University Task: Analyzing Potato Tissue Osmosis and Mass Changes
VerifiedAdded on 2023/04/23
|13
|3114
|96
Report
AI Summary
This report details an experiment investigating osmosis in potato tissue. The aim was to observe the change in mass of potato strips immersed in various solutions, including distilled water (hypotonic) and saltwater (hypertonic), over a 2.5-hour period, with measurements taken every 30 minutes. The research question focused on determining if 1 hour was sufficient for osmosis to occur. The experiment involved immersing potato strips in solutions of varying salt concentrations (0.0 M, 0.12 M, 0.25 M, 0.5 M, and 1.0 M) and measuring the mass changes. The results, presented in tables and graphs, demonstrated that the mass of the potato tissue generally decreased in hypertonic solutions and increased in the hypotonic solution. The report analyzed the percentage change in mass over time, illustrating the effects of osmosis on the potato cells. The findings indicated that as the concentration of the salt solution increased, the decrease in the potato's weight became more significant, with an isotonic point observed at a molarity of 1. The discussion highlighted the net change in mass and the movement of water molecules across the cell membrane, supporting the understanding of osmotic processes in plant cells. The experiment successfully demonstrated the principles of osmosis and its impact on plant tissue in different environments.

University
Task
Investigating the time potato tissue takes to reach osmotic equilibrium
Student Name
Unit
Tutor
Task
Investigating the time potato tissue takes to reach osmotic equilibrium
Student Name
Unit
Tutor
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Investigating the time potato tissue takes to reach osmotic equilibrium
Aim
To investigate the change in mass in potato strips over a period of two and a half hours
when immersed in a range of solutions from distilled water (hypotonic solution) and salty
water (hypertonic solution).
Research Question
Is 1-hour sufficient time for osmosis in potato tissue?
Background Information
Osmosis is one of the physiological processes in living organisms. It entails the movement of
water molecules from a region of low to high region of solute concentration across the
semi-permeable membrane. Osmosis entails the diffusion of water molecules from high to
lower water concentration across a selectively permeable membrane, in this way the water
movement is achieved down the concentration gradient. The permeability of the membrane
allows smaller substances to penetrate but prevents larger substances moving across the
membrane (Blanco-Marigorta, Lozano-Medina & Marcos, 2017). The cell wall is permeable
thus has no definite role in controlling what enters into the cells. Osmosis is important in
both plants and animals. In plants it makes cells to be turgid or flaccid while in animals it
offsets the osmotic pressures in the cell. Plant cells contain solutes because they have a cell
sap, so when they are put in distilled water (a hypotonic solution), they absorb water by
osmosis, swell up and become turgid (Binding, 2018). They do not burst because they have a
cell wall that develops a wall pressure that balances the turgor pressure used by turgid cells.
As the plant gains turgidity, its volume increases until it achieves maximum turgidity. Water
2
Aim
To investigate the change in mass in potato strips over a period of two and a half hours
when immersed in a range of solutions from distilled water (hypotonic solution) and salty
water (hypertonic solution).
Research Question
Is 1-hour sufficient time for osmosis in potato tissue?
Background Information
Osmosis is one of the physiological processes in living organisms. It entails the movement of
water molecules from a region of low to high region of solute concentration across the
semi-permeable membrane. Osmosis entails the diffusion of water molecules from high to
lower water concentration across a selectively permeable membrane, in this way the water
movement is achieved down the concentration gradient. The permeability of the membrane
allows smaller substances to penetrate but prevents larger substances moving across the
membrane (Blanco-Marigorta, Lozano-Medina & Marcos, 2017). The cell wall is permeable
thus has no definite role in controlling what enters into the cells. Osmosis is important in
both plants and animals. In plants it makes cells to be turgid or flaccid while in animals it
offsets the osmotic pressures in the cell. Plant cells contain solutes because they have a cell
sap, so when they are put in distilled water (a hypotonic solution), they absorb water by
osmosis, swell up and become turgid (Binding, 2018). They do not burst because they have a
cell wall that develops a wall pressure that balances the turgor pressure used by turgid cells.
As the plant gains turgidity, its volume increases until it achieves maximum turgidity. Water
2

will then start moving out of the cell to balance the pressure in the cells and the outside
environment, (Kramer & Myers, 2013).
When a plant cell is placed in the hypertonic solution, for example, a solution containing
NaCl (aq), it loses water due to osmosis, shrinks and become flaccid (plasmolysis). This is
because the salt concentration is higher than the concentration in the cell sap.
The process illustrates the physical process which solvents move across the permeable
membrane. Osmosis pressure functions as a required external pressure applied, there is a
net movement occurring on the solvent across the membrane. Osmosis pressure has
colligative properties which allow for the osmotic pressure to depend on the molar
concentration. When cells are submerged in water, the water molecules in the cell
membrane move in and out at the same rate. The mechanism responsible is aimed at
driving osmosis in a high concentration gradient, creating a thermodynamic effect (Raven,
2018).
Osmotic pressure is the key support of plants. Water entry increases turgor pressure which
exerts cell walls until it attains an equal osmotic pressure thus creating a steady state. When
plants are placed in hypertonic solutions which are relative to the cytoplasm, water tends to
move out of the cell and shrink (Khatibi et al., 2019). When this happens, cells become
flaccid. In other cases, the cell becomes plasmolysed as the cell membranes disengage from
the cell through a lack of water pressure on it. Placing the plant cell in a hypotonic solution,
water tends to move inside the cell and becomes turgid (Malphettes, Snowden & Yuk,
2018).
3
environment, (Kramer & Myers, 2013).
When a plant cell is placed in the hypertonic solution, for example, a solution containing
NaCl (aq), it loses water due to osmosis, shrinks and become flaccid (plasmolysis). This is
because the salt concentration is higher than the concentration in the cell sap.
The process illustrates the physical process which solvents move across the permeable
membrane. Osmosis pressure functions as a required external pressure applied, there is a
net movement occurring on the solvent across the membrane. Osmosis pressure has
colligative properties which allow for the osmotic pressure to depend on the molar
concentration. When cells are submerged in water, the water molecules in the cell
membrane move in and out at the same rate. The mechanism responsible is aimed at
driving osmosis in a high concentration gradient, creating a thermodynamic effect (Raven,
2018).
Osmotic pressure is the key support of plants. Water entry increases turgor pressure which
exerts cell walls until it attains an equal osmotic pressure thus creating a steady state. When
plants are placed in hypertonic solutions which are relative to the cytoplasm, water tends to
move out of the cell and shrink (Khatibi et al., 2019). When this happens, cells become
flaccid. In other cases, the cell becomes plasmolysed as the cell membranes disengage from
the cell through a lack of water pressure on it. Placing the plant cell in a hypotonic solution,
water tends to move inside the cell and becomes turgid (Malphettes, Snowden & Yuk,
2018).
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Osmosis has the ability to make the plant roots draw water from the soil. The plant absorbs
solutes in the roots through active transport and the water enters the roots through
osmosis. In this experiment, the osmosis effect has been demonstrated by using potato
slices placed in the media under different time frames.
