Deakin University HMM301: Pharmacology Report on Ivacaftor's Mechanism
VerifiedAdded on 2023/01/23
|7
|2392
|78
Report
AI Summary
This report delves into the pharmacology of Ivacaftor, a CFTR potentiator used in the treatment of cystic fibrosis. It begins with an introduction to Ivacaftor, its mechanism of action, and its role in treating cystic fibrosis, a genetic disorder characterized by thick mucus in the lungs and digestive system. The report then explores the drug's pharmacodynamics, detailing its interaction with the CFTR protein and its impact on chloride ion transport, which is essential for restoring the function of epithelial cells in the lungs, pancreas, and other organs. The report also discusses the drug's pharmacokinetics, including its absorption, distribution, metabolism, and elimination processes. It highlights the importance of administering Ivacaftor with fatty foods to enhance its bioavailability and describes how the liver metabolizes the drug, producing both active and inactive metabolites. The report concludes with a summary of Ivacaftor's effectiveness in treating cystic fibrosis and its potential for future research. This report is based on the assignment brief for HMM301 Principles of Pharmacology at Deakin University.

Pharmacology 1
PHARMACOLOGY
Student’s Name
Course
Professor’s Name
University
City (State)
Date
PHARMACOLOGY
Student’s Name
Course
Professor’s Name
University
City (State)
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharmacology 2
Introduction
Ivacaftor belongs to class drugs commonly referred to as cystic fibrosis transmembrane
conductance (CFTR) potentiators that boost the function of CFTR protein while treating cystic fibrosis.
Potentiators aim to reinstate the function of CFTR protein, an ion channel that pumps sodium and
chloride ions in and out of the membranes. The epithelial cells that contain CFTR include lungs,
pancreas, reproductive, and digestive tracts. Cystic fibrosis is a congenital condition that targets the
exocrine glands often leads to the production of abnormally thick and sticky mucus that blocks or
damages the epithelial cells. High sweat chloride concentration, premature deaths, and obstruction of
the lungs characterize cystic fibrosis (Grasemann & Ratjen 2013 p. 150). Notably, Ivacaftor targets the
cause rather than the symptoms of cystic fibrosis. Vertex Pharmaceuticals in union with the Cystic
Fibrosis Foundation developed Ivacaftor, which was approved by the Food and Drug Administration
(FDA) in 2012 following improved conditions during the clinical trial phases from 2010 (Fohner et al.
2017 p.41). The FDA approved its combination therapy in 2015 for the treatment of cystic fibrosis in
patients with G551D mutation (Quittner et al. 2015 p. 93). Under such conditions, the amino acid,
aspartic acid replaces glycine at position 551, which results in a defective CFTR protein. According to
Fohner et al. (2017), there about 70,000 cystic fibrosis patients globally, and Ivacaftor has been effective
in the treatment of the disease in children above two years and adults.
Pharmacodynamics
Ivacaftor restores the CFTR activity in individuals with mutations in one of the genetic variants of
the gene. Patients with mutant G551D develop severe cystic fibrosis condition due to defective chloride
gating channels that do not open when ATP binds to the CFTR channels (VanGoor et al. 2014 p. 30).
More also, the mutations of the genes on the CFTR protein results in the alteration of the protein
structure, abnormal fluid and ion movement within the cell membranes. Ivacaftor acts on the cystic
fibrosis transmembrane conductance regulator (CFTR), a cAMP-activated ATP ion gated channel present
in the epithelial cells. Often, the interactions between ligands or signal molecules to their respective
binding site sites open or loses the ion channels. As a potentiator, Ivacaftor forms a tight bond with the
CFTR protein-ion channel resulting in a conformational change that opens the ligand-gated ion channels
(VanGoor et al. 2014 p. 30). The difference in ionic concentration within the membrane triggers cellular
responses that open the pores allowing the chloride and water ions to flow: it facilitates the transport
water and chloride ions across the membranes, hence responsible for the production of thin mucus.
