legal aspect - confidential information.
The legal framework in place demands for the signing of very aggressive non-disclosure
agreements between parties and companies especially they join senior management ranks of the
companies they serve. Nevertheless, the law in Hong Kong is still a bit relaxed because it
requires the company to disclose some information that would in other terms be categorized as
trade secrets. For instance from the company ordinance act of Hong Kong, it is clear that ever
company needs to disclose information about mergers, profits, and specified information on its
products. In the PuraPharm's line of work, the law also demands that the products manufactured
must be thoroughly checked by the relevant bodies as a way to ascertain that they are fit for
human consumption (Tian et al, 2017). The laws therefore does not uphold the strictness in
maintaining trade secrecy in Hong Kong as it does in the larger China.
OTHER FORMS OF IPs
The more the laws become clearer together with the increased globalization of the
economy on China, then keeping trade secrets is getting more difficult (Wu, 2016). On the other
hand, there are other forms of intellectual properties that are making the entire approach the
pharmaceutical companies take very difficult. Some of these include the reliance on patents,
trademarks registrations and also the use of stakeholder plus third-party confidentiality
agreements geared towards protecting the company crucial information (Qiao and Zhu, 2009).
These forms have increased the secrecy but they have been noted to increase knowledge loss and

INTELLECTUAL PROPERTY MANAGEMENT 5
the over-reliance on a few persons and hence diluting the technical knowledge pool greatly. It is
due to these challenges that the companies have decided to come with better and more robust
approaches that will increase the knowledge pool while maintaining and controlling the spread of
crucial information. The different forms of protection in addition to proper shelving of trade
secrets provides comprehensive protection required in guarding proprietary information.
the over-reliance on a few persons and hence diluting the technical knowledge pool greatly. It is
due to these challenges that the companies have decided to come with better and more robust
approaches that will increase the knowledge pool while maintaining and controlling the spread of
crucial information. The different forms of protection in addition to proper shelving of trade
secrets provides comprehensive protection required in guarding proprietary information.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

INTELLECTUAL PROPERTY MANAGEMENT 6
REFERENCES
Effron, R. J. (2016). Trade Secrets, Extraterritoriality, and Jurisdiction. Wake Forest L.
Rev., 51, 765.
Menell, P. S. (2017). Tailoring a public policy exception to trade secret protection. Calif.
L. Rev., 105, 1.
PuraPharm Corporation Limited. (2018). Annual report 2017. Retrieved from
https://www.purapharm.com/wp-content/uploads/2018/04/EW01498_AR.pdf
Qiao, Y., and Zhu, X. (2009). Patent Protection of the Traditional Chinese Medicine and
Its Impact on the Related Industries in China. Retrieved from
https://www.researchgate.net/publication/247838230_Patent_Protection_of_the_Traditional_Chi
nese_Medicine_and_Its_Impact_on_the_Related_Industries_in_China
Schuman, M. (2017, August 23). How one company brought traditional Chinese
medicine to the modern world and made billions. Retrieved from
https://www.forbes.com/sites/michaelschuman/2017/08/23/yunnan-baiyao-asia-fab-50-medicine-
china/#28edeaf44a4e
Staff, C., & Sandeen, S. (2017). Transcript from 2017 Mitchell Hamline Trade Secret
Conference, Cybaris scholar symposium. Cybaris®, 8(2), 1.
Tian, E., Li, H., Cai, M., & Kong, M. (2017). A pressing need: Standardization of TCM
granule products. Research & Reviews: Journal of Botanical Sciences, 6(3), pp.75-77. Retrieved
from http://www.rroij.com/open-access/a-pressing-need-standardisation-of-tcm-granule-
products-.pdf
Wu, M. (2016). The China, Inc. Challenge to Global Trade Governance. Harv. Int'l
LJ, 57, 261.
REFERENCES
Effron, R. J. (2016). Trade Secrets, Extraterritoriality, and Jurisdiction. Wake Forest L.
Rev., 51, 765.
Menell, P. S. (2017). Tailoring a public policy exception to trade secret protection. Calif.
L. Rev., 105, 1.
PuraPharm Corporation Limited. (2018). Annual report 2017. Retrieved from
https://www.purapharm.com/wp-content/uploads/2018/04/EW01498_AR.pdf
Qiao, Y., and Zhu, X. (2009). Patent Protection of the Traditional Chinese Medicine and
Its Impact on the Related Industries in China. Retrieved from
https://www.researchgate.net/publication/247838230_Patent_Protection_of_the_Traditional_Chi
nese_Medicine_and_Its_Impact_on_the_Related_Industries_in_China
Schuman, M. (2017, August 23). How one company brought traditional Chinese
medicine to the modern world and made billions. Retrieved from
https://www.forbes.com/sites/michaelschuman/2017/08/23/yunnan-baiyao-asia-fab-50-medicine-
china/#28edeaf44a4e
Staff, C., & Sandeen, S. (2017). Transcript from 2017 Mitchell Hamline Trade Secret
Conference, Cybaris scholar symposium. Cybaris®, 8(2), 1.
Tian, E., Li, H., Cai, M., & Kong, M. (2017). A pressing need: Standardization of TCM
granule products. Research & Reviews: Journal of Botanical Sciences, 6(3), pp.75-77. Retrieved
from http://www.rroij.com/open-access/a-pressing-need-standardisation-of-tcm-granule-
products-.pdf
Wu, M. (2016). The China, Inc. Challenge to Global Trade Governance. Harv. Int'l
LJ, 57, 261.
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