Thus, the aim of this experiment is to assess the effect of hypertonic and hypotonic
solutions on potato over duration of less than two and a half hours.
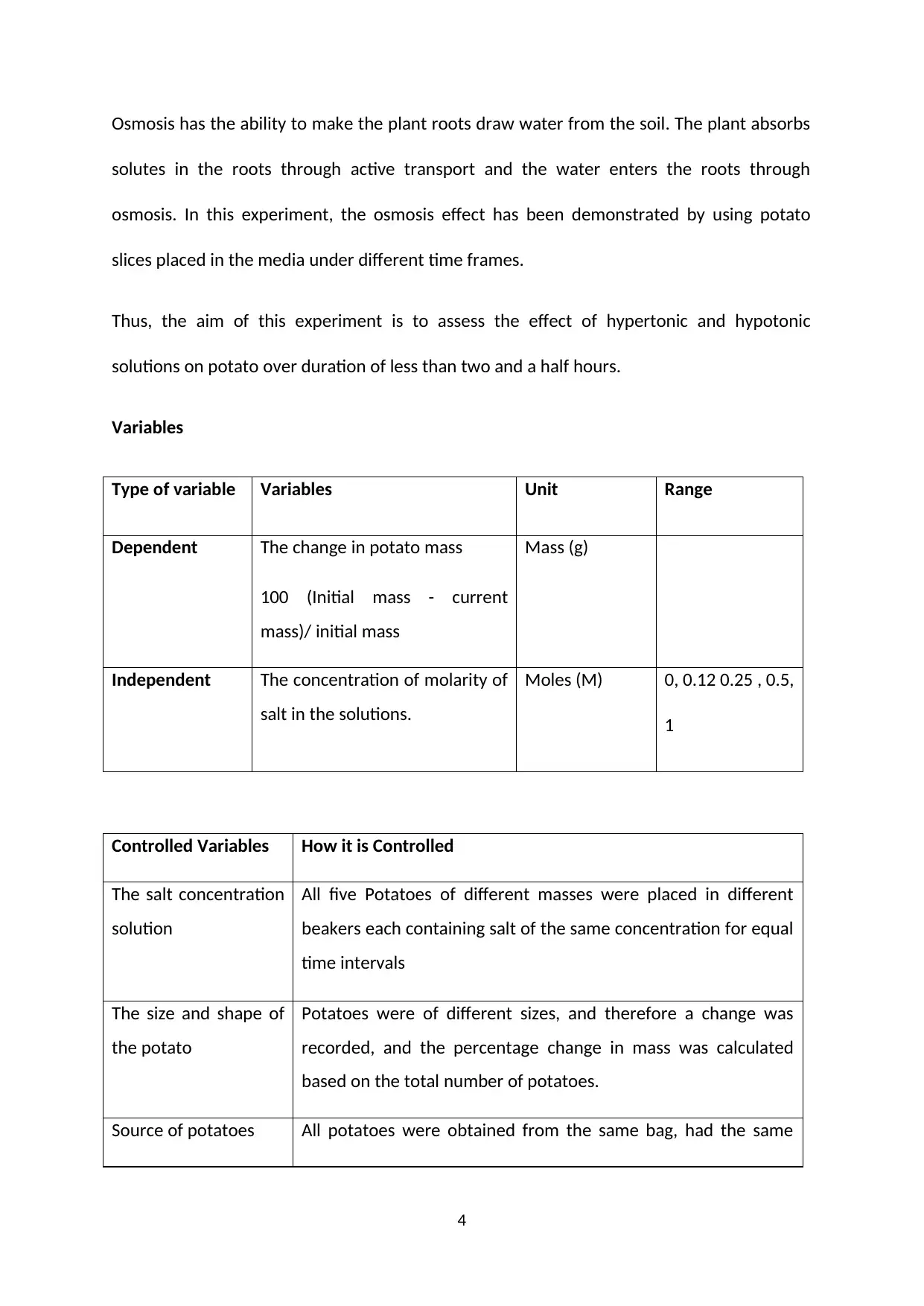
Variables
Type of variable Variables Unit Range
Dependent The change in potato mass
100 (Initial mass - current
mass)/ initial mass
Mass (g)
Independent The concentration of molarity of
salt in the solutions.
Moles (M) 0, 0.12 0.25 , 0.5,
1
Controlled Variables How it is Controlled
The salt concentration
solution
All five Potatoes of different masses were placed in different
beakers each containing salt of the same concentration for equal
time intervals
The size and shape of
the potato
Potatoes were of different sizes, and therefore a change was
recorded, and the percentage change in mass was calculated
based on the total number of potatoes.
Source of potatoes All potatoes were obtained from the same bag, had the same
4
solutes in the roots through active transport and the water enters the roots through
osmosis. In this experiment, the osmosis effect has been demonstrated by using potato
slices placed in the media under different time frames.
Thus, the aim of this experiment is to assess the effect of hypertonic and hypotonic
solutions on potato over duration of less than two and a half hours.
Variables
Type of variable Variables Unit Range
Dependent The change in potato mass
100 (Initial mass - current
mass)/ initial mass
Mass (g)
Independent The concentration of molarity of
salt in the solutions.
Moles (M) 0, 0.12 0.25 , 0.5,
1
Controlled Variables How it is Controlled
The salt concentration
solution
All five Potatoes of different masses were placed in different
beakers each containing salt of the same concentration for equal
time intervals
The size and shape of
the potato
Potatoes were of different sizes, and therefore a change was
recorded, and the percentage change in mass was calculated
based on the total number of potatoes.