According to Schmidt (2016), the administration of Ivacaftor during the clinical trials resulted in
markedly improved sweat chloride levels and lung functionality. The medication acts by opening the
chloride ligand and slowing down its closure rate (Schmidt et al. 2016 p.127). Similarly, it boosts the
functionality of both the normal and defective CFTR. Besides the open pores on the CFTR protein
controls the functions of other positively charged ion channels that are essential for the normal function
of various organs such as the pancreas. Ivacaftor medication acts on the CFTR anion channels
agonistically to trigger biological responses (Davies et al. 2016 p. 110). The drug has an affinity to its
receptors and affinity: the ability to alter a receptor to elicit a response. The quantity of CFTR protein
present on the membrane surfaces determines the effect of interaction between Ivacaftor and the CFTR
chloride gates. Also, the biological response is contingent on the responsiveness of the mutant protein
on the epithelial membranes (Quittner et al. 2015 p. 93).
Introduction
Ivacaftor belongs to class drugs commonly referred to as cystic fibrosis transmembrane
conductance (CFTR) potentiators that boost the function of CFTR protein while treating cystic fibrosis.
Potentiators aim to reinstate the function of CFTR protein, an ion channel that pumps sodium and
chloride ions in and out of the membranes. The epithelial cells that contain CFTR include lungs,
pancreas, reproductive, and digestive tracts. Cystic fibrosis is a congenital condition that targets the
exocrine glands often leads to the production of abnormally thick and sticky mucus that blocks or
damages the epithelial cells. High sweat chloride concentration, premature deaths, and obstruction of
the lungs characterize cystic fibrosis (Grasemann & Ratjen 2013 p. 150). Notably, Ivacaftor targets the
cause rather than the symptoms of cystic fibrosis. Vertex Pharmaceuticals in union with the Cystic
Fibrosis Foundation developed Ivacaftor, which was approved by the Food and Drug Administration
(FDA) in 2012 following improved conditions during the clinical trial phases from 2010 (Fohner et al.
2017 p.41). The FDA approved its combination therapy in 2015 for the treatment of cystic fibrosis in
patients with G551D mutation (Quittner et al. 2015 p. 93). Under such conditions, the amino acid,
aspartic acid replaces glycine at position 551, which results in a defective CFTR protein. According to
Fohner et al. (2017), there about 70,000 cystic fibrosis patients globally, and Ivacaftor has been effective
in the treatment of the disease in children above two years and adults.
Pharmacodynamics
Ivacaftor restores the CFTR activity in individuals with mutations in one of the genetic variants of
the gene. Patients with mutant G551D develop severe cystic fibrosis condition due to defective chloride
gating channels that do not open when ATP binds to the CFTR channels (VanGoor et al. 2014 p. 30).
More also, the mutations of the genes on the CFTR protein results in the alteration of the protein
structure, abnormal fluid and ion movement within the cell membranes. Ivacaftor acts on the cystic
fibrosis transmembrane conductance regulator (CFTR), a cAMP-activated ATP ion gated channel present
in the epithelial cells. Often, the interactions between ligands or signal molecules to their respective
binding site sites open or loses the ion channels. As a potentiator, Ivacaftor forms a tight bond with the
CFTR protein-ion channel resulting in a conformational change that opens the ligand-gated ion channels
(VanGoor et al. 2014 p. 30). The difference in ionic concentration within the membrane triggers cellular
responses that open the pores allowing the chloride and water ions to flow: it facilitates the transport
water and chloride ions across the membranes, hence responsible for the production of thin mucus.
According to Schmidt (2016), the administration of Ivacaftor during the clinical trials resulted in
markedly improved sweat chloride levels and lung functionality. The medication acts by opening the
chloride ligand and slowing down its closure rate (Schmidt et al. 2016 p.127). Similarly, it boosts the
functionality of both the normal and defective CFTR. Besides the open pores on the CFTR protein
controls the functions of other positively charged ion channels that are essential for the normal function
of various organs such as the pancreas. Ivacaftor medication acts on the CFTR anion channels
agonistically to trigger biological responses (Davies et al. 2016 p. 110). The drug has an affinity to its
receptors and affinity: the ability to alter a receptor to elicit a response. The quantity of CFTR protein
present on the membrane surfaces determines the effect of interaction between Ivacaftor and the CFTR
chloride gates. Also, the biological response is contingent on the responsiveness of the mutant protein
on the epithelial membranes (Quittner et al. 2015 p. 93).

Pharmacology 3
Moreover, the signaling pathways that result in the treatment of cystic fibrosis rely on cyclic-
AMP, a secondary messenger. These messengers are the small water-soluble molecules that spread
throughout the cells via diffusion. Binding of Ivacaftor to the respective receptor activates an inactive G
protein that subsequently activates adenylyl cyclase, ATP, c-AMP, protein kinase A, phosphorylase
kinase, glycogen phosphorylase, and glycogen: the outcome is increased activated molecules and
biological response.
Initial clinical trials showed that Ivacaftor improved weight gain for persons between 6 to 10
years old as patients above 12 years showed markedly improved the transport of chloride ions (Maiuri
et al. 2015). Besides, the patients placed on a 2-week treatment with the drug recounted improved
respiratory conditions. However, patients with F508del alleles showed no improvement in their
symptoms following treatment with Ivacaftor; instead, they were responsive to combination therapy of
Ivacaftor and Lumacaftor (Maiuri et al. 2015). Lumacaftor repairs F508del folding while Ivacaftor
potentiates resulting in about 31% enhancement of the CFTR protein in air passage epithelial cells
obtained from patients with F508del alleles (Maiuri et al. 2015).
Pharmacokinetics
Physicians administer Ivacaftor through the oral route, which is often convenient. However, the
drug must cross several barriers between the ileum and blood circulatory system to reach the site of
action. Several factors that affect the absorption of the drug include its chemical stability, disintegration,
dissolution, formulation, and passage through the gastrointestinal tracts (Ferl, Theil & Wong 2016 p. 76).
Therefore, it’s recommended to administer Ivacaftor with fatty foods to increase its bioavailability: the
fraction of Ivacaftor that will be present in the systemic circulation. There is an enhanced absorption and
increased AUC by 2.5 folds following its administration with fatty diets. More also, peak plasma
concentrations (Tmax) were reached in about four hours after its administration.
Following its oral ingestion, Ivacaftor interacts with various blood constituents and processes as
it moves to the target membranes. The binding of Ivacaftor to blood components, receptors, and
passage through lipid barriers affects the concentration at the target sites and its elimination (Davies et
al. 20161 p.13). Notably, an equilibrium state exists between bound drugs and freely circulation ones;
the freely circulating portion is available for distribution and elimination. Free active drugs determine
both efficacy and toxicity. Ivacaftor is 99% bound to plasma proteins that account for the 1% free
molecules present for its effectiveness. It predominantly binds to alpha-1-acid glycoprotein and albumin
for its distribution to the epithelial membrane epithelial cells.
The liver is the primary site of metabolism of Ivacaftor. Enzymes present in the liver act on drugs
to increase or decrease its action through Phase I and II processes. Cytochrome P450 enzymes modify
the drugs in Phase I to create sites Phase II reactions (Fohner et al. 40). However, the metabolic
processes can take place in other different sites other than the liver such as the lungs and kidneys. At
these sites, the medications may be converted into less or more toxic metabolites; effective or less
effective products; or metabolites with different toxicity or efficacy. Cytochrome P450 enzymes act on
Ivacaftor converting it into hydroxymethyl-ivacaftor (M1), which is more potent than the initial. Besides,
the process produces an inactive metabolite ivacaftor-carboxylate (M6) whose activity is about 1/50th of
the original drug. The figure below depicts the metabolism of Ivacaftor in the liver.
Moreover, the signaling pathways that result in the treatment of cystic fibrosis rely on cyclic-
AMP, a secondary messenger. These messengers are the small water-soluble molecules that spread
throughout the cells via diffusion. Binding of Ivacaftor to the respective receptor activates an inactive G
protein that subsequently activates adenylyl cyclase, ATP, c-AMP, protein kinase A, phosphorylase
kinase, glycogen phosphorylase, and glycogen: the outcome is increased activated molecules and
biological response.
Initial clinical trials showed that Ivacaftor improved weight gain for persons between 6 to 10
years old as patients above 12 years showed markedly improved the transport of chloride ions (Maiuri
et al. 2015). Besides, the patients placed on a 2-week treatment with the drug recounted improved
respiratory conditions. However, patients with F508del alleles showed no improvement in their
symptoms following treatment with Ivacaftor; instead, they were responsive to combination therapy of
Ivacaftor and Lumacaftor (Maiuri et al. 2015). Lumacaftor repairs F508del folding while Ivacaftor
potentiates resulting in about 31% enhancement of the CFTR protein in air passage epithelial cells
obtained from patients with F508del alleles (Maiuri et al. 2015).