Source of potatoes All potatoes were obtained from the same bag, had the same
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
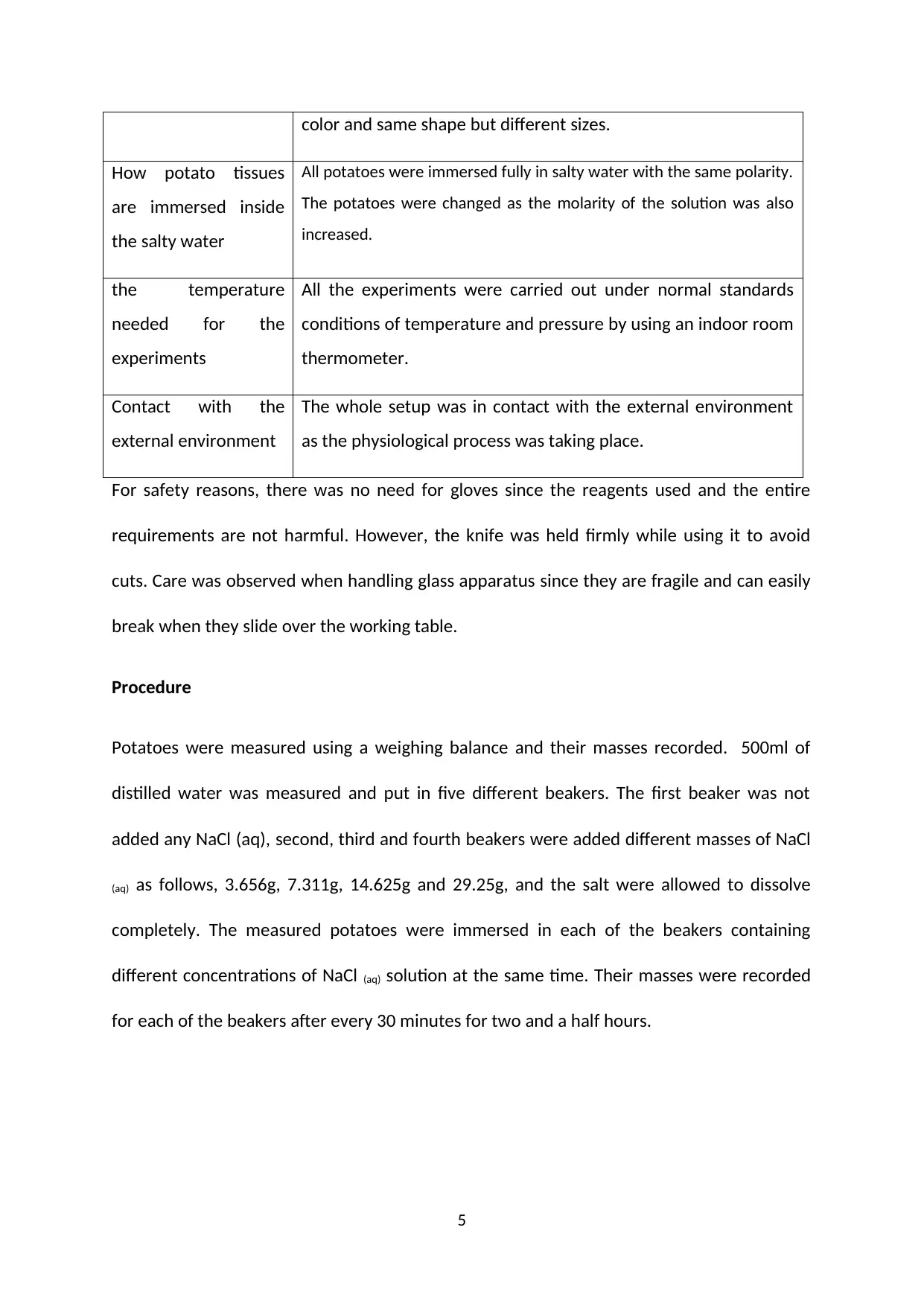
color and same shape but different sizes.
How potato tissues
are immersed inside
the salty water
All potatoes were immersed fully in salty water with the same polarity.
The potatoes were changed as the molarity of the solution was also
increased.
the temperature
needed for the
experiments
All the experiments were carried out under normal standards
conditions of temperature and pressure by using an indoor room
thermometer.
Contact with the
external environment
The whole setup was in contact with the external environment
as the physiological process was taking place.
For safety reasons, there was no need for gloves since the reagents used and the entire
requirements are not harmful. However, the knife was held firmly while using it to avoid
cuts. Care was observed when handling glass apparatus since they are fragile and can easily
break when they slide over the working table.
Procedure
Potatoes were measured using a weighing balance and their masses recorded. 500ml of
distilled water was measured and put in five different beakers. The first beaker was not
added any NaCl (aq), second, third and fourth beakers were added different masses of NaCl
(aq) as follows, 3.656g, 7.311g, 14.625g and 29.25g, and the salt were allowed to dissolve
completely. The measured potatoes were immersed in each of the beakers containing
different concentrations of NaCl (aq) solution at the same time. Their masses were recorded
for each of the beakers after every 30 minutes for two and a half hours.
5
How potato tissues
are immersed inside
the salty water
All potatoes were immersed fully in salty water with the same polarity.
The potatoes were changed as the molarity of the solution was also
increased.
the temperature
needed for the
experiments
All the experiments were carried out under normal standards
conditions of temperature and pressure by using an indoor room
thermometer.
Contact with the
external environment
The whole setup was in contact with the external environment
as the physiological process was taking place.
For safety reasons, there was no need for gloves since the reagents used and the entire
requirements are not harmful. However, the knife was held firmly while using it to avoid
cuts. Care was observed when handling glass apparatus since they are fragile and can easily
break when they slide over the working table.
Procedure
Potatoes were measured using a weighing balance and their masses recorded. 500ml of
distilled water was measured and put in five different beakers. The first beaker was not
added any NaCl (aq), second, third and fourth beakers were added different masses of NaCl
(aq) as follows, 3.656g, 7.311g, 14.625g and 29.25g, and the salt were allowed to dissolve
completely. The measured potatoes were immersed in each of the beakers containing
different concentrations of NaCl (aq) solution at the same time. Their masses were recorded
for each of the beakers after every 30 minutes for two and a half hours.