Pharmacokinetics
Physicians administer Ivacaftor through the oral route, which is often convenient. However, the
drug must cross several barriers between the ileum and blood circulatory system to reach the site of
action. Several factors that affect the absorption of the drug include its chemical stability, disintegration,
dissolution, formulation, and passage through the gastrointestinal tracts (Ferl, Theil & Wong 2016 p. 76).
Therefore, it’s recommended to administer Ivacaftor with fatty foods to increase its bioavailability: the
fraction of Ivacaftor that will be present in the systemic circulation. There is an enhanced absorption and
increased AUC by 2.5 folds following its administration with fatty diets. More also, peak plasma
concentrations (Tmax) were reached in about four hours after its administration.
Following its oral ingestion, Ivacaftor interacts with various blood constituents and processes as
it moves to the target membranes. The binding of Ivacaftor to blood components, receptors, and
passage through lipid barriers affects the concentration at the target sites and its elimination (Davies et
al. 20161 p.13). Notably, an equilibrium state exists between bound drugs and freely circulation ones;
the freely circulating portion is available for distribution and elimination. Free active drugs determine
both efficacy and toxicity. Ivacaftor is 99% bound to plasma proteins that account for the 1% free
molecules present for its effectiveness. It predominantly binds to alpha-1-acid glycoprotein and albumin
for its distribution to the epithelial membrane epithelial cells.
The liver is the primary site of metabolism of Ivacaftor. Enzymes present in the liver act on drugs
to increase or decrease its action through Phase I and II processes. Cytochrome P450 enzymes modify
the drugs in Phase I to create sites Phase II reactions (Fohner et al. 40). However, the metabolic
processes can take place in other different sites other than the liver such as the lungs and kidneys. At
these sites, the medications may be converted into less or more toxic metabolites; effective or less
effective products; or metabolites with different toxicity or efficacy. Cytochrome P450 enzymes act on
Ivacaftor converting it into hydroxymethyl-ivacaftor (M1), which is more potent than the initial. Besides,
the process produces an inactive metabolite ivacaftor-carboxylate (M6) whose activity is about 1/50th of
the original drug. The figure below depicts the metabolism of Ivacaftor in the liver.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
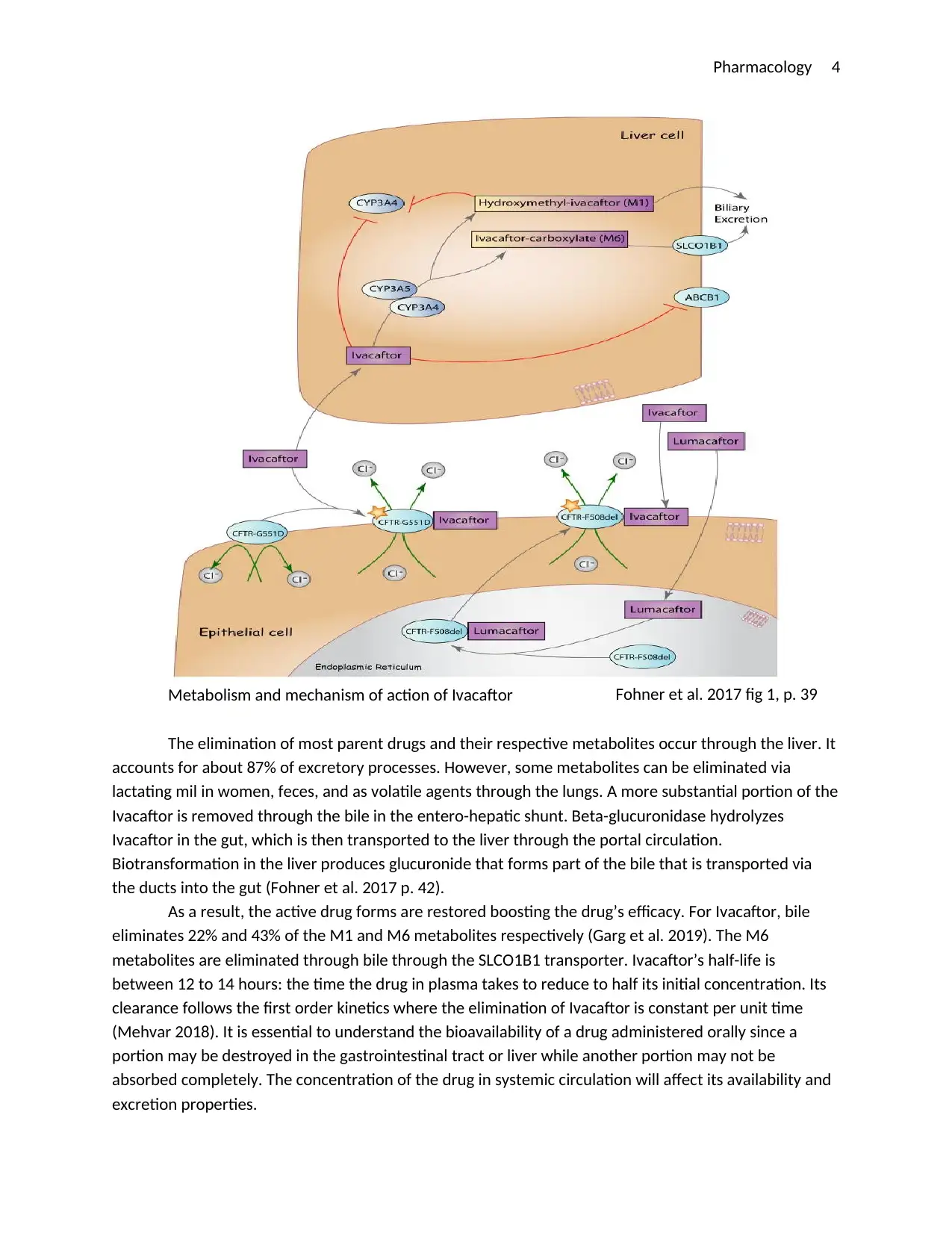
Pharmacology 4
Metabolism and mechanism of action of Ivacaftor
The elimination of most parent drugs and their respective metabolites occur through the liver. It
accounts for about 87% of excretory processes. However, some metabolites can be eliminated via
lactating mil in women, feces, and as volatile agents through the lungs. A more substantial portion of the
Ivacaftor is removed through the bile in the entero-hepatic shunt. Beta-glucuronidase hydrolyzes
Ivacaftor in the gut, which is then transported to the liver through the portal circulation.
Biotransformation in the liver produces glucuronide that forms part of the bile that is transported via
the ducts into the gut (Fohner et al. 2017 p. 42).
As a result, the active drug forms are restored boosting the drug’s efficacy. For Ivacaftor, bile
eliminates 22% and 43% of the M1 and M6 metabolites respectively (Garg et al. 2019). The M6
metabolites are eliminated through bile through the SLCO1B1 transporter. Ivacaftor’s half-life is
between 12 to 14 hours: the time the drug in plasma takes to reduce to half its initial concentration. Its
clearance follows the first order kinetics where the elimination of Ivacaftor is constant per unit time
(Mehvar 2018). It is essential to understand the bioavailability of a drug administered orally since a
portion may be destroyed in the gastrointestinal tract or liver while another portion may not be
absorbed completely. The concentration of the drug in systemic circulation will affect its availability and
excretion properties.
Fohner et al. 2017 fig 1, p. 39
Metabolism and mechanism of action of Ivacaftor
The elimination of most parent drugs and their respective metabolites occur through the liver. It
accounts for about 87% of excretory processes. However, some metabolites can be eliminated via
lactating mil in women, feces, and as volatile agents through the lungs. A more substantial portion of the
Ivacaftor is removed through the bile in the entero-hepatic shunt. Beta-glucuronidase hydrolyzes
Ivacaftor in the gut, which is then transported to the liver through the portal circulation.
Biotransformation in the liver produces glucuronide that forms part of the bile that is transported via
the ducts into the gut (Fohner et al. 2017 p. 42).
As a result, the active drug forms are restored boosting the drug’s efficacy. For Ivacaftor, bile
eliminates 22% and 43% of the M1 and M6 metabolites respectively (Garg et al. 2019). The M6
metabolites are eliminated through bile through the SLCO1B1 transporter. Ivacaftor’s half-life is
between 12 to 14 hours: the time the drug in plasma takes to reduce to half its initial concentration. Its
clearance follows the first order kinetics where the elimination of Ivacaftor is constant per unit time
(Mehvar 2018). It is essential to understand the bioavailability of a drug administered orally since a
portion may be destroyed in the gastrointestinal tract or liver while another portion may not be
absorbed completely. The concentration of the drug in systemic circulation will affect its availability and
excretion properties.