5
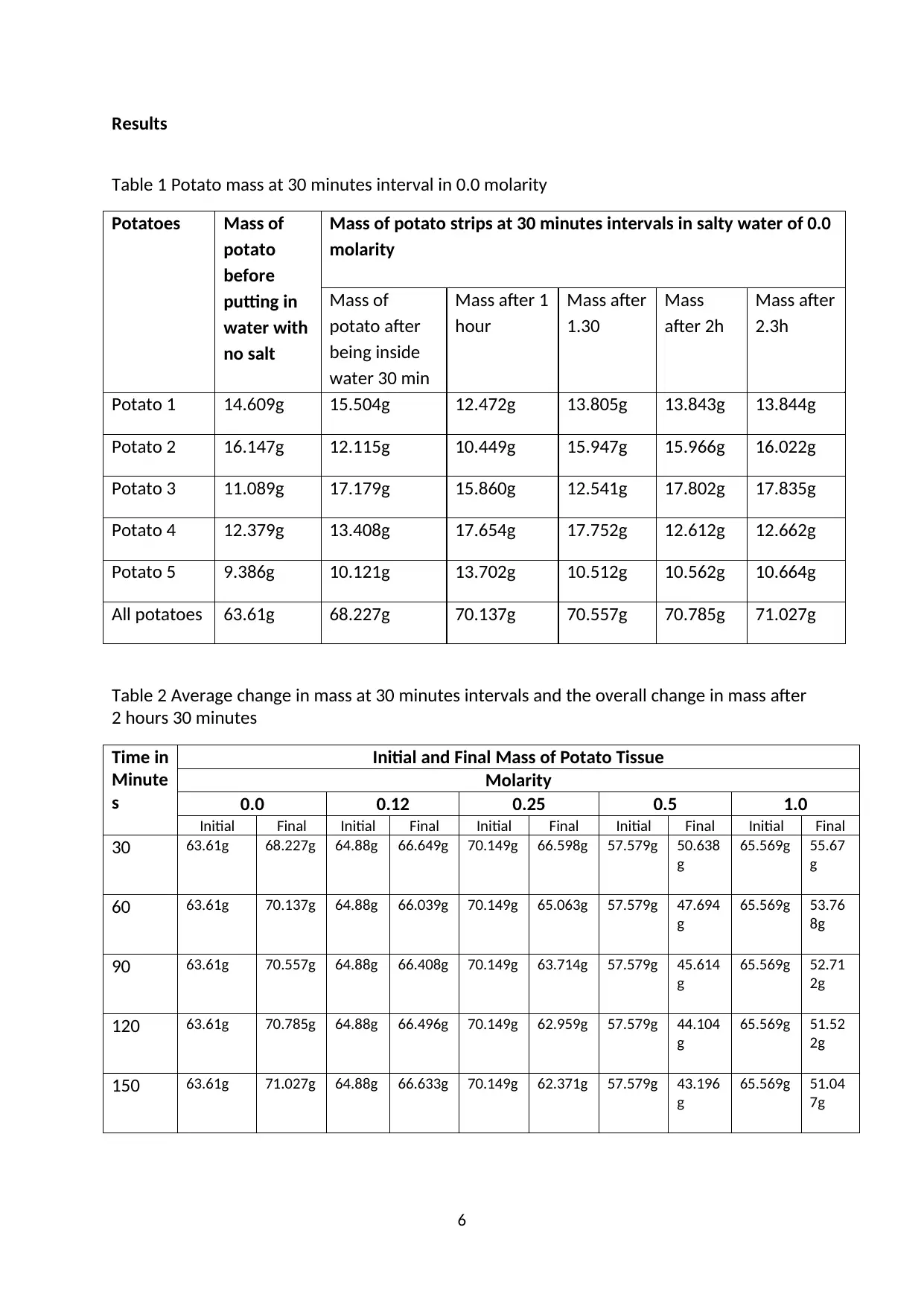
Results
Table 1 Potato mass at 30 minutes interval in 0.0 molarity
Potatoes Mass of
potato
before
putting in
water with
no salt
Mass of potato strips at 30 minutes intervals in salty water of 0.0
molarity
Mass of
potato after
being inside
water 30 min
Mass after 1
hour
Mass after
1.30
Mass
after 2h
Mass after
2.3h
Potato 1 14.609g 15.504g 12.472g 13.805g 13.843g 13.844g
Potato 2 16.147g 12.115g 10.449g 15.947g 15.966g 16.022g
Potato 3 11.089g 17.179g 15.860g 12.541g 17.802g 17.835g
Potato 4 12.379g 13.408g 17.654g 17.752g 12.612g 12.662g
Potato 5 9.386g 10.121g 13.702g 10.512g 10.562g 10.664g
All potatoes 63.61g 68.227g 70.137g 70.557g 70.785g 71.027g
Table 2 Average change in mass at 30 minutes intervals and the overall change in mass after
2 hours 30 minutes
Time in
Minute
s
Initial and Final Mass of Potato Tissue
Molarity
0.0 0.12 0.25 0.5 1.0
Initial Final Initial Final Initial Final Initial Final Initial Final
30 63.61g 68.227g 64.88g 66.649g 70.149g 66.598g 57.579g 50.638
g
65.569g 55.67
g
60 63.61g 70.137g 64.88g 66.039g 70.149g 65.063g 57.579g 47.694
g
65.569g 53.76
8g
90 63.61g 70.557g 64.88g 66.408g 70.149g 63.714g 57.579g 45.614
g
65.569g 52.71
2g
120 63.61g 70.785g 64.88g 66.496g 70.149g 62.959g 57.579g 44.104
g
65.569g 51.52
2g
150 63.61g 71.027g 64.88g 66.633g 70.149g 62.371g 57.579g 43.196
g
65.569g 51.04
7g
6
Table 1 Potato mass at 30 minutes interval in 0.0 molarity
Potatoes Mass of
potato
before
putting in
water with
no salt
Mass of potato strips at 30 minutes intervals in salty water of 0.0
molarity
Mass of
potato after
being inside
water 30 min
Mass after 1
hour
Mass after
1.30
Mass
after 2h
Mass after
2.3h
Potato 1 14.609g 15.504g 12.472g 13.805g 13.843g 13.844g
Potato 2 16.147g 12.115g 10.449g 15.947g 15.966g 16.022g
Potato 3 11.089g 17.179g 15.860g 12.541g 17.802g 17.835g
Potato 4 12.379g 13.408g 17.654g 17.752g 12.612g 12.662g
Potato 5 9.386g 10.121g 13.702g 10.512g 10.562g 10.664g
All potatoes 63.61g 68.227g 70.137g 70.557g 70.785g 71.027g
Table 2 Average change in mass at 30 minutes intervals and the overall change in mass after
2 hours 30 minutes
Time in
Minute
s
Initial and Final Mass of Potato Tissue
Molarity