Fohner et al. 2017 fig 1, p. 39
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharmacology 5
Conclusion
Cystic fibrosis is a genetic disorder that continues to threaten most children and adults.
Abnormally high levels of thick mucus in the respiratory and gastrointestinal tracts, which results in
recurring infections characterize the disorder. Defective CFTR proteins cause ineffective transport of
chloride ions between membranes. The movement of chloride and water molecules between epithelial
membrane cells is responsible for the production of clear and runny mucus. Further research projects
and a better understanding of the defective CFTR proteins has borne the development of site-specific
molecules that target dysfunctional proteins. Ivacaftor, the first licensed medication by the Food and
Drug Administration, has proven to be effective in treating the cause of cystic fibrosis rather than its
symptoms. It targets CFTR gating mutation (G551D) and acts as a potentiator. Ivacaftor binds to the
defective chloride-gated channel, causing it to open while slowing its closure rate. As a result, it restores
the homeostatic balance of chloride ions between membranes. Therefore, it improves lung and
pulmonary function as well as decreasing sweat chloride. Ivacaftor is given orally twice daily. Ivacaftor
binds to plasma proteins. Since drugs bound to plasma proteins cannot be removed through the
glomeruli, Ivacaftor releases its therapeutic agents slowly hence a longer half-life. A longer half-life (12-
14 hours) dictates Ivacaftor’s duration of action. The drug shows a more considerable promise to the
treatment of cystic fibrosis.
Reference List
Conclusion
Cystic fibrosis is a genetic disorder that continues to threaten most children and adults.
Abnormally high levels of thick mucus in the respiratory and gastrointestinal tracts, which results in
recurring infections characterize the disorder. Defective CFTR proteins cause ineffective transport of
chloride ions between membranes. The movement of chloride and water molecules between epithelial
membrane cells is responsible for the production of clear and runny mucus. Further research projects
and a better understanding of the defective CFTR proteins has borne the development of site-specific
molecules that target dysfunctional proteins. Ivacaftor, the first licensed medication by the Food and
Drug Administration, has proven to be effective in treating the cause of cystic fibrosis rather than its
symptoms. It targets CFTR gating mutation (G551D) and acts as a potentiator. Ivacaftor binds to the
defective chloride-gated channel, causing it to open while slowing its closure rate. As a result, it restores
the homeostatic balance of chloride ions between membranes. Therefore, it improves lung and
pulmonary function as well as decreasing sweat chloride. Ivacaftor is given orally twice daily. Ivacaftor
binds to plasma proteins. Since drugs bound to plasma proteins cannot be removed through the
glomeruli, Ivacaftor releases its therapeutic agents slowly hence a longer half-life. A longer half-life (12-
14 hours) dictates Ivacaftor’s duration of action. The drug shows a more considerable promise to the
treatment of cystic fibrosis.
Reference List

Pharmacology 6
Davies, JC, Cunningham, S, Harris, WT, Lapey, A, Regelmann, WE, Sawicki, GS, Southern, KW, Robertson,
S, Green, Y & Rosenfeld, M 2016, Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in
patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm
study. The Lancet Respiratory Medicine, vol 4, no. 2, pp. 107-115.
Ferl, GZ, Theil, FP & Wong, H 2016, Physiologically based pharmacokinetic models of small molecules
and therapeutic antibodies: a mini review on fundamental concepts and applications.‐ Biopharmaceutics
& drug disposition, vol. 37, no. 2, pp. 75-92.
Fohner, AE, McDonagh, EM, Clancy, JP, Whirl, CM, Altman, RB & Klein, TE 2017, PharmGKB summary:
ivacaftor pathway, pharmacokinetics/pharmacodynamics. Pharmacogenetics and genomics,, vol. 27, no.
1, pp. 39-42.
Garg, V, Shen, J, Li, C, Agarwal, S, Gebre, A, Robertson, S, Huang, J, Han, L, Stephan, K & Wang, LT 2019,
Pharmacokinetic and Drug–Drug Interaction Profiles of the Combination of Tezacaftor/Ivacaftor. Clinical
and translational science.
Grasemann, H & Ratjen, F 2013, Early lung disease in cystic fibrosis. The Lancet Respiratory Medicine,
vol. 1, no. 2, pp. 148-157.
Maiuri, L, De Stefno, D, Raia, V & Kroemer, G 2015, The holy grail of cystic fibrosis research:
pharmacological repair of the F508del-CFTR mutation. Annals of translational medicine, vol. 3.
Mehvar, R 2018. Fundamentals and Application to Pharmacokinetic Behavior of Drugs.
Davies, JC, Cunningham, S, Harris, WT, Lapey, A, Regelmann, WE, Sawicki, GS, Southern, KW, Robertson,
S, Green, Y & Rosenfeld, M 2016, Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in
patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm
study. The Lancet Respiratory Medicine, vol 4, no. 2, pp. 107-115.
Ferl, GZ, Theil, FP & Wong, H 2016, Physiologically based pharmacokinetic models of small molecules
and therapeutic antibodies: a mini review on fundamental concepts and applications.‐ Biopharmaceutics
& drug disposition, vol. 37, no. 2, pp. 75-92.
Fohner, AE, McDonagh, EM, Clancy, JP, Whirl, CM, Altman, RB & Klein, TE 2017, PharmGKB summary:
ivacaftor pathway, pharmacokinetics/pharmacodynamics. Pharmacogenetics and genomics,, vol. 27, no.
1, pp. 39-42.
Garg, V, Shen, J, Li, C, Agarwal, S, Gebre, A, Robertson, S, Huang, J, Han, L, Stephan, K & Wang, LT 2019,
Pharmacokinetic and Drug–Drug Interaction Profiles of the Combination of Tezacaftor/Ivacaftor. Clinical
and translational science.
Grasemann, H & Ratjen, F 2013, Early lung disease in cystic fibrosis. The Lancet Respiratory Medicine,
vol. 1, no. 2, pp. 148-157.
Maiuri, L, De Stefno, D, Raia, V & Kroemer, G 2015, The holy grail of cystic fibrosis research:
pharmacological repair of the F508del-CFTR mutation. Annals of translational medicine, vol. 3.
Mehvar, R 2018. Fundamentals and Application to Pharmacokinetic Behavior of Drugs.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pharmacology 7
Quittner, A, Suthoff, E, Rendas-Baum, R, Bayliss, MS, Sermet-Gaudelus, I, Castiglione, B & Vera-Llonch, M
2015, Effect of ivacaftor treatment in patients with cystic fibrosis and the G551D-CFTR mutation:
patient-reported outcomes in the STRIVE randomized, controlled trial. Health and quality of life
outcomes, vol. 13, p. 93.
Schmidt, BZ, Haaf, JB, Leal, T & Noel, S 2016, Cystic fibrosis transmembrane conductance regulator
modulators in cystic fibrosis: current perspectives. Clinical pharmacology: advances and applications,
vol. 8, pp. 127-140.
Van Goor, F, Yu, H, Burton, B & Hoffman, BJ 2014, Effect of ivacaftor on CFTR forms with missense
mutations associated with defects in protein processing or function. Journal of Cystic Fibrosis, vol. 13,
no. 1, pp. 29-36.
Quittner, A, Suthoff, E, Rendas-Baum, R, Bayliss, MS, Sermet-Gaudelus, I, Castiglione, B & Vera-Llonch, M
2015, Effect of ivacaftor treatment in patients with cystic fibrosis and the G551D-CFTR mutation:
patient-reported outcomes in the STRIVE randomized, controlled trial. Health and quality of life
outcomes, vol. 13, p. 93.
Schmidt, BZ, Haaf, JB, Leal, T & Noel, S 2016, Cystic fibrosis transmembrane conductance regulator
modulators in cystic fibrosis: current perspectives. Clinical pharmacology: advances and applications,
vol. 8, pp. 127-140.
Van Goor, F, Yu, H, Burton, B & Hoffman, BJ 2014, Effect of ivacaftor on CFTR forms with missense
mutations associated with defects in protein processing or function. Journal of Cystic Fibrosis, vol. 13,
no. 1, pp. 29-36.
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