0.0 0.12 0.25 0.5 1.0
Initial Final Initial Final Initial Final Initial Final Initial Final
30 63.61g 68.227g 64.88g 66.649g 70.149g 66.598g 57.579g 50.638
g
65.569g 55.67
g
60 63.61g 70.137g 64.88g 66.039g 70.149g 65.063g 57.579g 47.694
g
65.569g 53.76
8g
90 63.61g 70.557g 64.88g 66.408g 70.149g 63.714g 57.579g 45.614
g
65.569g 52.71
2g
120 63.61g 70.785g 64.88g 66.496g 70.149g 62.959g 57.579g 44.104
g
65.569g 51.52
2g
150 63.61g 71.027g 64.88g 66.633g 70.149g 62.371g 57.579g 43.196
g
65.569g 51.04
7g
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
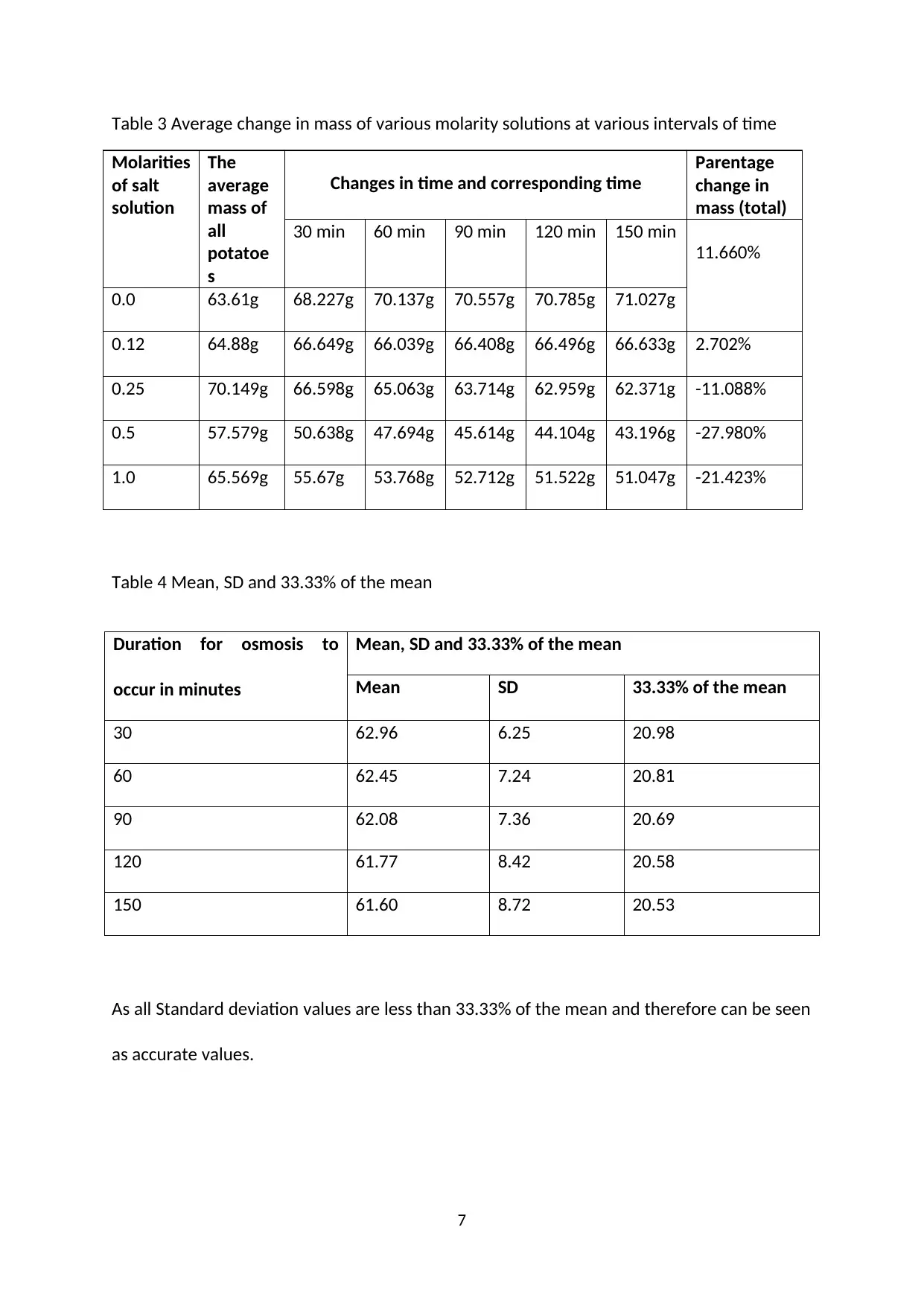
Table 3 Average change in mass of various molarity solutions at various intervals of time
Molarities
of salt
solution
The
average
mass of
all
potatoe
s
Changes in time and corresponding time
Parentage
change in
mass (total)
30 min 60 min 90 min 120 min 150 min
11.660%
0.0 63.61g 68.227g 70.137g 70.557g 70.785g 71.027g
0.12 64.88g 66.649g 66.039g 66.408g 66.496g 66.633g 2.702%
0.25 70.149g 66.598g 65.063g 63.714g 62.959g 62.371g -11.088%
0.5 57.579g 50.638g 47.694g 45.614g 44.104g 43.196g -27.980%
1.0 65.569g 55.67g 53.768g 52.712g 51.522g 51.047g -21.423%
Table 4 Mean, SD and 33.33% of the mean
Duration for osmosis to
occur in minutes
Mean, SD and 33.33% of the mean
Mean SD 33.33% of the mean
30 62.96 6.25 20.98
60 62.45 7.24 20.81
90 62.08 7.36 20.69
120 61.77 8.42 20.58
150 61.60 8.72 20.53
As all Standard deviation values are less than 33.33% of the mean and therefore can be seen
as accurate values.
7
Molarities
of salt
solution
The
average
mass of
all
potatoe
s
Changes in time and corresponding time
Parentage
change in
mass (total)
30 min 60 min 90 min 120 min 150 min
11.660%
0.0 63.61g 68.227g 70.137g 70.557g 70.785g 71.027g
0.12 64.88g 66.649g 66.039g 66.408g 66.496g 66.633g 2.702%
0.25 70.149g 66.598g 65.063g 63.714g 62.959g 62.371g -11.088%
0.5 57.579g 50.638g 47.694g 45.614g 44.104g 43.196g -27.980%
1.0 65.569g 55.67g 53.768g 52.712g 51.522g 51.047g -21.423%
Table 4 Mean, SD and 33.33% of the mean
Duration for osmosis to
occur in minutes
Mean, SD and 33.33% of the mean
Mean SD 33.33% of the mean
30 62.96 6.25 20.98
60 62.45 7.24 20.81
90 62.08 7.36 20.69
120 61.77 8.42 20.58
150 61.60 8.72 20.53
As all Standard deviation values are less than 33.33% of the mean and therefore can be seen
as accurate values.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
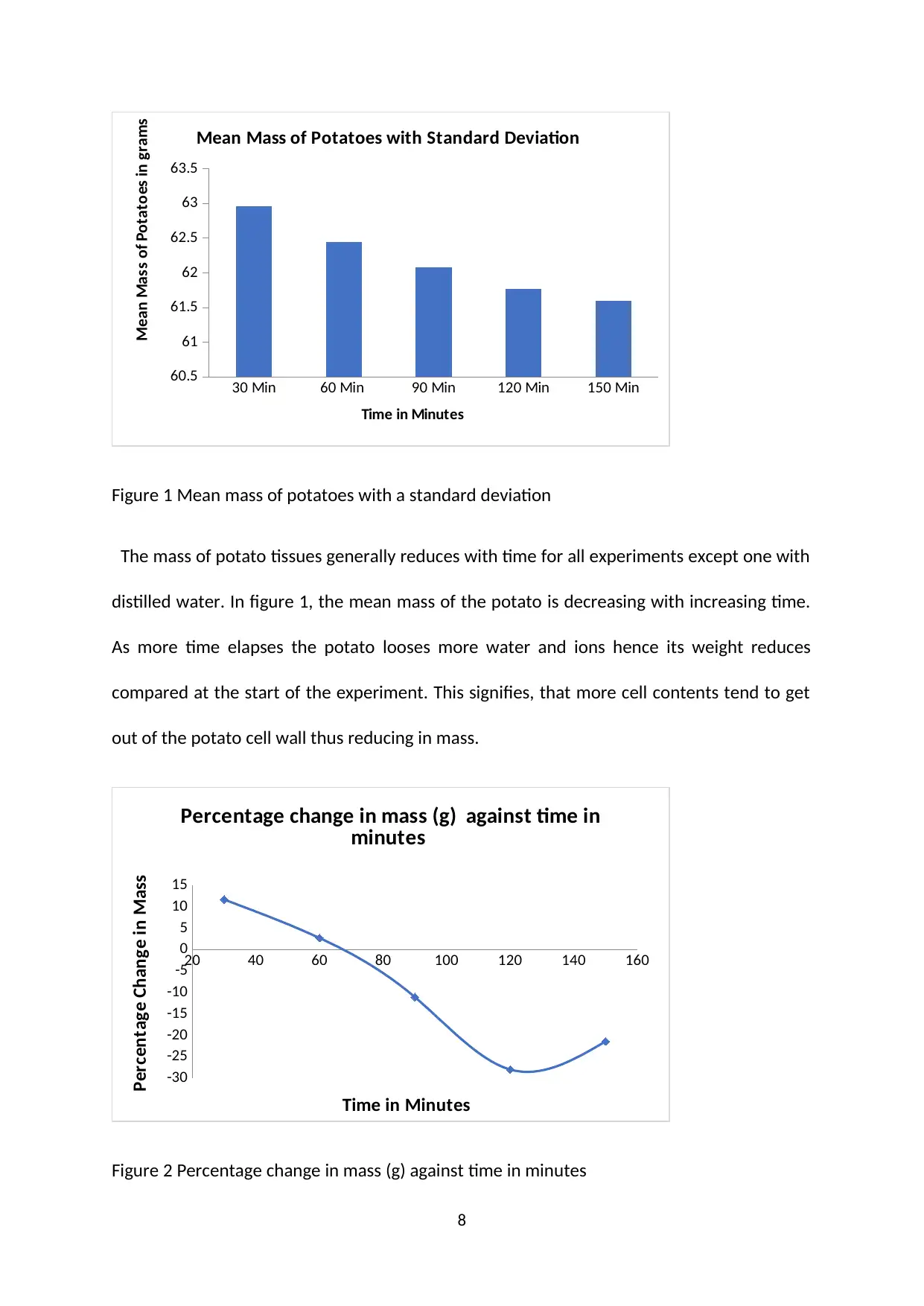
30 Min 60 Min 90 Min 120 Min 150 Min
60.5
61
61.5
62
62.5
63
63.5
Mean Mass of Potatoes with Standard Deviation
Time in Minutes
Mean Mass of Potatoes in grams
Figure 1 Mean mass of potatoes with a standard deviation
The mass of potato tissues generally reduces with time for all experiments except one with
distilled water. In figure 1, the mean mass of the potato is decreasing with increasing time.
As more time elapses the potato looses more water and ions hence its weight reduces
compared at the start of the experiment. This signifies, that more cell contents tend to get
out of the potato cell wall thus reducing in mass.
20 40 60 80 100 120 140 160
-30
-25
-20
-15
-10
-5
0
5
10
15
Percentage change in mass (g) against time in
minutes
Time in Minutes
Percentage Change in Mass
Figure 2 Percentage change in mass (g) against time in minutes
8
60.5
61
61.5
62
62.5
63
63.5
Mean Mass of Potatoes with Standard Deviation
Time in Minutes
Mean Mass of Potatoes in grams
Figure 1 Mean mass of potatoes with a standard deviation
The mass of potato tissues generally reduces with time for all experiments except one with
distilled water. In figure 1, the mean mass of the potato is decreasing with increasing time.
As more time elapses the potato looses more water and ions hence its weight reduces
compared at the start of the experiment. This signifies, that more cell contents tend to get
out of the potato cell wall thus reducing in mass.
20 40 60 80 100 120 140 160
-30
-25
-20
-15
-10
-5
0
5
10
15
Percentage change in mass (g) against time in
minutes
Time in Minutes
Percentage Change in Mass
Figure 2 Percentage change in mass (g) against time in minutes
8
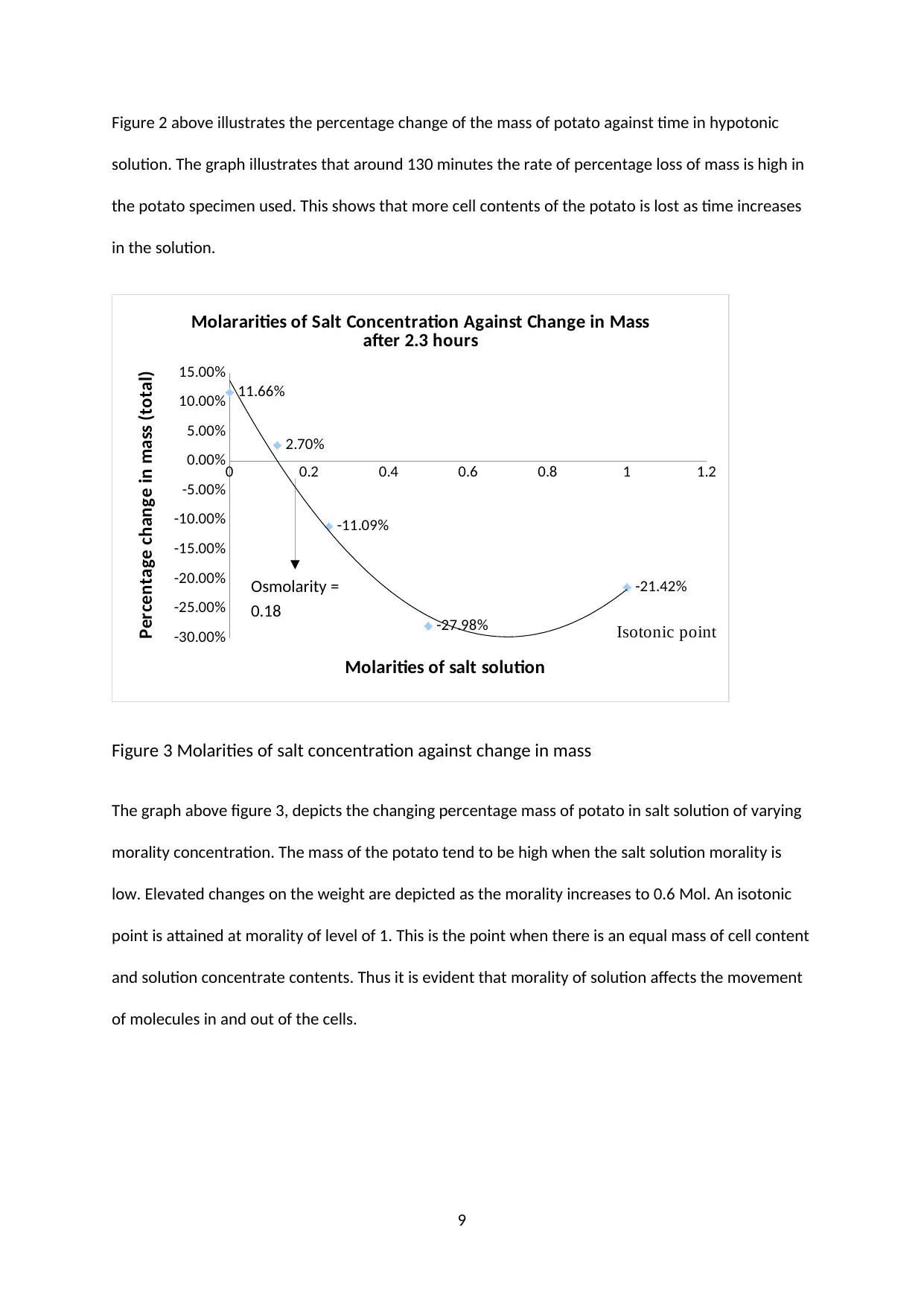
Figure 2 above illustrates the percentage change of the mass of potato against time in hypotonic
solution. The graph illustrates that around 130 minutes the rate of percentage loss of mass is high in
the potato specimen used. This shows that more cell contents of the potato is lost as time increases
in the solution.
0 0.2 0.4 0.6 0.8 1 1.2
-30.00%
-25.00%
-20.00%
-15.00%
-10.00%
-5.00%
0.00%
5.00%
10.00%
15.00%
11.66%
2.70%
-11.09%
-27.98%
-21.42%
Molararities of Salt Concentration Against Change in Mass
after 2.3 hours
Molarities of salt solution
Percentage change in mass (total)
Isotonic point
Figure 3 Molarities of salt concentration against change in mass
The graph above figure 3, depicts the changing percentage mass of potato in salt solution of varying
morality concentration. The mass of the potato tend to be high when the salt solution morality is
low. Elevated changes on the weight are depicted as the morality increases to 0.6 Mol. An isotonic
point is attained at morality of level of 1. This is the point when there is an equal mass of cell content
and solution concentrate contents. Thus it is evident that morality of solution affects the movement
of molecules in and out of the cells.
9
Osmolarity =
0.18
solution. The graph illustrates that around 130 minutes the rate of percentage loss of mass is high in
the potato specimen used. This shows that more cell contents of the potato is lost as time increases
in the solution.
0 0.2 0.4 0.6 0.8 1 1.2
-30.00%
-25.00%
-20.00%
-15.00%
-10.00%
-5.00%
0.00%
5.00%
10.00%
15.00%
11.66%
2.70%
-11.09%
-27.98%
-21.42%
Molararities of Salt Concentration Against Change in Mass
after 2.3 hours
Molarities of salt solution
Percentage change in mass (total)
Isotonic point
Figure 3 Molarities of salt concentration against change in mass
The graph above figure 3, depicts the changing percentage mass of potato in salt solution of varying
morality concentration. The mass of the potato tend to be high when the salt solution morality is
low. Elevated changes on the weight are depicted as the morality increases to 0.6 Mol. An isotonic
point is attained at morality of level of 1. This is the point when there is an equal mass of cell content
and solution concentrate contents. Thus it is evident that morality of solution affects the movement
of molecules in and out of the cells.
9
Osmolarity =
0.18
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
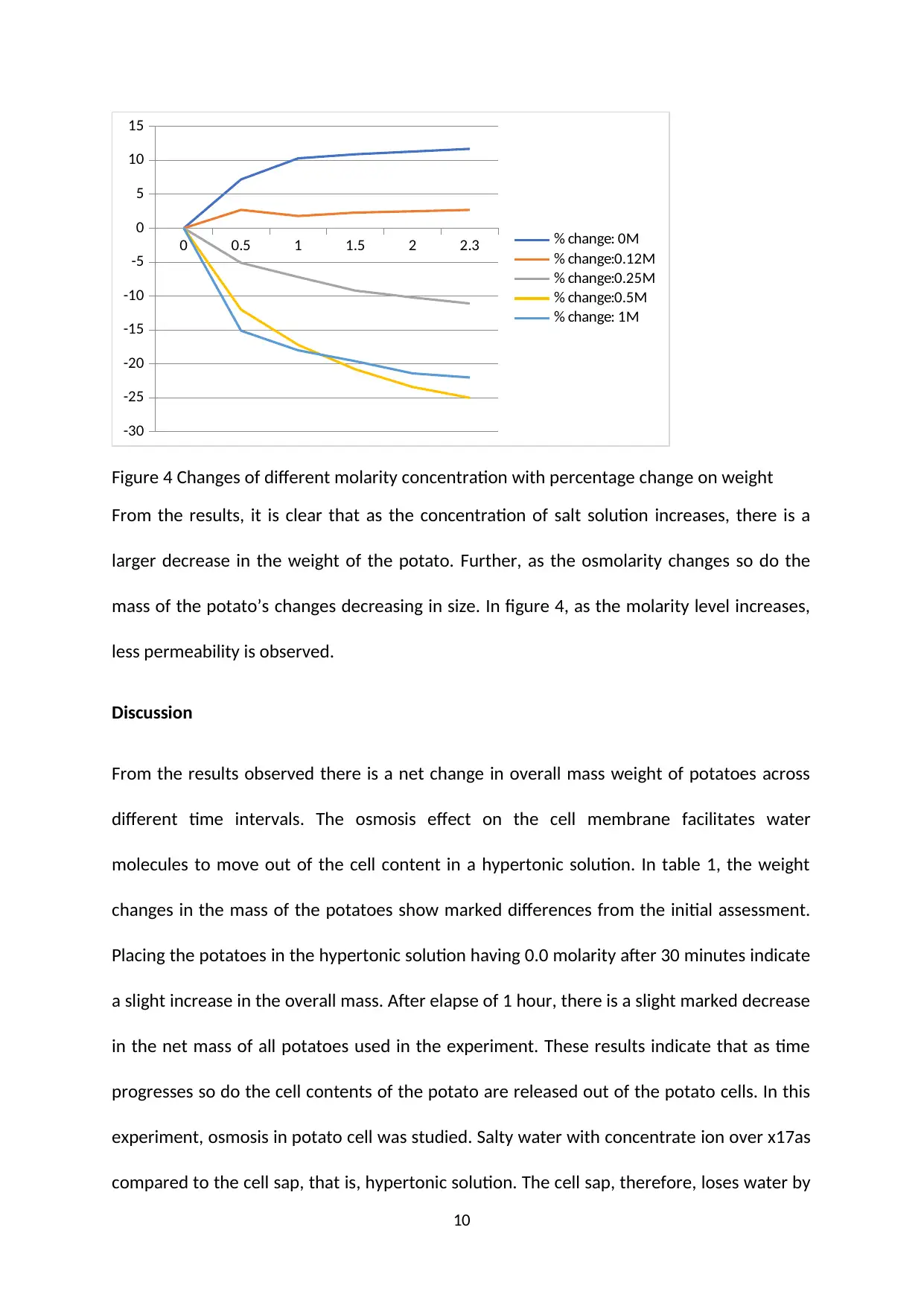
0 0.5 1 1.5 2 2.3
-30
-25
-20
-15
-10
-5
0
5
10
15
% change: 0M
% change:0.12M
% change:0.25M
% change:0.5M
% change: 1M
Figure 4 Changes of different molarity concentration with percentage change on weight
From the results, it is clear that as the concentration of salt solution increases, there is a
larger decrease in the weight of the potato. Further, as the osmolarity changes so do the
mass of the potato’s changes decreasing in size. In figure 4, as the molarity level increases,
less permeability is observed.
Discussion
From the results observed there is a net change in overall mass weight of potatoes across
different time intervals. The osmosis effect on the cell membrane facilitates water
molecules to move out of the cell content in a hypertonic solution. In table 1, the weight
changes in the mass of the potatoes show marked differences from the initial assessment.
Placing the potatoes in the hypertonic solution having 0.0 molarity after 30 minutes indicate
a slight increase in the overall mass. After elapse of 1 hour, there is a slight marked decrease
in the net mass of all potatoes used in the experiment. These results indicate that as time
progresses so do the cell contents of the potato are released out of the potato cells. In this
experiment, osmosis in potato cell was studied. Salty water with concentrate ion over x17as
compared to the cell sap, that is, hypertonic solution. The cell sap, therefore, loses water by
10
-30
-25
-20
-15
-10
-5
0
5
10
15
% change: 0M
% change:0.12M
% change:0.25M
% change:0.5M
% change: 1M
Figure 4 Changes of different molarity concentration with percentage change on weight
From the results, it is clear that as the concentration of salt solution increases, there is a
larger decrease in the weight of the potato. Further, as the osmolarity changes so do the
mass of the potato’s changes decreasing in size. In figure 4, as the molarity level increases,
less permeability is observed.
Discussion
From the results observed there is a net change in overall mass weight of potatoes across
different time intervals. The osmosis effect on the cell membrane facilitates water
molecules to move out of the cell content in a hypertonic solution. In table 1, the weight
changes in the mass of the potatoes show marked differences from the initial assessment.
Placing the potatoes in the hypertonic solution having 0.0 molarity after 30 minutes indicate
a slight increase in the overall mass. After elapse of 1 hour, there is a slight marked decrease
in the net mass of all potatoes used in the experiment. These results indicate that as time
progresses so do the cell contents of the potato are released out of the potato cells. In this
experiment, osmosis in potato cell was studied. Salty water with concentrate ion over x17as
compared to the cell sap, that is, hypertonic solution. The cell sap, therefore, loses water by
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

osmosis and shrinks. As the concentration of salts increases, the mass of potato reduces.
During the first 30 minutes, there is an observed increase in the mass of the potatoes in the
solution with 0.0 molarity of NaCl (aq), signifying plasmolysis effect (Odom et al, 2017).
Varying the concentration gradient of the solutes decreases the overall mass of the
potatoes, facilitating the osmosis effect. In table 2, the weight of the potatoes mass tissues
is observed to be changing with varying molarity concentration. As time increases a slight
mass difference in the overall mass is observed with increasing molarity concentration (Xu
et al, 2017). At 30 min, all of them showed a decrease in mass because water is drawn from
potato tissue to the salt solution. As time increases, the cell is continually losing water. The
overall change in mass is a decrease, in the end, the solutions become isotonic, that is, all
solutions have attained the same concentration, and there is no net movement of water
molecules from one solution to another, (Garcia-Neto et al., 2017).
Figure 1, has shown that the mean mass of the potato decreases with time. At 1 hour time
in hypertonic solution, there is a slight weight change; however, as time progress at 120
minutes and 150 minutes, there is a marked decline in the mean mass of the potato. Figure
2 illustrates the percentage of the mean mass of the potato against time factor. The results
indicate that at around 130 minutes there is a higher negative change on the weight of the
potato in the hypertonic solution. The effect of molarity concentration affects as depicted in
figure 4.
The water located in the potato diffuses out of the solution making the potato to shrink and
thus losing its turgor pressure. The higher the concentration of a salt solution, the bigger the
differences it is observed in terms of its size and weight. Essentially, when a cell is placed in
a solution which has a higher concentration than its own, the cell will swell and even bursts.
This is due to the created factors such as osmotic pressure and gradient. Osmotic gradient
11
During the first 30 minutes, there is an observed increase in the mass of the potatoes in the
solution with 0.0 molarity of NaCl (aq), signifying plasmolysis effect (Odom et al, 2017).
Varying the concentration gradient of the solutes decreases the overall mass of the
potatoes, facilitating the osmosis effect. In table 2, the weight of the potatoes mass tissues
is observed to be changing with varying molarity concentration. As time increases a slight
mass difference in the overall mass is observed with increasing molarity concentration (Xu
et al, 2017). At 30 min, all of them showed a decrease in mass because water is drawn from
potato tissue to the salt solution. As time increases, the cell is continually losing water. The
overall change in mass is a decrease, in the end, the solutions become isotonic, that is, all
solutions have attained the same concentration, and there is no net movement of water
molecules from one solution to another, (Garcia-Neto et al., 2017).
Figure 1, has shown that the mean mass of the potato decreases with time. At 1 hour time
in hypertonic solution, there is a slight weight change; however, as time progress at 120
minutes and 150 minutes, there is a marked decline in the mean mass of the potato. Figure
2 illustrates the percentage of the mean mass of the potato against time factor. The results
indicate that at around 130 minutes there is a higher negative change on the weight of the
potato in the hypertonic solution. The effect of molarity concentration affects as depicted in
figure 4.
The water located in the potato diffuses out of the solution making the potato to shrink and
thus losing its turgor pressure. The higher the concentration of a salt solution, the bigger the
differences it is observed in terms of its size and weight. Essentially, when a cell is placed in
a solution which has a higher concentration than its own, the cell will swell and even bursts.
This is due to the created factors such as osmotic pressure and gradient. Osmotic gradient
11

refers to differences in solutions on either side of the cell while osmotic pressure regulates
the force in high concentration region to lower concentration, (Gigli-Besceglia & Hamann,
2018).
Conclusion
From the results above it is evident that time and molarity of the solute affects the rate of
osmosis in potato tissues. One hour is not a sufficient time frame to conduct an effective
osmosis protocol in potato tissues. This is observed through the marked minimal change in
weight after 1 hour compared to the range of between 120-150 minutes, where there is a
significant change in mass, signifying effect of osmosis on the cell contents of the potato
tissue. There is an increase change on the weight of the mass of potato as time increases;
the cell loses more water thus losing its cell contents declining in mass. Hence, the duration
of two and a half hours is an efficient time to observe effective osmosis changes on potato
tissue.
12
the force in high concentration region to lower concentration, (Gigli-Besceglia & Hamann,
2018).
Conclusion
From the results above it is evident that time and molarity of the solute affects the rate of
osmosis in potato tissues. One hour is not a sufficient time frame to conduct an effective
osmosis protocol in potato tissues. This is observed through the marked minimal change in
weight after 1 hour compared to the range of between 120-150 minutes, where there is a
significant change in mass, signifying effect of osmosis on the cell contents of the potato
tissue. There is an increase change on the weight of the mass of potato as time increases;
the cell loses more water thus losing its cell contents declining in mass. Hence, the duration
of two and a half hours is an efficient time to observe effective osmosis changes on potato
tissue.
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





